Key Points
-
Patients co-infected with HCV and HIV are at increased risk of accelerated disease progression, resulting in higher rates of liver decompensation and death compared with patients monoinfected with HCV
-
HIV accelerates HCV-related fibrosis progression through multiple mechanisms
-
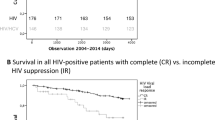
HIV suppression seems to reduce fibrosis progression and decrease rates of hepatic decompensation among co-infected patients
-
Successful HCV therapy is associated with a halting of fibrosis progression and decreased complications from end-stage liver disease, but historical rates of sustained virologic response have been significantly lower among co-infected patients than those for chronic HCV monoinfection
-
Promising data exist for all-oral direct-acting antiviral agents suggesting improved efficacy and tolerability, which supports their use in co-infection
Abstract
HCV and HIV co-infection is associated with accelerated hepatic fibrosis progression and higher rates of liver decompensation and death compared to HCV monoinfection, and liver disease is a leading cause of non-AIDS-related mortality among HIV-infected patients. New insights have revealed multiple mechanisms by which HCV and HIV lead to accelerated disease progression, specifically that HIV infection increases HCV replication, augments HCV-induced hepatic inflammation, increases hepatocyte apoptosis, increases microbial translocation from the gut and leads to an impairment of HCV-specific immune responses. Treatment of HIV with antiretroviral therapy and treatment of HCV have independently been shown to delay the progression of fibrosis and reduce complications from end-stage liver disease among co-infected patients. However, rates of sustained virologic response with PEG-IFN and ribavirin have been significantly inferior among co-infected patients compared with HCV-monoinfected patients, and treatment uptake has remained low given the limited efficacy and tolerability of current HCV regimens. With multiple direct-acting antiviral agents in development to treat HCV, a unique opportunity exists to redefine the treatment paradigm for co-infected patients, which incorporates data on fibrosis stage as well as potential drug interactions with antiretroviral therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
World Health Organization. Global Health Observatory: HIV/AIDS. WHO [online], (2014).
Kim, A. Y. & Chung, R. T. Coinfection with HIV-1 and HCV—a one-two punch. Gastroenterology 137, 795–814 (2009).
Rotman, Y. & Liang, T. J. Coinfection with hepatitis C virus and human immunodeficiency virus: virological, immunological, and clinical outcomes. J. Virol. 83, 7366–7374 (2009).
Koziel, M. J. & Peters, M. G. Viral hepatitis in HIV infection. N. Engl. J. Med. 356, 1445–1454 (2007).
Smith, C. et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 24, 1537–1548 (2010).
Gerberding, J. L. Incidence and prevalence of human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and cytomegalovirus among health care personnel at risk for blood exposure: final report from a longitudinal study. J. Infect. Dis. 170, 1410–1417 (1994).
Garten, R. J. et al. Rapid transmission of hepatitis C virus among young injecting heroin users in Southern China. Int. J. Epidemiol. 33, 182–188 (2004).
Quan, V. M. et al. Risks for HIV, HBV, and HCV infections among male injection drug users in northern Vietnam: a case–control study. AIDS Care 21, 7–16 (2009).
Rauch, A. et al. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV Cohort Study. Clin. Infect. Dis. 41, 395–402 (2005).
Eyster, M. E. et al. Heterosexual co-transmission of hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Ann. Intern. Med. 115, 764–768 (1991).
Thomas, D. L. et al. Perinatal transmission of hepatitis C virus from human immunodeficiency virus type 1-infected mothers. Women and Infants Transmission Study. J. Infect. Dis. 177, 1480–1488 (1998).
Hershow, R. C. et al. Increased vertical transmission of human immunodeficiency virus from hepatitis C virus-coinfected mothers. Women and Infants Transmission Study. J. Infect. Dis. 176, 414–420 (1997).
Polis, C. B. et al. Impact of maternal HIV coinfection on the vertical transmission of hepatitis C virus: a meta-analysis. Clin. Infect. Dis. 44, 1123–1131 (2007).
Pappalardo, B. L. Influence of maternal human immunodeficiency virus (HIV) co-infection on vertical transmission of hepatitis C virus (HCV): a meta-analysis. Int. J. Epidemiol. 32, 727–734 (2003).
A significant sex—but not elective cesarean section—effect on mother-to-child transmission of hepatitis C virus infection. J. Infect. Dis. 192, 1872–1879 (2005).
Marine-Barjoan, E. et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS 21, 1811–1815 (2007).
Sulkowski, M. S. & Thomas, D. L. Hepatitis C in the HIV-infected person. Ann. Intern. Med. 138, 197–207 (2003).
Gotz, H. M. et al. A cluster of acute hepatitis C virus infection among men who have sex with men—results from contact tracing and public health implications. AIDS 19, 969–974 (2005).
van de Laar, T. et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology 136, 1609–1617 (2009).
Giraudon, I. et al. Increase in diagnosed newly acquired hepatitis C in HIV-positive men who have sex with men across London and Brighton, 2002–2006: is this an outbreak? Sex. Transm. Infect. 84, 111–115 (2008).
van de Laar, T. J. et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J. Infect. Dis. 196, 230–238 (2007).
Gamage, D. G. et al. Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect. Dis. 11, 39 (2011).
Wandeler, G. et al. Hepatitis C virus infections in the Swiss HIV Cohort Study: a rapidly evolving epidemic. Clin. Infect. Dis. 55, 1408–1416 (2012).
Brook, G. et al. British HIV Association guidelines for the management of coinfection with HIV-1 and hepatitis B or C virus 2010. HIV Med. 11, 1–30 (2010).
Rockstroh, J. K. et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med. 9, 82–88 (2008).
European AIDS Treatment Network (NEAT) Acute Hepatitis C Infection Consensus Panel. Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference. AIDS 25, 399–409 (2011).
Rein, D. B. et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in US primary care settings. Ann. Intern. Med. 156, 263–270 (2012).
Hernando, V. et al. All-cause and liver-related mortality in HIV positive subjects compared to the general population: differences by HCV co-infection. J. Hepatol. 57, 743–751 (2012).
Chen, T. Y. et al. Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin. Infect. Dis. 49, 1605–1615 (2009).
Eyster, M. E. et al. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Multicenter Hemophilia Cohort Study. Blood 84, 1020–1023 (1994).
Alter, M. J. et al. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N. Engl. J. Med. 327, 1899–1905 (1992).
Thomas, D. L. et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 284, 450–456 (2000).
Benhamou, Y. et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 30, 1054–1058 (1999).
Sulkowski, M. S. et al. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS 21, 2209–2216 (2007).
Ghany, M. G. et al. Progression of fibrosis in chronic hepatitis C. Gastroenterology 124, 97–104 (2003).
Ryder, S. D. et al. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut 53, 451–455 (2004).
Macias, J. et al. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology 50, 1056–1063 (2009).
Brau, N. et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J. Hepatol. 44, 47–55 (2006).
Verma, S. et al. Do type and duration of antiretroviral therapy attenuate liver fibrosis in HIV-hepatitis C virus-coinfected patients? Clin. Infect. Dis. 42, 262–270 (2006).
Sterling, R. K. et al. Similar progression of fibrosis between HIV/HCV-infected and HCV-infected patients: analysis of paired liver biopsy samples. Clin. Gastroenterol. Hepatol. 8, 1070–1076 (2010).
Thein, H. H. et al. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 22, 1979–1991 (2008).
Graham, C. S. et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin. Infect. Dis. 33, 562–569 (2001).
Pineda, J. A. et al. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology 41, 779–789 (2005).
Pineda, J. A. et al. Natural history of compensated hepatitis C virus-related cirrhosis in HIV-infected patients. Clin. Infect. Dis. 49, 1274–1282 (2009).
Giron-Gonzalez, J. A. et al. Natural history of compensated and decompensated HCV-related cirrhosis in HIV-infected patients: a prospective multicentre study. Antivir. Ther. 12, 899–907 (2007).
Brau, N. et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a US–Canadian multicenter study. J. Hepatol. 47, 527–537 (2007).
Verma, S. HAART attenuates liver fibrosis in patients with HIV/HCV co-infection: fact or fiction? J. Antimicrob. Chemother. 58, 496–501 (2006).
Lin, W. et al. HIV increases HCV replication in a TGF-β1-dependent manner. Gastroenterology 134, 803–811 (2008).
Jang, J. Y. et al. HIV infection increases HCV-induced hepatocyte apoptosis. J. Hepatol. 54, 612–620 (2011).
Zheng, S. J. et al. Critical roles of TRAIL in hepatic cell death and hepatic inflammation. J. Clin. Invest. 113, 58–64 (2004).
Lin, W. et al. Hepatitis C virus regulates transforming growth factor β1 production through the generation of reactive oxygen species in a nuclear factor κB-dependent manner. Gastroenterology 138, 2509–2518 (2010).
Lin, W. et al. HIV and HCV cooperatively promote hepatic fibrogenesis via induction of reactive oxygen species and NFκB. J. Biol. Chem. 286, 2665–2674 (2011).
Tuyama, A. C. et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology 52, 612–622 (2010).
Veazey, R. S. et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280, 427–431 (1998).
Brenchley, J. M. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371 (2006).
Seki, E. et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332 (2007).
Balagopal, A. et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology 135, 226–233 (2008).
Flynn, J. K. et al. Impaired hepatitis C virus (HCV)-specific interferon-γ responses in individuals with HIV who acquire HCV infection: correlation with CD4(+) T-cell counts. J. Infect. Dis. 206, 1568–1576 (2012).
Kim, A. Y. et al. Impaired hepatitis C virus-specific T cell responses and recurrent hepatitis C virus in HIV coinfection. PLoS Med. 3, e492 (2006).
Morishima, C. et al. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology 43, 573–580 (2006).
Glassner, A. et al. Impaired CD4(+) T cell stimulation of NK cell anti-fibrotic activity may contribute to accelerated liver fibrosis progression in HIV/HCV patients. J. Hepatol. 59, 427–433 (2013).
Palmateer, N. et al. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction 105, 844–859 (2010).
Blome, M. A. et al. Minimal transmission of HIV despite persistently high transmission of hepatitis C virus in a Swedish needle exchange program. J. Viral Hepat. 18, 831–839 (2011).
Paintsil, E., He, H., Peters, C., Lindenbach, B. D. & Heimer, R. Survival of hepatitis C virus in syringes: implication for transmission among injection drug users. J. Infect. Dis. 202, 984–990 (2010).
Martin, T. C. et al. Hepatitis C virus reinfection incidence and treatment outcome among HIV-positive MSM. AIDS 27, 2551–2557 (2013).
Walensky, R. P. et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N. Engl. J. Med. 369, 1715–1725 (2013).
Thomas, D. L. Global control of hepatitis C: where challenge meets opportunity. Nat. Med. 19, 850–858 (2013).
Qurishi, N. et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet 362, 1708–1713 (2003).
Limketkai, B. N. et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA 308, 370–378 (2012).
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. AIDSinfo [online].
European AIDS Clinical Society. Guidelines, Version 7.0, October 2013. European AIDS Clinical Society [online], (2013).
Aranzabal, L. et al. Influence of liver fibrosis on highly active antiretroviral therapy-associated hepatotoxicity in patients with HIV and hepatitis C virus coinfection. Clin. Infect. Dis. 40, 588–593 (2005).
Dorward, J. et al. Successful treatment of acute hepatitis C virus in HIV positive patients using the European AIDS Treatment Network guidelines for treatment duration. J. Clin. Virol. 52, 367–369 (2011).
Webster, D. P. et al. Spontaneous clearance and treatment of acute hepatitis C infection in HIV-positive men with 48 weeks of interferon-α and ribavirin. Int. J. STD AIDS 24, 179–183 (2013).
Broers, B. et al. Barriers to interferon-α therapy are higher in intravenous drug users than in other patients with acute hepatitis C. J. Hepatol. 42, 323–328 (2005).
Wiegand, J. et al. Early monotherapy with pegylated interferon α-2b for acute hepatitis C infection: the HEP-NET acute HCV-II study. Hepatology 43, 250–256 (2006).
Fierer, D. S. et al. Telaprevir in the treatment of acute hepatitis C infection in HIV-infected men. Clin. Infect. Dis. http://dx.doi.org/10.1093/cid/cit799.
van der Meer, A. J. et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 308, 2584–2593 (2012).
Backus, L. I. et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin. Gastroenterol. Hepatol. 9, 509–516.e1 (2011).
Imazeki, F. et al. Favorable prognosis of chronic hepatitis C after interferon therapy by long-term cohort study. Hepatology 38, 493–502 (2003).
Shiratori, Y. et al. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann. Intern. Med. 142, 105–114 (2005).
Veldt, B. J. et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann. Intern. Med. 147, 677–684 (2007).
Berenguer, J. et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology 50, 407–413 (2009).
Mira, J. A. et al. Benefits from sustained virologic response to pegylated interferon plus ribavirin in HIV/hepatitis C virus-coinfected patients with compensated cirrhosis. Clin. Infect. Dis. 56, 1646–1653 (2013).
Fierer, D. S. et al. Rapid progression to decompensated cirrhosis, liver transplant, and death in HIV-infected men after primary hepatitis C virus infection. Clin. Infect. Dis. 56, 1038–1043 (2013).
Macias, J. et al. Risk of liver decompensation among HIV/hepatitis C virus-coinfected individuals with advanced fibrosis: implications for the timing of therapy. Clin. Infect. Dis. 57, 1401–1408 (2013).
Laguno, M. et al. Randomized trial comparing pegylated interferon α-2b versus pegylated interferon α-2a, both plus ribavirin, to treat chronic hepatitis C in human immunodeficiency virus patients. Hepatology 49, 22–31 (2009).
Torriani, F. J. et al. Peginterferon α-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N. Engl. J. Med. 351, 438–450 (2004).
Chung, R. T. et al. Peginterferon α-2a plus ribavirin versus interferon α-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N. Engl. J. Med. 351, 451–459 (2004).
Carrat, F. et al. Pegylated interferon α-2b vs standard interferon α-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA 292, 2839–2848 (2004).
Voigt, E. et al. Pegylated interferon α-2b plus ribavirin for the treatment of chronic hepatitis C in HIV-coinfected patients. J. Infect. 53, 36–42 (2006).
Rodriguez-Torres, M. et al. Peginterferon α-2a plus ribavirin for HIV–HCV genotype 1 coinfected patients: a randomized international trial. HIV Clin. Trials 13, 142–152 (2012).
Zylberberg, H. et al. Safety and efficacy of interferon-ribavirin combination therapy in HCV–HIV coinfected subjects: an early report. Gut 47, 694–697 (2000).
Manns, M. P. et al. Peginterferon α-2b plus ribavirin compared with interferon α-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358, 958–965 (2001).
Barcaui, H. S. et al. Low rates of sustained virologic response with peginterferon plus ribavirin for chronic hepatitis C virus infection in HIV infected patients in Rio de Janeiro, Brazil. PLoS ONE 8, e67734 (2013).
Nunez, M. et al. Role of weight-based ribavirin dosing and extended duration of therapy in chronic hepatitis C in HIV-infected patients: the PRESCO trial. AIDS Res. Hum. Retroviruses 23, 972–982 (2007).
Moreno, A. et al. High rate of didanosine-related mitochondrial toxicity in HIV/HCV-coinfected patients receiving ribavirin. Antivir. Ther. 9, 133–138 (2004).
Bani-Sadr, F. et al. Risk factors for symptomatic mitochondrial toxicity in HIV/hepatitis C virus-coinfected patients during interferon plus ribavirin-based therapy. J. Acquir. Immune Defic. Syndr. 40, 47–52 (2005).
Brau, N. Epoetin α treatment for acute anaemia during interferon plus ribavirin combination therapy for chronic hepatitis C. J. Viral Hepat. 11, 191–197 (2004).
Mauss, S. et al. Risk factors for hepatic decompensation in patients with HIV/HCV coinfection and liver cirrhosis during interferon-based therapy. AIDS 18, F21–F25 (2004).
Lafeuillade, A., Hittinger, G. & Chadapaud, S. Increased mitochondrial toxicity with ribavirin in HIV/HCV coinfection. Lancet 357, 280–281 (2001).
Sulkowski, M. S. et al. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann. Intern. Med. 159, 86–96 (2013).
Sulkowski, M. et al. Boceprevir versus placebo with pegylated interferon α-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect. Dis. 13, 597–605 (2013).
Jacobson, I. M. et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 364, 2405–2416 (2011).
Montes, M. et al. Telaprevir combination therapy in treatment-naive and experienced patients co-infected with HCV and HIV [abstract 38]. Presented at the 64th Annual Meeting of the American Association for the Study of Liver Diseases, Washington DC (2013).
Cotte, L. et al. High End-Of-Treatment (EOT) response rate with Telaprevir-PegIFN-RBV in treatment-experienced HIV coinfected patients with HCV genotype 1: ANRS HC26 TelapreVIH study [Poster 1108]. Presented at the 64th Annual Meeting of the American Association for the Study of Liver Diseases, Washington DC (2013).
Poizot-Martin, I. et al. W48 Response rate of Boceprevir-PegIFN-RBV in treatment-experienced HIV coinfected patients with HCV genotype 1: ANRS-HC27 BocepreVIH study [Poster 1105]. Presented at the 64th Annual Meeting of the American Association for the Study of Liver Diseases, Washington, DC (2013).
Dieterich, D. et al. Simeprevir (TMC435) plus peginterferon/ribavirin in patients co-infected with HCV genotype-1 and HIV-1: primary analysis of the C212 study [abstract LBPS9/5]. Presented at the 14th European AIDS Conference (EACS), Brussels (2013).
Rockstroh, J. K. et al. STARTVerso4 Phase III tiral of faldaprevir plus peg interferon alfa-2a and ribavirin (PR) in patients with HIV and HCV genotype 1 coinfection: end of treatment response [Poster 1099]. Presented at the 64th Annual Meeting of the American Association for the Study of Liver Diseases, Washington, DC (2013).
Lawitz, E. et al. Sofosbuvir in combination with peginterferon α-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect. Dis. 13, 401–408 (2013).
Lawitz, E. & Gane, E. J. Sofosbuvir for previously untreated chronic hepatitis C infection. N. Engl. J. Med. 369, 678–679 (2013).
Jacobson, I. M. et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N. Engl. J. Med. 368, 1867–1877 (2013).
Sulkowski, M. S. et al. All-oral therapy with sofosbuvir plus ribavirin for the treatment of HCV genotype 1, 2, and 3 infection in patients co-infected with HIV (PHOTON-1) [abstract 212]. Presented at the 64th Annual Meeting of the American Association for the Study of Liver Diseases, Washington DC (2013).
Osinusi, A. et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA 310, 804–811 (2013).
Lawitz, E. et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet http://dx.doi.org/10.1016/S0140-6737(13)62121-2.
Jacobson, I. M. et al. SVR results of a once-daily regimen of simeprevir (SMV, TMC435) plus sofosbuvir (SOF, GS-7977) with or without ribavirin in cirrhotic and non-cirrhotic HCV genotype 1 treatment-naïve and prior null responder patients: the COSMOS study [abstract LB-3]. Presented at the 64th Annual Meeting of the American Association for the Study of Liver Diseases, Washington, DC (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Ge, D. et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461, 399–401 (2009).
Tanaka, Y. et al. Genome-wide association of IL28B with response to pegylated interferon-α and ribavirin therapy for chronic hepatitis C. Nat. Genet. 41, 1105–1109 (2009).
Rallon, N. I. et al. Association of a single nucleotide polymorphism near the interleukin-28B gene with response to hepatitis C therapy in HIV/hepatitis C virus-coinfected patients. AIDS 24, F23–F29 (2010).
Thompson, A. J. et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 139, 120–129 (2010).
Rivero-Juarez, A. et al. The IL28B effect on hepatitis C virus kinetics among HIV patients after the first weeks of pegylated-interferon/ribavirin treatment varies according to hepatitis C virus-1 subtype. AIDS 27, 1941–1947 (2013).
Resino, S., Sanchez-Conde, M. & Berenguer, J. Coinfection by human immunodeficiency virus and hepatitis C virus: noninvasive assessment and staging of fibrosis. Curr. Opin. Infect. Dis. 25, 564–569 (2012).
Bambha, K. et al. Assessing mortality in women with hepatitis C virus and HIV using indirect markers of fibrosis. AIDS 26, 599–607 (2012).
Nunes, D. et al. HIV infection does not affect the performance of noninvasive markers of fibrosis for the diagnosis of hepatitis C virus-related liver disease. J. Acquir. Immune Defic. Syndr. 40, 538–544 (2005).
Fernandez-Montero, J. V. et al. Liver stiffness predicts liver-related complications and mortality in HIV patients with chronic hepatitis C on antiretroviral therapy. AIDS 27, 1129–1134 (2013).
Soriano, V. et al. Regression of liver fibrosis in hepatitis C virus/HIV-co-infected patients after treatment with pegylated interferon plus ribavirin. AIDS 20, 2225–2227 (2006).
Macias, J. et al. Changes in liver stiffness in patients with chronic hepatitis C with and without HIV co-infection treated with pegylated interferon plus ribavirin. J. Antimicrob. Chemother. 65, 2204–2211 (2010).
Hernandez-Gea, V. & Friedman, S. L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 6, 425–456 (2011).
Choi, J. Oxidative stress, endogenous antioxidants, alcohol, and hepatitis C: pathogenic interactions and therapeutic considerations. Free Radic. Biol. Med. 52, 1135–1150 (2012).
Guicciardi, M. E. & Gores, G. J. Apoptosis: a mechanism of acute and chronic liver injury. Gut 54, 1024–1033 (2005).
Zhan, S. S. et al. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology 43, 435–443 (2006).
Canbay, A. et al. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab. Invest. 83, 655–663 (2003).
Wang, K. et al. Hepatic apoptosis can modulate liver fibrosis through TIMP1 pathway. Apoptosis 18, 566–577 (2013).
Acknowledgements
The work of the authors is supported by HHS/NIH grants T32DK007191 (J.Y.C.), DK098079 and DA033541 (R.T.C.) and by the Harvard University Center for AIDS Research (HU CFAR NIH/NIAID fund 5P30AI060354-09 to E.R.F.).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
R.T.C. has acted as a consultant for Abbvie and has received research grant support from Gilead Sciences. J.Y.C. and E.R.F. declare no competing interests.
Rights and permissions
About this article
Cite this article
Chen, J., Feeney, E. & Chung, R. HCV and HIV co-infection: mechanisms and management. Nat Rev Gastroenterol Hepatol 11, 362–371 (2014). https://doi.org/10.1038/nrgastro.2014.17
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2014.17
This article is cited by
-
Polarity protein AF6 functions as a modulator of necroptosis by regulating ubiquitination of RIPK1 in liver diseases
Cell Death & Disease (2023)
-
Strategies of Managing Repeated Measures: Using Synthetic Random Forest to Predict HIV Viral Suppression Status Among Hospitalized Persons with HIV
AIDS and Behavior (2023)
-
Combination antiretroviral therapy is associated with reduction in liver fibrosis scores in patients with HIV and HBV co-infection
AIDS Research and Therapy (2021)
-
Hepatitis B, Hepatitis C, tuberculosis and sexually-transmitted infections among HIV positive patients in Kazakhstan
Scientific Reports (2021)
-
Liver function following hepatitis C virus eradication by direct acting antivirals in patients with liver cirrhosis: data from the PITER cohort
BMC Infectious Diseases (2021)