Abstract
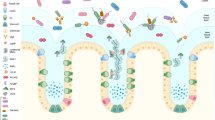
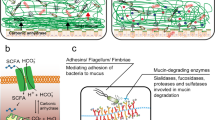
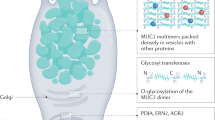
Mucins—large, highly glycosylated proteins—are important for the luminal protection of the gastrointestinal tract. Enterocytes have their apical surface covered by transmembrane mucins and goblet cells produce the secreted gel-forming mucins that form mucus. The small intestine has a single unattached mucus layer, which in cystic fibrosis becomes attached, accounting for the intestinal manifestations of this disease. The stomach and colon have two layers of mucus; the inner layer is attached and the outer layer is less dense and unattached. In the colon, the outer mucus layer is the habitat for commensal bacteria. The inner mucus layer is impervious to bacteria and is renewed every hour by surface goblet cells. The crypt goblet cells have the ability to restitute the mucus layer by secretion, for example after an ischaemic challenge. Proteases of certain parasites and some bacteria can cleave mucins and dissolve the mucus as part of their pathogenicity. The inner mucus layer can, however, also become penetrable to bacteria by several other mechanisms, including aberrations in the immune system. When bacteria reach the epithelial surface, the immune system is activated and inflammation is triggered. This mechanism might occur in some types of ulcerative colitis.
Key Points
-
Gastrointestinal mucus is the first line of defence against bacteria
-
The mucus layer in the small intestine is freely movable and carries bacteria distally
-
In cystic fibrosis, the small intestinal mucus is not freely movable, which might explain the intestinal symptoms of this disease
-
The colon handles its large bacterial load with a two-layered mucus system, in which the inner layer normally remains impenetrable to bacteria.
-
Defective functioning of the inner mucus layer of the colon might be a pathophysiological mechanism for colitis and infectious diseases
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kim, Y. S. & Ho, S. B. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr. Gastroenterol. Rep. 12, 319–330 (2010).
Neutra, M. R., O'Malley, L. J. & Specian, R. D. Regulation of intestinal goblet cell secretion. II. A survey of potentid secretugogues. Am. J. Physiol. Gastroint. Liver Physiol. 242, G380–G387 (1982).
Ito, S. Structure and function of the glycocalyx. Fed. Proc. 28, 12–25 (1969).
Soergel, K. H. & Ingelfinger, F. J Composition of rectal mucus in normal subjects and patients with ulcerative colitis. Gastroenterology 47, 610–616 (1964).
Rhodes, J. M. Colonic mucus and mucosal glycoproteins: the key to colitis and cancer? Gut 30, 1660–1666 (1989).
Hollingsworth, M. A. & Swanson, B. J. Mucin in cancer: protection and control of the cell surface. Nat. Rev. Cancer 4, 45–60 (2004).
Hattrup, C. L. & Gendler, S. J. Structure and function of the cell surface (tethered) mucins. Ann. Rev. Physiol. 70, 431–457 (2008).
Thornton, D. J., Rousseau, K. & McGuckin, M. A. Structure and function of the polymeric mucins in airways mucus. Ann. Rev. Physiol. 70, 459–486 (2008).
McGuckin, M. A., Linden, S. K., Sutton, P. & Florin, T. H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 9, 265–278 (2011).
Johansson, M. E. V. et al. Composition and functional role of the mucus layers in the intestine. Cell Mol. Life Sci. 68, 3535–3641 (2011).
Lang, T., Hansson, G. C. & Samuelsson, T. Gel-forming mucins appeared early in metazoan evolution. Proc. Natl Acad. Sci. USA 104, 16209–16214 (2007).
Fowler, J., Vinall, L. & Swallow, D. Polymorphism of the human MUC genes. Front. Biosci. 6, D1207–D1215 (2001).
Gum, J. R., Hicks, J. W., Toribara, N. W., Siddiki, B. & Kim, Y. S. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J. Biol. Chem. 269, 2440–2446 (1994).
Bennett, E. P. et al. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22, 736–756 (2012).
Jensen, P. H., Kolarich, D. & Packer, N. H. Mucin-type O-glycosylation—putting the pieces together. FEBS J. 277, 81–94 (2010).
Larsson, J. M., Karlsson, H., Sjovall, H. & Hansson, G. C. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology 19, 756–766 (2009).
Ambort, D. et al. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl Acad. Sci. USA 109, 5645–5650 (2012).
Ligtenberg, M. J. L. et al. Cell-associated episialin is a complex containing two proteins derived from a common precursor. J. Biol. Chem. 267, 6171–6177 (1992).
Palmai-Pallag, T. et al. The role of the SEA (sea urchin sperm protein, enterokinase and agrin) module in cleavage of membrane-tethered mucins. FEBS J. 272, 2901–2911 (2005).
Levitin, F. et al. The MUC1 SEA module is a self-cleaving domain. J. Biol. Chem. 280, 33374–33386 (2005).
Macao, B., Johansson, D. G. A., Hansson, G. C. & Härd, T. Auto-proteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat. Struct. Mol. Biol. 13, 71–76 (2006).
Pelaseyed, T. et al. Unfolding dynamics of the mucin SEA domain probed by force spectroscopy suggest that it acts as a cell protective device. FEBS J. http://dx.doi.org/10.1111/febs.12144.
Soto, P., Zhang, J. & Carraway, K. L. Enzymatic cleavage as a processing step in the maturation of Muc4/sialomucin complex. J. Cell. Biochem. 97, 1267–1274 (2006).
Lidell, M. E. & Hansson, G. C. Cleavage in the GDPH sequence of the C-terminal cysteine-rich part of the human MUC5AC mucin. Biochem. J. 399, 121–129 (2006).
Weiss, A. A., Babyatsky, M. W., Ogata, S., Chen, A. & Itzkowitz, S. H. Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissues. J. Histochem. Cytochem. 44, 1161–1166 (1996).
Williams, S. J. et al. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res. 59, 4083–4089 (1999).
Williams, S. J. et al. MUC13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J. Biol. Chem. 276, 18327–18336 (2001).
Button, B. et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337, 937–941 (2012).
Linden, S. K. et al. MUC1 limits helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 5, e1000617 (2009).
Gendler, S. Muc1, The renaissance molecule. J. Mammary Gland Biol. Neoplasia 6, 339–353 (2001).
Yamamoto, M., Bharti, A., Li, Y. & Kufe, D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J. Biol. Chem. 272, 12492–12494 (1997).
Wen, Y. F., Caffrey, T. C., Wheelock, M. J., Johnson, K. R. & Hollingsworth, M. A. Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. J. Biol. Chem. 278, 38029–38039 (2003).
Schroeder, J. C. et al. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene 23, 5739–5747 (2004).
Malmberg, E. K. et al. The transmembrane MUC17 mucin C-terminus binds to the scaffold protein PDZK1 that stably localizes it to the enterocyte apical membrane in the small intestine. Biochem. J. 410, 283–289 (2008).
LaLonde, D. P., Garbett, D. & Bretscher, A. A. Regulated complex of the scaffolding proteins PDZK1 and EBP50 with ezrin contribute to microvillar organization. Mol. Biol. Cell 21, 1519–1529 (2010).
Donowitz, M. & Li, X. Regulatory binding partners and complexes of NHE3. Physiol. Rev. 87, 825–872 (2007).
Singh, P. K. & Hollingsworth, M. A. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 16, 467–476 (2006).
Caldara, M. et al. Mucin biopolymers prevent bacterial aggregation by retaining cells in the free-swimming state. Curr. Biol. 22, 2325–2330 (2012).
Atuma, C., Strugula, V., Allen, A. & Holm, L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. 280, G922–G929 (2001).
Khan, S. H., Aguirre, A. & Bobek, L. A. In-situ hybridization localized MUC7 mucin gene expression to the mucous acinar cells of human and MUC7-transgenic mouse salivary glands. Glycoconj. J. 15, 1125–1132 (1998).
Bobek, L. A., Tsai, H., Biesbrock, A. R. & Levine, M. J. Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7). J. Biol. Chem. 268, 20563–20569 (1993).
Nielsen, P. A., Mandel, U., Therkildsen, M. H. & Clausen, H. Differential expression of human high-molecular-weight salivary mucin (MG1) and low-molecular-weight salivary mucin (MG2). J. Dental Res. 75, 1820–1826 (1996).
Thornton, D. J. et al. Salivary mucin MG1 is comprised almost entirely of different glycosylated forms of the MUC5B gene product. Glycobiology 9, 293–302 (1999).
Wickstrom, C., Davies, J. R., Eriksen, G. V., Veerman, E. C. I. & Carlstedt, I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem. J. 334, 685–693 (1998).
HO, S. B. et al. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology 109, 735–747 (1995).
Nordman, H. et al. Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem. J. 364, 191–200 (2002).
Debolos, C., Garrido, M. & Real, F. X. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology 109, 723–734 (1995).
Bartman, A. E. et al. The MUC6 secretory mucin genets expressed in a wide variety of epithelial tissues. J. Pathol. 186, 398–405 (1998).
Audie, J. P. et al. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J. Histochem. Cytochem. 41, 1479–1485 (1993).
Johansson, M. E. V. et al. The inner of the two Muc2 mucin dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008).
Godl, K. et al. The N-termini of the MUC2 mucin form trimers that are held together within a trypsin-resistant core fragment. J. Biol. Chem. 277, 47248–47256 (2002).
Lidell, M. E. et al. The recombinant C-terminus of the human MUC2 mucin forms dimers in CHO cells and heterodimers with full-length MUC2 in LS 174T cells. Biochem. J. 372, 335–345 (2003).
Round, A. N. et al. Lamellar structures of MUC2-rich mucin: a potential role in governing the barrier and lubricating functions of intestinal mucus. Biomacromolecules 13, 3253–3261 (2012).
Johansson, M. E. V. Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. PLoS ONE 7, e41009 (2012).
Schade, C., Flemstrom, G. & Holm, L. Hydrogen ion concentration in the mucus layer on top of acid-stimulated and -inhibited rat gastric mucosa. Gastroenterology 107, 180–188 (1994).
Phillipson, M., Atuma, C., Henriksnas, J. & Holm, L. The importance of mucus layers and bicarbonate transport in preservation of gastric juxtamucosal pH. Am. J. Physiol. 282, G211–G219 (2002).
Allen, A. & Flemstrom, G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am. J. Physiol. Cell Physiol. 288, C1–C19 (2005).
Bhaskar, K. R. et al. Viscous fingering of HCl through gastric mucin. Nature 360, 458–461 (1992).
Johansson, M., Synnerstad, I. & Holm, L. Acid transport through channels in the mucous layer of rat stomach. Gastroenterology 119, 1297–1304 (2000).
Ho, S. B. et al. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig. Dis. Sci. 49, 1598–1606 (2004).
Phillipson, M. et al. The gastric mucus layers: constituents and regulation of accumulation. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G806–G812 (2008).
Baxter, P. S. et al. Abnormal jejunal potential difference in cystic fibrosis. Lancet 333, 464–466 (1989).
Deloose, E., Janssen, P., Depoortere, I. & Tack, J. The migrating motor complex: control mechanisms and its role in health and disease. Nat. Rev. Gastroenterol. Hepatol. 9, 271–285 (2012).
Ouellette, A. J. Paneth cells and innate mucosal immunity. Curr. Opin. Gastroenterol. 26, 547–553 (2010).
Vaishnava, S. et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258 (2011).
Chu, H. et al. Human defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science 337, 477–481 (2012).
Johansson, M. E. V. & Hansson, G. C. Keeping bacteria at a distance. Science 334, 182–183 (2011).
Rosenstiel, P. et al. Regulation of DMBT1 via NOD2 and TLR4 in intestinal epithelial cells modulates bacterial recognition and invasion. J. Immunol. 178, 8203–8211 (2007).
Riordan, J. R. CFTR function and prospects for therapy. Annu. Rev. Biochem. 77, 701–726 (2008).
O'Sullivan, B. P. & Freedman, S. D. Cystic fibrosis. Lancet 373, 1891–1904 (2009).
Houwen, R. H. et al. Defining DIOS and constipation in cystic fibrosis with a multicentre study on the incidence, characteristics, and treatment of DIOS. J. Pediatr. Gastroenterol. Nutr. 50, 38–42 (2010).
Grubb, B. R. & Gabriel, S. E. Intestinal physiology and pathology in gene-targeted mouse models of cystic fibrosis. Am. J. Physiol. 273, G258–G266 (1997).
French, P. J. et al. A Delta F508 mutation in mouse cystic fibrosis transmembrane conductance regulator results in a temperature-sensitive processing defect in vivo. J. Clin. Invest. 98, 1304–1312 (1996).
Rogers, C. S. et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321, 1837–1841 (2008).
Gustafsson, J. K. et al. Bicarbonate and functional CFTR channel is required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J. Exp. Med. 209, 1263–1272 (2012).
De Lisle, R. C., Roach, E. & Jansson, K. Effects of laxative and N-acetylcysteine on mucus accumulation, bacterial load, transit, and inflammation in the cystic fibrosis mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G577–G584 (2007).
Fridge, J. L., Conrad, C., Gerson, L., Castillo, R. O. & Cox, K. Risk factors for small bowel bacterial overgrowth in cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 44, 212–218 (2007).
Malmberg, E. K. et al. Increased levels of mucins in the cystic fibrosis mouse small intestine and modulator effects of the Muc1 mucin expression. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G203–G210 (2006).
Delisle, R. C., Roach, E. A. & Norkina, O. Eradicaion of small intestinal bacterial overgrowth in the cystic fibrosis mouse reduces mucus accumulation. J. Pediatr. Gastroenterol. Nutr. 42, 46–52 (2006).
Backhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A. & Gordon, J. I. Host–bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005).
Sonnenburg, J. L. et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307, 1955–1959 (2005).
Wong, J. M., de Souza, R., Kendall, C. W., Emam, A. & Jenkins, D. J. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 40, 235–243 (2006).
Rawls, J. F., Mahowald, M. A., Ley, R. E. & Gordon, J. I. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 127, 423–433 (2006).
Bergstrom, K. S. B. et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 6, e1000902 (2010).
Moore, M. E., Boren, T. & Solnick, J. V. Life at the margins: modulation of attachment proteins in Helicobacter pylori. Gut Microbes 2, 42–46 (2011).
Else, K. J. & Finkelman, F. D. Intestinal nematode parasites, cytokines and effector mechanisms. Int. J. Parasitol. 28, 1145–1158 (1998).
Ishikawa, N., Horii, Y. & Nawa, Y. Immune-mediated alteration of the terminal sugars of goblet cell mucins in the small intestine of Nippostrongylus brasiliensis-infected rats. Immunology 78, 303–307 (1993).
Ishikawa, N., Wakelin, D. & Mahida, Y. R. Role of T helper 2 cells in intestinal goblet cell hyperplasia in mice infected with Trichinella spiralis. Gastroenterology 113, 542–549 (1997).
Koninkx, J. F., Mirck, M. H., Hendriks, H. G., Mouwen, J. M. & van Dijk, J. E. Nippostrongylus brasiliensis: histochemical changes in the composition of mucins in goblet cells during infection in rats. Exp. Parasitol. 65, 84–90 (1988).
McKay, D. M. et al. Hymenolepis diminuta: intestinal goblet cell response to infection in male C57 mice. Exp. Parasitol. 71, 9–20 (1990).
Hasnain, S. Z. et al. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology 138, 1763–1771 (2010).
Hasnain, S. Z. et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp. Med. 208, 893–900 (2011).
Finkelman, F. D. et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 201, 139–155 (2004).
Biton, M. et al. Epithelial microRNAs regulate gut mucosal immunity via epithelium-T cell crosstalk. Nat. Immunol. 12, 239–246 (2011).
Petri, W. A., Haque, R. & Mann, B. J. The bittersweet interface of parasite and host: lectin-carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annu. Rev. Microbiol. 56, 39–64 (2002).
Moncada, D., Keller, K. & Chadee, K. Entamoeba histolytica cysteine proteinases disrupt the polymeric structure of colonic mucin and alter its protective function. Infec. Immun. 71, 838–844 (2003).
Lidell, M. E., Moncada, D. M., Chadee, K. & Hansson, G. C. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal part and dissolves the protective colonic mucus gel. Proc. Natl Acad. Sci. USA 103, 9298–9393 (2006).
Brandt, L. J., Feuerstadt, P. & Blaszka, M. C. Anatomic patterns, patient characteristics, and clinical outcomes in ischemic colitis: a study of 313 cases supported by histology. Am. J. Gastroenterol. 105, 2245–2252 (2010).
[No authors listed] American Gastroenterological Association Medical Position Statement: Guidelines on intestinal ischemia. Gastroenterology 118, 951–953 (2000).
Grootjans, J. et al. Ischemia-induced mucus barrier loss and bacterial penetration are rapidly counteracted by increased goblet cell secretory activity in human and rat colon. Gut 62, 259–258 (2013).
Specian, D. & Neutra, M. R. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J. Cell Biol. 85, 626–640 (1980).
Plaisancie, P. et al. Effects of neurotransmitters, gut hormones, and inflammatory mediators on mucus discharge in rat colon. Am. J. Physiol. 275, G1073–G1084 (1998).
Johansson, M. E. V. & Hansson, G. C. The goblet cell: a key player in ischemia–reperfusion injury. Gut 62, 188–189 (2012).
Ordas, I., Eckmann, L., Talamini, M., Baumgart, D. C. & Sandborn, W. J. Ulcerative colitis. The Lancet 380, 1609–1619 (2012).
Swidsinski, A. et al. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 56, 343–350 (2007).
Velcich, A. et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295, 1726–1729 (2002).
Van der Sluis, M. et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129 (2006).
Heazlewood, C. K. et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5, e54 (2008).
Dawson, P. A. et al. Impaired intestinal function in the hyposulphataemic NaS1 null mouse. Gut 58, 910–919 (2009).
Fu, J. et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis. J. Clin. Invest. 121, 1657–1666 (2011).
Johansson, M. E. V. et al. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 5, e12238 (2010).
Johansson, M. E. V. et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and in patients with ulcerative colitis. Gut http://dx.doi.org/10.1136/gutjnl-2012-303207.
Gustafsson, J. K., Hansson, G. C. & Sjovall, H. Ulcerative colitis patients in remission have an altered secretory capacity in the proximal colon despite macroscopically normal mucosa. Neurogastroenterol. Motil. 24, e381–e391 (2012).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Eri, R. D. et al. An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol. 4, 354–364 (2011).
Park, S. W. et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl Acad. Sci. USA 106, 6950–6955 (2009).
Zhao, F. et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2-/- mice. Develop. Biol. 338, 270–279 (2010).
Lan, M. S., Batra, S. K., Qi, W., Metzgar, R. S. & Hollingsworth, M. A. Cloning and sequencing of a human pancreatic tumor mucin cDNA. J. Biol. Chem. 265, 15294–15299 (1990).
Ligtenberg, M. J. L., Vos, H. L., Gennissen, A. M. C. & Hilkens, J. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J. Biol. Chem. 265, 5573–5578 (1990).
Gendler, S. J. et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J. Biol. Chem. 265, 15286–15293 (1990).
Lancaster, C. A. et al. Structure and expression of the human polymorphic epithelial mucin gene: an expressed VNTR unit. Biochem. Biophys. Res. Commun. 173, 1019–1029 (1990).
Wreschner, D. H. et al. Human epithelial tumor antigen cDNA sequences. Differential splicing may generate multiple protein forms. Eur. J. Biochem. 189, 463–473 (1990).
McAuley, J. L. et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Invest. 117, 2313–2324 (2007).
Linden, S. K., Florin, T. H. & McGuckin, M. A. Mucin dynamics in intestinal bacterial infection. PLoS ONE 3, e3952 (2008).
Gum, J. R. et al. Molecular cloning of human intestinal mucin cDNAs sequence analysis and evidence for genetic polymorphism. J. Biol. Chem. 264, 6480–6487 (1989).
Gum, J. R. et al. The human MUC2 intestinal mucin has cysteine-rich subdomains located both upstream and downstream of its central repetitive region. J. Biol. Chem. 267, 21375–21383 (1992).
Gum, J. R. et al. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem. Biophys. Res. Commun. 171, 407–415 (1990).
Gum, J. R. et al. MUC3 human intestinal mucin. J. Biol. Chem. 272, 26678–26686 (1997).
Crawley, S. C. et al. Genomic organization and structure of the 3' region of human MUC3: alternative splicing predicts membrane-bound and soluble forms of the mucin. Biochem. Biophys. Res. Commun. 263, 728–736 (1999).
Moniaux, N., Nollet, S., Degand, P., Laine, A. & Aubert, J. P. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem. J. 338, 325–333 (1999).
Escande, F. et al. Genomic organization of MUC4 mucin gene. Towards the characterization of splice variants. Eur. J. Biochem. 269, 3637–3644 (2002).
Price-Schiavi, S. A., Perez, A., Barco, R. & Carraway, K. L. Cloning and characterization of the 5' flanking region of the sialomucin complex/rat Muc4 gene: promoter activity in cultured cells. Biochem. J. 349, 641–649 (2000).
Rong, M. et al. Expression and localization of Muc4/sialomucin complex (SMC) in the adult and developing rat intestine: implications for Muc4/SMC function. J. Cell. Physiol 202, 275–284 (2005).
Buisine, M. P. et al. Genomic organization of the 3'-region of the human MUC5AC mucin gene: additional evidence for a common ancestral gene for the 11p15.5 mucin gene family. Biochem. J. 332, 729–738 (1998).
Escande, F., Aubert, J. P., Porchet, N. & Buisine, M. P. Human mucin gene MUC5AC: organization of its 5'-region and central repetitive region. Biochem. J. 358, 763–772 (2001).
Li, D. Z., Gallup, M., Fan, N., Szymkowski, D. E. & Basbaum, C. B. Cloning of the amino-terminal and 5'-flanking region of the human MUC5AC mucin gene and transcriptional up-regulation by bacterial exoproducts. J. Biol. Chem. 273, 6812–6820 (1998).
Klomp, L. W. J., Vanrens, L. & Strous, G. J. Cloning and analysis of human gastric mucin cDNA reveals two types of conserved cysteine-rich domains. Biochem. J. 308, 831–838 (1995).
Meerzaman, D. et al. Cloning and analysis of cDNA encoding a major airway glycoprotein, human tracheobronchial mucin (Muc5). J. Biol. Chem. 269, 12932–12939 (1994).
Keates, A. C., Nunes, D. P., Afdhal, N. H., Troxler, R. F. & Offner, G. D. Molecular cloning of a major human gall bladder mucin: complete C-terminal sequence and genomic organization of MUC5B. Biochem. J. 324, 295–303 (1997).
Offner, G. D., Nunes, D. P., Keates, A. C., Afdhal, N. H. & Troxler, R. F. The amino-terminal sequence of MUC5B contains conserved multifunctional D domains: implications for tissue-specific mucin functions. Biochem. Biophys. Res. Commun. 251, 350–355 (1998).
Troxler, R. F., Offner, G. D., Zhang, F., Iontcheva, I. & Oppenheim, F. G. Molecular cloning of a novel high molecular weight mucin (MG1) from human sublingual gland. Biochem. Biophys. Res. Commun. 217, 1112–1119 (1995).
Toribara, N. W. et al. Human gastric mucin. J. Biol. Chem. 268, 5879–5885 (1993).
Toribara, N. W. et al. The carboxyl-terminal sequence of the human secretory mucin, MUC6. J. Biol. Chem. 272, 16398–16403 (1997).
Rousseau, K. et al. The complete genomic organization of the human MUC6 and MUC2 mucin genes. Genomics 83, 936–939 (2004).
Situ, H., Wei, G., Smith, C. J., Mashhoon, S. & Bobek, L. A. Human salivary MUC7 mucin peptides: effect of size, charge and cysteine residues on antifungal activity. Biochem. J. 375, 175–182 (2003).
Sheng, Y. H. et al. The MUC13 cell-surface mucin protects against intestinal inflammation by inhibiting epithelial cell apoptosis. Gut 60, 1661–1670 (2011).
Yin, B. W. T. & Lloyd, K. Molecular cloning of the CA125 ovarian cancer antigen. J. Biol. Chem. 276, 27371–27375 (2001).
O'Brien, T. J., Beard, J. B., Underwood, L. J. & Shigemasa, K. The CA125 gene: a newly discovered extesion of the glycosylated N-terminal domain doubles the size of this extracellular superstructure. Tumor Biol. 23, 154–169 (2004).
Gum, J. R., Crawley, S. C., Hicks, J. W., Szymkowski, D. E. & Kim, Y. S. MUC17, a novel membrane-tethered mucin. Biochem. Biophys. Res. Commun. 291, 466–475 (2002).
Pratt, W. S. et al. Multiple transcripts of MUC3: evidence for two genes MUC3A and MUC3B. Biochem. Biophys. Res. Commun. 275, 916–923 (2000).
Pullan, R. D. et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 35, 353–359 (1994).
Debailleul, V. et al. Human mucin genes MUC2, MUC3, MUC4, MUC5AC, MUC5B, and MUC6 express stable and extremely large mRNAs and exhibit a variable length polymorphism. J. Biol. Chem. 273, 881–890 (1998).
Ogata, S., Uehara, H., Chen, A. & Itzkowitz, S. H. Mucin gene expression in colonic tissues and cell lines. Cancer Res. 52, 5971–5978 (1992).
Vanklinken, B. J. W. et al. MUC5B is the prominent mucin in human gallbladder and is also expressed in a subset of colonic goblet cells. Am. J. Physiol. 37, G871–G878 (1998).
Peat, N., Gendler, S. J., Lalani, N., Duhig, T. & Taylor-Papadimitriou, J. Tissue-specific expression of a human polymorphic epithelial mucin (MUC1) in transgenic mice. Cancer Res. 52, 1954–1960 (1992).
Zhang, J., Yasin, M., Carraway, C. A. & Carraway, K. L. MUC4 expression and localization in gastrointestinal tract and skin of human embryos. Tissue Cell 38, 271–275 (2006).
Gonzalez-Begne, M. et al. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT). J. Proteome Res. 8, 1304–1314 (2009).
Senapati, S. et al. Expression of intestinal MUC17 membrane-bound mucin in inflammatory and neoplastic diseases of the colon. J. Clin. Pathology 63, 702–707 (2010).
Acknowledgements
The authors' research is supported by the Swedish Research Council, The Swedish Cancer Foundation, The Knut and Alice Wallenberg Foundation, IngaBritt and Arne Lundberg Foundation, Sahlgren's University Hospital (LUA-ALF), Wilhelm and Martina Lundgren's Foundation, Torsten och Ragnar Söderbergs Stiftelser, The Sahlgrenska Academy, National Institute of Allergy and Infectious Diseases (U01AI095473, the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH), and The Swedish Foundation for Strategic Research—The Mucus–Bacteria–Colitis Center (MBC) of the Innate Immunity Program.
Author information
Authors and Affiliations
Contributions
All authors equally contributed to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Johansson, M., Sjövall, H. & Hansson, G. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol 10, 352–361 (2013). https://doi.org/10.1038/nrgastro.2013.35
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2013.35
This article is cited by
-
Solobacterium moorei promotes the progression of adenomatous polyps by causing inflammation and disrupting the intestinal barrier
Journal of Translational Medicine (2024)
-
Metabolic network of the gut microbiota in inflammatory bowel disease
Inflammation and Regeneration (2024)
-
Single nuclear RNA sequencing of terminal ileum in patients with cirrhosis demonstrates multi-faceted alterations in the intestinal barrier
Cell & Bioscience (2024)
-
β-carotene inhibits MAPKs signaling pathways on rat colonic epithelial cells to attenuate TNF-α-induced intestinal inflammation and injury
Cell Biochemistry and Biophysics (2024)
-
Deciphering mucin degrading ability and safety aspects of enterococcus strain from human feces
Biologia (2024)