Key Points
-
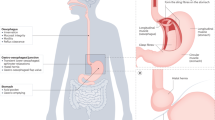
An organic process must be ruled out by endoscopy in all patients presenting with dysphagia symptoms
-
Eosinophilic oesophagitis is one of the most prevalent causes of dysphagia in adults and children
-
Oesophageal biopsy samples should be obtained in all patients with unexplained dysphagia symptoms
-
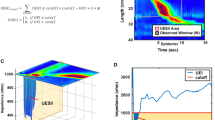
High-resolution manometry is the gold-standard investigation for diagnosis of oesophageal motor disorders
-
According to the Chicago classification algorithm, major oesophageal motor disorders are achalasia, oesophagogastric junction outflow obstruction, diffuse oesophageal spasm, hypercontractile 'jackhammer' oesophagus and absent peristalsis
-
Sophisticated investigations (for example integrated pressure impedance analysis and impedance planimetry) could reveal subtle abnormalities and help distinguish between neuromechanical dysfunction and functional dysphagia
Abstract
Oesophageal dysphagia is a common symptom, which might be related to severe oesophageal diseases such as carcinomas. Therefore, an organic process must be ruled out in the first instance by endoscopy in all patients presenting with dysphagia symptoms. The most prevalent obstructive aetiologies are oesophageal cancer, peptic strictures and eosinophilic oesophagitis. Eosinophilic oesophagitis is one of the most common causes of dysphagia in adults and children, thus justifying the need to obtain oesophageal biopsy samples from all patients presenting with unexplained dysphagia. With the advent of standardized high-resolution manometry and specific metrics to characterize oesophageal motility, the Chicago classification has become a gold-standard algorithm for manometric diagnosis of oesophageal motor disorders. In addition, sophisticated investigations and analysis methods that combine pressure and impedance measurement are currently in development. In the future, these techniques might be able to detect subtle pressure abnormalities during bolus transport, which could further explain pathophysiology and symptoms. The degree to which novel approaches will help distinguish dysphagia caused by motor abnormalities from functional dysphagia still needs to be determined.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Eslick, G. D. & Talley, N. J. Dysphagia: epidemiology, risk factors and impact on quality of life—a population-based study. Aliment. Pharmacol. Ther. 27, 971–979 (2008).
Lindgren, S. & Janzon, L. Prevalence of swallowing complaints and clinical findings among 50–79 year-old men and women in an urban population. Dysphagia 6, 187–192 (1991).
Galmiche, J. P. et al. Functional esophageal disorders. Gastroenterology 130, 1459–1465 (2006).
Roeder, B. E., Murray, J. A. & Dierkhising, R. A. Patient localization of esophageal dysphagia. Dig. Dis. Sci. 49, 697–701 (2004).
Chen, C. L. & Orr, W. C. Comparison of esophageal motility in patients with solid dysphagia and mixed dysphagia. Dysphagia 20, 261–265 (2005).
Zografos, G. N., Georgiadou, D., Thomas, D., Kaltsas, G. & Digalakis, M. Drug-induced esophagitis. Dis. Esophagus 22, 633–637 (2009).
Dellon, E. S. et al. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am. J. Gastroenterol. 108, 679–692 (2013).
Belafsky, P. C. et al. Validity and reliability of the eating assessment tool (EAT-10). Ann. Otol. Rhinol. Laryngol. 117, 919–924 (2008).
McElhiney, J. et al. The mayo dysphagia questionnaire-30: documentation of reliability and validity of a tool for interventional trials in adults with esophageal disease. Dysphagia 25, 221–230 (2010).
Rommel, N., De Meyer, A. M., Feenstra, L. & Veereman-Wauters, G. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J. Pediatr. Gastroenterol. Nutr. 37, 75–84 (2003).
Singendonk, M. M., Rommel, N., Omari, T. I., Benninga, M. A. & van Wijk, M. P. Upper gastrointestinal motility: prenatal development and problems in infancy. Nat. Rev. Gastroenterol. Hepatol. 11, 545–555 (2014).
Muller, M. et al. Is the Schatzki ring a unique esophageal entity? World J. Gastroenterol. 17, 2838–2843 (2011).
Hirano, I. et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 62, 489–495 (2013).
Muller, S., Puhl, S., Vieth, M. & Stolte, M. Analysis of symptoms and endoscopic findings in 117 patients with histological diagnoses of eosinophilic esophagitis. Endoscopy 39, 339–344 (2007).
Ponsot, P. et al. Chronic esophagitis dissecans: an unrecognized clinicopathologic entity? Gastrointest. Endosc. 45, 38–45 (1997).
Hokama, A. et al. Esophagitis dissecans superficialis and autoimmune bullous dermatoses: A review. World J. Gastrointest. Endosc. 2, 252–256 (2010).
Katzka, D. A. et al. Variations in presentations of esophageal involvement in lichen planus. Clin. Gastroenterol. Hepatol. 8, 777–782 (2010).
Vaezi, M. F., Baker, M. E., Achkar, E. & Richter, J. E. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut 50, 765–770 (2002).
Vaezi, M. F., Baker, M. E. & Richter, J. E. Assessment of esophageal emptying post-pneumatic dilation: use of the timed barium esophagram. Am. J. Gastroenterol. 94, 1802–1807 (1999).
Bredenoord, A. J. et al. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol. Motil. 24 (Suppl. 1), 57–65 (2012).
Pandolfino, J. E., Kwiatek, M. A., Ho, K., Scherer, J. R. & Kahrilas, P. J. Unique features of esophagogastric junction pressure topography in hiatus hernia patients with dysphagia. Surgery 147, 57–64 (2010).
Kahrilas, P. J. & Peters, J. H. Evaluation of the esophagogastric junction using high resolution manometry and esophageal pressure topography. Neurogastroenterol. Motil. 24 (Suppl. 1), 11–19 (2012).
Xiao, Y. et al. Lack of correlation between HRM metrics and symptoms during the manometric protocol. Am. J. Gastroenterol. 109, 521–526 (2014).
Daum, C. et al. Failure to respond to physiologic challenge characterizes esophageal motility in erosive gastro-esophageal reflux disease. Neurogastroenterol. Motil. 23, 517–e200 (2011).
Sweis, R. et al. Normative values and inter-observer agreement for liquid and solid bolus swallows in upright and supine positions as assessed by esophageal high-resolution manometry. Neurogastroenterol. Motil. 23, 509–e198 (2011).
Singendonk, M. M. J. et al. Applying the Chicago Classification criteria of esophageal motility to a pediatric cohort: effects of patient age and size. Neurogastroenterol. Motil. 26, 1333–1341 (2014).
Chumpitazi, B. & Nurko, S. Pediatric gastrointestinal motility disorders: challenges and a clinical update. Gastroenterol. Hepatol. (N.Y.) 4, 140–148 (2008).
Fox, M. et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol. Motil. 16, 533–542 (2004).
Omari, T., Tack, J. & Rommel, N. Impedance as an adjunct to manometric testing: What it has failed to do and what it may tell us in the future. United European Gastroenterol. J. 2, 355–366 (2014).
Omari, T., Kritas, S. & Cock, C. New insights into pharyngo-esophageal bolus transport revealed by pressure-impedance measurement. Neurogastroenterol. Motil. 24, 549–556 (2012).
Imam, H., Marrero, F. & Shay, S. Impedance nadir values correlate with barium bolus amount. Dis. Esophagus 25, 600–607 (2012).
Kim, J. H., Mittal, R. K., Patel, N., Ledgerwood, M. & Bhargava, V. Esophageal distension during bolus transport: can it be detected by intraluminal impedance recordings? Neurogastroenterol. Motil. 26, 1122–1130 (2014).
Omari, T. I. et al. Upper esophageal sphincter impedance as a marker of sphincter opening diameter. Am. J. Physiol. Gastrointest. Liver Physiol. 302, 909–913 (2012).
Lin, Z. et al. Flow time through esophagogastric junction derived during high-resolution impedance-manometry studies: a novel parameter for assessing esophageal bolus transit. Am. J. Physiol. Gastrointest. Liver Physiol. 307, 158–163 (2014).
Omari, T. I., Wauters, L., Rommel, N., Kritas, S. & Myers, J. C. Oesophageal pressure-flow metrics in relation to bolus volume, bolus consistency, and bolus perception. United European Gastroenterol. J. 1, 249–258 (2013).
Chen, C. L., Yi, C. H., Liu, T. T., Hsu, C. S. & Omari, T. I. Characterization of esophageal pressure-flow abnormalities in patients with non-obstructive dysphagia and normal manometry findings. J. Gastroenterol. Hepatol. 28, 946–953 (2013).
Cho, Y. K. et al. Assessing bolus retention in achalasia using high-resolution manometry with impedance: a comparator study with timed barium esophagram. Am. J. Gastroenterol. 109, 829–835 (2014).
Conchillo, J. M., Selimah, M., Bredenoord, A. J., Samsom, M. & Smout, A. J. Assessment of oesophageal emptying in achalasia patients by intraluminal impedance monitoring. Neurogastroenterol. Motil. 18, 971–977 (2006).
Rohof, W. O., Hirsch, D. P., Kessing, B. F. & Boeckxstaens, G. E. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology 143, 328–335 (2012).
Nicodeme, F. et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 11, 1101–1107 (2013).
Gregersen, H., Kwiatek, M. A., Schwizer, W. & Tutuian, R. Contribution of sensitivity, volume and tone to visceral perception in the upper gastrointestinal tract in man: emphasis on testing. Neurogastroenterol. Motil. 19, 47–61 (2007).
Lin, Z. et al. Regional variation in distal esophagus distensibility assessed using the functional luminal imaging probe (FLIP). Neurogastroenterol. Motil. 25, 765–771 (2013).
Vakil, N., van Zanten, S. V., Kahrilas, P., Dent, J. & Jones, R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am. J. Gastroenterol. 101, 1900–1920 (2006).
Armstrong, D. et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 111, 85–92 (1996).
Vakil, N. B., Traxler, B. & Levine, D. Dysphagia in patients with erosive esophagitis: prevalence, severity, and response to proton pump inhibitor treatment. Clin. Gastroenterol. Hepatol. 2, 665–668 (2004).
Rubenstein, J. H. & Taylor, J. B. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment. Pharmacol. Ther. 32, 1222–1227 (2010).
Fransen, G. A., Janssen, M. J., Muris, J. W., Laheij, R. J. & Jansen, J. B. Meta-analysis: the diagnostic value of alarm symptoms for upper gastrointestinal malignancy. Aliment. Pharmacol. Ther. 20, 1045–1052 (2004).
El-Serag, H. B. & Lau, M. Temporal trends in new and recurrent oesophageal strictures in a Medicare population. Aliment. Pharmacol. Ther. 25, 1223–1229 (2007).
Savarino, E. et al. Oesophageal motility and bolus transit abnormalities increase in parallel with the severity of gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 34, 476–486 (2011).
Broeders, J. A. et al. Systematic review and meta-analysis of laparoscopic Nissen (posterior total) versus Toupet (posterior partial) fundoplication for gastro-oesophageal reflux disease. Br. J. Surg. 97, 1318–1330 (2010).
Galmiche, J. P. et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 305, 1969–1977 (2011).
Loots, C. et al. Gastroesophageal reflux, esophageal function, gastric emptying, and the relationship to dysphagia before and after antireflux surgery in children. J. Pediatr. 162, 566–573 (2013).
Myers, J. C. et al. Susceptibility to dysphagia after fundoplication revealed by novel automated impedance manometry analysis. Neurogastroenterol. Motil. 24, 812–e393 (2012).
Stoikes, N. et al. The value of multiple rapid swallows during preoperative esophageal manometry before laparoscopic antireflux surgery. Surg. Endosc. 26, 3401–3407 (2012).
Kidambi, T., Toto, E., Ho, N., Taft, T. & Hirano, I. Temporal trends in the relative prevalence of dysphagia etiologies from 1999–2009. World J. Gastroenterol. 18, 4335–4341 (2012).
Syed, A. A. et al. The rising incidence of eosinophilic oesophagitis is associated with increasing biopsy rates: a population-based study. Aliment. Pharmacol. Ther. 36, 950–958 (2012).
Rothenberg, M. E. et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat. Genet. 42, 289–291 (2010).
Gonsalves, N. et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 142, 1451–1459 (2012).
Lucendo, A. J. et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J. Allergy Clin. Immunol. 131, 797–804 (2013).
Molina-Infante, J. et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin. Gastroenterol. Hepatol. 9, 110–117 (2011).
van Rhijn, B. D. et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 12, 1815–1823 (2014).
Roman, S. et al. Manometric features of eosinophilic esophagitis in esophageal pressure topography. Neurogastroenterol. Motil. 23, 208–214 (2011).
Kwiatek, M. A. et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology 140, 82–90 (2011).
van Rhijn, B. D., Verheij, J., Smout, A. J. & Bredenoord, A. J. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol. Motil. 25, 47–52 (2013).
Furuta, G. T. et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 133, 1342–1363 (2007).
Desai, T. K. et al. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest. Endosc. 61, 795–801 (2005).
Carlson, D. A. & Pandolfino, J. E. The Chicago criteria for esophageal motility disorders: what has changed in the past 5 years? Curr. Opin. Gastroenterol. 28, 395–402 (2012).
Kahrilas, P. J. Esophageal motor disorders in terms of high-resolution esophageal pressure topography: what has changed? Am. J. Gastroenterol. 105, 981–987 (2010).
Kahrilas, P. J. & Boeckxstaens, G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology 145, 954–965 (2013).
Pandolfino, J. E. et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology 135, 1526–1533 (2008).
Nicodeme, F. et al. Adding a radial dimension to the assessment of esophagogastric junction relaxation: validation studies of the 3D-eSleeve. Am. J. Physiol. Gastrointest. Liver Physiol. 303, 275–280 (2012).
Bogte, A., Bredenoord, A. J., Oors, J., Siersema, P. D. & Smout, A. J. Relationship between esophageal contraction patterns and clearance of swallowed liquid and solid boluses in healthy controls and patients with dysphagia. Neurogastroenterol. Motil. 24, 364–372 (2012).
Sweis, R., Anggiansah, A., Wong, T., Brady, G. & Fox, M. Assessment of esophageal dysfunction and symptoms during and after a standardized test meal: development and clinical validation of a new methodology utilizing high-resolution manometry. Neurogastroenterol. Motil. 26, 215–228 (2014).
Nguyen, N. Q., Holloway, R. H., Smout, A. J. & Omari, T. I. Automated impedance-manometry analysis detects esophageal motor dysfunction in patients who have non-obstructive dysphagia with normal manometry. Neurogastroenterol. Motil. 25, 238–245 (2013).
Rommel, N., Van Oudenhove, L., Tack, J. & Omari, T. I. Automated impedance manometry analysis as a method to assess esophageal function. Neurogastroenterol. Motil. 26, 636–645 (2014).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
F.Z. is a consultant and speaker for Given Imaging. T.O. holds patents on pressure-flow analysis methods.
Rights and permissions
About this article
Cite this article
Zerbib, F., Omari, T. Oesophageal dysphagia: manifestations and diagnosis. Nat Rev Gastroenterol Hepatol 12, 322–331 (2015). https://doi.org/10.1038/nrgastro.2014.195
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2014.195
This article is cited by
-
Oesophageal cancer awareness and anticipated time to help-seeking: results from a population-based survey
British Journal of Cancer (2024)
-
Esophageal dysphagia in neuromuscular disorder patients with validity and reliability study of the brief esophageal dysphagia questionnaire
Acta Neurologica Belgica (2022)
-
Validation in French of the Brief Esophageal Dysphagia Questionnaire in Patients Referred For Esophageal Manometry
Dysphagia (2022)
-
High-Resolution Manometry Improves the Diagnosis of Esophageal Motility Disorders in Patients With Dysphagia: A Randomized Multicenter Study
American Journal of Gastroenterology (2016)
-
Therapeutic options in oesophageal dysphagia
Nature Reviews Gastroenterology & Hepatology (2015)