Key Points
-
Despite almost three decades of research on artificial and bioartificial liver support, appropriate, randomized, controlled, and adequately powered studies are rare
-
Compared with the effect of a suitable orthotopic liver graft on a patient's clinical course, the clinical effects of extracorporeal liver support systems seem to be very limited
-
The main issues for all liver support therapy concepts are the need for tissue vascularization and integration into the host circulation, and a lack of reliable sources of safe and metabolically active cells
-
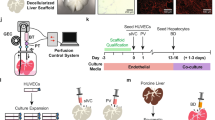
Strategies to overcome the conceptual limitations of (bio)artificial liver support devices include hepatocyte transplantation and various tissue engineering approaches (for example repopulation of decellularized organs, organ printing and induced organogenesis)
-
Hepatocyte transplantation seems to be at least a temporary alternative treatment strategy in certain metabolic liver disorders, and might have a role in the treatment of acute liver failure and chronic liver disease
-
Translation of regenerative medicine into the clinical routine, especially transplantation of recellularized liver grafts, seems possible but further intense research is needed
Abstract
The treatment of end-stage liver disease and acute liver failure remains a clinically relevant issue. Although orthotopic liver transplantation is a well-established procedure, whole-organ transplantation is invasive and increasingly limited by the unavailability of suitable donor organs. Artificial and bioartificial liver support systems have been developed to provide an alternative to whole organ transplantation, but despite three decades of scientific efforts, the results are still not convincing with respect to clinical outcome. In this Review, conceptual limitations of clinically available liver support therapy systems are discussed. Furthermore, alternative concepts, such as hepatocyte transplantation, and cutting-edge developments in the field of liver support strategies, including the repopulation of decellularized organs and the biofabrication of entirely new organs by printing techniques or induced organogenesis are analysed with respect to clinical relevance. Whereas hepatocyte transplantation shows promising clinical results, at least for the temporary treatment of inborn metabolic diseases, so far data regarding implantation of engineered hepatic tissue have only emerged from preclinical experiments. However, the evolving techniques presented here raise hope for bioengineered liver support therapies in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dutkowski, P. et al. The model for end-stage liver disease allocation system for liver transplantation saves lives, but increases morbidity and cost: a prospective outcome analysis. Liver Transpl. 17, 674–684 (2011).
Axelrod, D. A. Economic and financial outcomes in transplantation: whose dime is it anyway? Curr. Opin. Organ Transplant. 18, 222–228 (2013).
Dutkowski, P. et al. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann. Surg. 254, 745–753 (2011).
Mitzner, S. R. Extracorporeal liver support-albumin dialysis with the Molecular Adsorbent Recirculating System (MARS). Ann. Hepatol. 10 (Suppl. 1), S21–S28 (2011).
Rifai, K. Fractionated plasma separation and adsorption: current practice and future options. Liver Int. 31 (Suppl. 3), 13–15 (2011).
Sauer, I. M. et al. In vitro comparison of the molecular adsorbent recirculation system (MARS) and single-pass albumin dialysis (SPAD). Hepatology 39, 1408–1414 (2004).
Al-Chalabi, A. et al. Evaluation of the Hepa Wash® treatment in pigs with acute liver failure. BMC Gastroenterol. 13, 83 (2013).
Rozga, J., Umehara, Y., Trofimenko, A., Sadahiro, T. & Demetriou, A. A. A novel plasma filtration therapy for hepatic failure: preclinical studies. Ther. Apher. Dial. 10, 138–144 (2006).
Ash, S. R. et al. Push-pull sorbent-based pheresis and hemodiabsorption in the treatment of hepatic failure: preliminary results of a clinical trial with the BioLogic-DTPF System. Ther. Apher. 4, 218–228 (2000).
Otto, J. J., Pender, J. C., Cleary, J. H., Sensenig, D. M. & Welch, C. S. The use of a donor liver in experimental animals with elevated blood ammonia. Surgery 43, 301–309 (1958).
Eiseman, B., Liem, D. S. & Raffucci, F. Heterologous liver perfusion in treatment of hepatic failure. Ann. Surg. 162, 329–345 (1965).
Sen, P. K. et al. Use of isolated perfused cadaveric liver in the management of hepatic failure. Surgery 59, 774–781 (1966).
Pascher, A., Sauer, I. M., Hammer, C., Gerlach, J. C. & Neuhaus, P. Extracorporeal liver perfusion as hepatic assist in acute liver failure: a review of world experience. Xenotransplantation 9, 309–324 (2002).
Demetriou, A. A. et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann. Surg. 239, 660–667 (2004).
Ellis, A. J. et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology 24, 1446–1451 (1996).
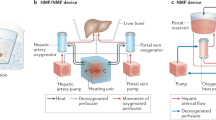
Sauer, I. M., Obermeyer, N., Kardassis, D., Theruvath, T. & Gerlach, J. C. Development of a hybrid liver support system. Ann. NY Acad. Sci. 944, 308–319 (2001).
Sauer, I. M. et al. Clinical extracorporeal hybrid liver support—phase I study with primary porcine liver cells. Xenotransplantation 10, 460–469 (2003).
Sauer, I. M. et al. Extracorporeal liver support based on primary human liver cells and albumin dialysis—treatment of a patient with primary graft non-function. J. Hepatol. 39, 649–653 (2003).
van de Kerkhove, M. P. et al. Phase I clinical trial with the AMC-bioartificial liver. Int. J. Artif. Organs 25, 950–959 (2002).
Mitzner, S. R. et al. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 6, 277–286 (2000).
Heemann, U. et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology 36, 949–958 (2002).
Sen, S. et al. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: a randomized controlled study. Liver Transpl. 10, 1109–1119 (2004).
Hassanein, T. I. et al. Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology 46, 1853–1862 (2007).
Banares, R. et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology 57, 1153–1162 (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2012).
Kribben, A. et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology 142, 782–789.e783 (2012).
Kortgen, A. et al. Albumin dialysis in liver failure: comparison of molecular adsorbent recirculating system and single pass albumin dialysis—a retrospective analysis. Ther. Apher. Dial. 13, 419–425 (2009).
Ringe, H. et al. Continuous veno-venous single-pass albumin hemodiafiltration in children with acute liver failure. Pediatr. Crit. Care Med. 12, 257–264 (2011).
Karvellas, C. J. et al. A case-control study of single-pass albumin dialysis for acetaminophen-induced acute liver failure. Blood Purif. 28, 151–158 (2009).
US National Library of Medicine. ClinicalTrials.gov[online], (2012).
US National Library of Medicine. ClinicalTrials.gov[online], (2012).
Millis, J. M. et al. Initial experience with the modified extracorporeal liver-assist device for patients with fulminant hepatic failure: system modifications and clinical impact. Transplantation 74, 1735–1746 (2002).
van de Kerkhove, M. P. et al. Bridging a patient with acute liver failure to liver transplantation by the AMC-bioartificial liver. Cell Transplant. 12, 563–568 (2003).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
Kjaergard, L. L., Liu, J., Als-Nielsen, B. & Gluud, C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure. JAMA 289, 217–222 (2003).
Liu, J. P., Gluud, L. L., Als-Nielsen, B. & Gluud, C. Artificial and bioartificial support systems for liver failure. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD003628. http://dx.doi.org/10.1002/14651858.CD003628.pub2.
Stutchfield, B. M., Simpson, K. & Wigmore, S. J. Systematic review and meta-analysis of survival following extracorporeal liver support. Br. J. Surg. 98, 623–631 (2011).
Pascher, A., Sauer, I. M. & Neuhaus, P. Analysis of allogeneic versus xenogeneic auxiliary organ perfusion in liver failure reveals superior efficacy of human livers. Int. J. Artif. Organs 25, 1006–1012 (2002).
Iwata, H. & Ueda, Y. Pharmacokinetic considerations in development of a bioartificial liver. Clin. Pharmacokinet. 43, 211–25 (2004).
Dhawan, A., Strom, S. C., Sokal, E. & Fox, I. J. Human hepatocyte transplantation. Methods Mol. Biol. 640, 525–534 (2010).
Gupta, S. et al. Entry and integration of transplanted hepatocytes in rat liver plates occur by disruption of hepatic sinusoidal endothelium. Hepatology 29, 509–519 (1999).
Laconi, E. et al. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am. J. Pathol. 153, 319–329 (1998).
Hughes, R. D., Mitry, R. R. & Dhawan, A. Current status of hepatocyte transplantation. Transplantation 93, 342–347 (2012).
Jorns, C. et al. Hepatocyte transplantation for inherited metabolic diseases of the liver. J. Intern. Med. 272, 201–223 (2012).
Mito, M. et al. Studies on ectopic liver utilizing hepatocyte transplantation into the rat spleen. Transplant. Proc. 11, 585–591 (1979).
Saito, R. et al. Transplantation of liver organoids in the omentum and kidney. Artif. Organs 35, 80–83 (2011).
Meyburg, J. et al. One liver for four children: first clinical series of liver cell transplantation for severe neonatal urea cycle defects. Transplantation 87, 636–641 (2009).
Meyburg, J., Schmidt, J. & Hoffmann, G. F. Liver cell transplantation in children. Clin. Transplant. 23, 75–82 (2009).
Dhawan, A., Puppi, J., Hughes, R. D. & Mitry, R. R. Human hepatocyte transplantation: current experience and future challenges. Nat. Rev. Gastroenterol. Hepatol. 7, 288–298 (2010).
Bosma, P. J. Inherited disorders of bilirubin metabolism. J. Hepatol. 38, 107–117 (2003).
Fox, I. J. et al. Treatment of the Crigler–Najjar syndrome type I with hepatocyte transplantation. N. Engl. J. Med. 338, 1422–1427 (1998).
Darwish, A. A. et al. Permanent access to the portal system for cellular transplantation using an implantable port device. Liver Transpl. 10, 1213–1215 (2004).
Ambrosino, G. et al. Isolated hepatocyte transplantation for Crigler–Najjar syndrome type 1. Cell Transplant. 14, 151–157 (2005).
Dhawan, A., Mitry, R. R. & Hughes, R. D. Hepatocyte transplantation for liver-based metabolic disorders. J. Inherit. Metab. Dis. 29, 431–435 (2006).
Allen, K. J., Mifsud, N. A., Williamson, R., Bertolino, P. & Hardikar, W. Cell-mediated rejection results in allograft loss after liver cell transplantation. Liver Transpl. 14, 688–694 (2008).
Lysy, P. A. et al. Liver cell transplantation for Crigler-Najjar syndrome type I: update and perspectives. World J. Gastroenterol. 14, 3464 (2008).
Mitry, R. R. et al. One liver, three recipients: segment IV from split-liver procedures as a source of hepatocytes for cell transplantation. Transplantation 77, 1614–1616 (2004).
Strom, S. C. et al. Transplantation of human hepatocytes. Transplant. Proc. 29, 2103–2106 (1997).
Horslen, S. P. et al. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics 111, 1262–1267 (2003).
Stephenne, X. et al. Cryopreserved liver cell transplantation controls ornithine transcarbamylase deficient patient while awaiting liver transplantation. Am. J. Transplant. 5, 2058–2061 (2005).
Puppi, J. et al. Hepatocyte transplantation followed by auxiliary liver transplantation—a novel treatment for ornithine transcarbamylase deficiency. Am. J. Transplant. 8, 452–457 (2008).
Stéphenne, X. et al. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology 130, 1317–1323 (2006).
Fisher, R. A. & Strom, S. C. Human hepatocyte transplantation: worldwide results. Transplantation 82, 441–449 (2006).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2012).
US National Library of Medicine. ClinicalTrials.gov[online], (2011).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2011).
Bilir, B. M. et al. Hepatocyte transplantation in acute liver failure. Liver Transpl. 6, 32–40 (2000).
Strom, S. C. et al. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation 63, 559–569 (1997).
Mito, M., Kusano, M. & Kawaura, Y. Hepatocyte transplantation in man. Transplant. Proc. 24, 3052–3053 (1992).
Soltys, K. A. et al. Barriers to the successful treatment of liver disease by hepatocyte transplantation. J. Hepatol. 53, 769 (2010).
Quaglia, A. et al. Liver after hepatocyte transplantation for liver-based metabolic disorders in children. Cell Transplant. 17, 1403–1414 (2008).
Puppi, J. & Modo, M. Use of magnetic resonance imaging contrast agents to detect transplanted liver cells. Top. Magn. Reson. Imaging 20, 113–120 (2009).
Hughes, R. D. et al. Isolation of hepatocytes from livers from non-heart-beating donors for cell transplantation. Liver Transpl. 12, 713–717 (2006).
Erker, L. et al. Therapeutic liver reconstitution with murine cells isolated long after death. Gastroenterology 139, 1019–1029 (2010).
Nagata, H. et al. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology 132, 321–329 (2007).
Gupta, S. Hog heaven on the road to liver cell therapy. Gastroenterology 132, 450–453 (2007).
Yamanouchi, K. et al. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology 49, 258–267 (2009).
Dagher, I. et al. Efficient hepatocyte engraftment and long-term transgene expression after reversible portal embolization in nonhuman primates. Hepatology 49, 950–959 (2009).
Zhou, H. et al. Single liver lobe repopulation with wildtype hepatocytes using regional hepatic irradiation cures jaundice in gunn rats. PLoS ONE 7, e46775 (2012).
Mei, J. et al. Improved survival of fulminant liver failure by transplantation of microencapsulated cryopreserved porcine hepatocytes in mice. Cell Transplant. 18, 101–110 (2009).
Zhou, P. et al. Decellularized liver matrix as a carrier for the transplantation of human fetal and primary hepatocytes in mice. Liver Transpl. 17, 418–427 (2011).
Yovchev, M. I., Xue, Y., Shafritz, D. A., Locker, J. & Oertel, M. Repopulation of the fibrotic/cirrhotic rat liver by transplanted hepatic stem/progenitor cells and mature hepatocytes. Hepatology http://dx.doi.org/10.1002/hep.26615.
Hoppo, T., Komori, J., Manohar, R., Stolz, D. B. & Lagasse, E. Rescue of lethal hepatic failure by hepatized lymph nodes in mice. Gastroenterology 140, 656–666 (2011).
Komori, J., Boone, L., DeWard, A., Hoppo, T. & Lagasse, E. The mouse lymph node as an ectopic transplantation site for multiple tissues. Nat. Biotechnol. 30, 976–983 (2012).
Baptista, P. M. et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 53, 604–617 (2011).
Folkman, J. & Hochberg, M. Self-regulation of growth in three dimensions. J. Exp. Med. 138, 745–753 (1973).
Bernard, M. P. et al. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry 22, 5213–5223 (1983).
Bernard, M. P. et al. Structure of a cDNA for the pro alpha 2 chain of human type I procollagen. Comparison with chick cDNA for pro alpha 2(I) identifies structurally conserved features of the protein and the gene. Biochemistry 22, 1139–1145 (1983).
Badylak, S. F., Taylor, D. & Uygun, K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 13, 27–53 (2011).
Bissell, M. J. & Aggeler, J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog. Clin. Biol. Res. 249, 251–262 (1987).
Sellaro, T. L. et al. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng. Part A 16, 1075–1082 (2010).
Wang, Y. et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology 53, 293–305 (2011).
Wang, X. et al. Decellularized liver scaffolds effectively support the proliferation and differentiation of mouse fetal hepatic progenitors. J. Biomed. Mater. Res. A http://doi.dx.org/10.1002/jbm.a.34764.
Cortiella, J. et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng. Part A 16, 2565–2580 (2010).
Badylak, S. F., Weiss, D. J., Caplan, A. & Macchiarini, P. Engineered whole organs and complex tissues. Lancet 379, 943–952 (2012).
Crapo, P. M., Gilbert, T. W. & Badylak, S. F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233–3243 (2011).
Nagata, S., Hanayama, R. & Kawane, K. Autoimmunity and the clearance of dead cells. Cell 140, 619–630 (2010).
Zheng, M. H. et al. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J. Biomed. Mater. Res. B Appl. Biomater. 73, 61–67 (2005).
Park, K. M., Park, S. M., Yang, S. R., Hong, S. H. & Woo, H. M. Preparation of immunogen-reduced and biocompatible extracellular matrices from porcine liver. J. Biosci. Bioeng. 115, 207–215 (2012).
Shirakigawa, N., Ijima, H. & Takei, T. Decellularized liver as a practical scaffold with a vascular network template for liver tissue engineering. J. Biosci. Bioeng. 114, 546–551 (2012).
Uygun, B. E. et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 16, 814–820 (2010).
Barakat, O. et al. Use of decellularized porcine liver for engineering humanized liver organ. J. Surg. Res. 173, e11–e25 (2012).
Soto-Gutierrez, A. et al. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng. Part C Methods 17, 677–686 (2011).
Ott, H. C. et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med. 14, 213–221 (2008).
Bao, J. et al. Construction of a portal implantable functional tissue-engineered liver using perfusion-decellularized matrix and hepatocytes in rats. Cell Transplant. 20, 753–766 (2011).
Olausson, M. et al. Transplantation of an allogeneic vein bioengineered with autologous stem cells: a proof-of-concept study. Lancet 380, 230–237 (2012).
Macchiarini, P. et al. Clinical transplantation of a tissue-engineered airway. Lancet 372, 2023–2030 (2008).
Elliott, M. J. et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet 380, 994–1000 (2012).
Baiguera, S., Birchall, M. A. & Macchiarini, P. Tissue-engineered tracheal transplantation. Transplantation 89, 485–491 (2010).
Horst, M. et al. Engineering functional bladder tissues. J. Tissue Eng. Regen. Med. 7, 515–522 (2013).
Mase, V. J. Jr et al. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics 33, 511 (2010).
Vogel, G. Trachea transplants test the limits. Science 340, 266–268 (2013).
Katsuda, T., Sakai, Y. & Ochiya, T. Induced pluripotent stem cell-derived hepatocytes as an alternative to human adult hepatocytes. J. Stem Cells 7, 1–17 (2012).
Wu, X. B. & Tao, R. Hepatocyte differentiation of mesenchymal stem cells. Hepatobiliary Pancreat. Dis. Int. 11, 360–371 (2012).
Espejel, S. et al. Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. J. Clin. Invest. 120, 3120 (2010).
Quante, M. & Wang, T. C. Stem cells in gastroenterology and hepatology. Nat. Rev. Gastroenterol. Hepatol. 6, 724–737 (2009).
Guillemot, F., Mironov, V. & Nakamura, M. Bioprinting is coming of age: Report from the International Conference on Bioprinting and Biofabrication in Bordeaux (3B'09). Biofabrication 2, 010201 (2010).
Klebe, R. Cytoscribing: A method for micropositioning cells and the construction of two- and three-dimensional synthetic tissues. Exp. Cell Res. 179, 362–373 (1988).
Derby, B. Printing and prototyping of tissues and scaffolds. Science 338, 921–926 (2012).
Villar, G., Graham, A. D. & Bayley, H. A tissue-like printed material. Science 340, 48–52 (2013).
Mironov, V. et al. Organ printing: tissue spheroids as building blocks. Biomaterials 30, 2164–2174 (2009).
Norotte, C., Marga, F. S., Niklason, L. E. & Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30, 5910–5917 (2009).
Visconti, R. P. et al. Towards organ printing: engineering an intra-organ branched vascular tree. Expert Opin. Biol. Ther. 10, 409–420 (2010).
Pérez-Pomares, J. M. & Foty, R. A. Tissue fusion and cell sorting in embryonic development and disease: biomedical implications. Bioessays 28, 809–821 (2006).
Forgacs, G., Foty, R. A., Shafrir, Y. & Steinberg, M. S. Viscoelastic properties of living embryonic tissues: a quantitative study. Biophys. J. 74, 2227–2234 (1998).
Cui, X. & Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 30, 6221–6227 (2009).
Khatiwala, C., Law, R., Shepherd, B., Dorfman, S. & Csete, M. 3D cell bioprinting for regenerative medicine research and therapies. Gene Ther. Regul. 7, 1230004 (2012).
Robbins, J. B., Gorgen, V., Min, P., Shepherd, B. R. & Presnell, S. C. A novel in vitro three-dimensional bioprinted liver tissue system for drug development [abstract 7979]. Presented at Experimental Biology, 2013, Boston, MA.
Chang, R., Emami, K., Wu, H. & Sun, W. Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2, 045004 (2010).
Snyder, J. et al. Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication 3, 034112 (2011).
Butcher, J. T., Sedmera, D., Guldberg, R. E. & Markwald, R. R. Quantitative volumetric analysis of cardiac morphogenesis assessed through micro-computed tomography. Dev. Dyn. 236, 802–809 (2007).
Little, T. S. et al. Engineering a 3D, biological construct: representative research in the South Carolina Project for Organ Biofabrication. Biofabrication 3, 030202 (2011).
Neagu, A., Jakab, K., Jamison, R. & Forgacs, G. Role of physical mechanisms in biological self-organization. Phys. Rev. Lett. 95, 178104 (2005).
Yang, X., Mironov, V. & Wang, Q. Modeling fusion of cellular aggregates in biofabrication using phase field theories. J. Theor. Biol. 303, 110–118 (2012).
Khan, F. & Ahmand, S. in Biomaterials and Stem Cells in Regenerative Medicine (eds Ramalingam, M., Ramakrishna, S. & Best, S.) 101–122 (CRC Press, 2012).
Parsa, S., Gupta, M., Loizeau, F. & Cheung, K. C. Effects of surfactant and gentle agitation on inkjet dispensing of living cells. Biofabrication 2, 025003 (2010).
Guillotin, B. & Guillemot, F. Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol. 29, 183–190 (2011).
Miller, J. S. et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 (2012).
Takebe, T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Struecker, B., Raschzok, N. & Sauer, I. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol 11, 166–176 (2014). https://doi.org/10.1038/nrgastro.2013.204
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2013.204
This article is cited by
-
Acute-on-chronic liver failure: far to go—a review
Critical Care (2023)
-
Integration of ATAC-Seq and RNA-Seq reveals FOSL2 drives human liver progenitor-like cell aging by regulating inflammatory factors
BMC Genomics (2023)
-
Perfusion-Based Recellularization of Rat Livers with Islets of Langerhans
Journal of Medical and Biological Engineering (2022)
-
Adult stem cell transplantation combined with conventional therapy for the treatment of end-stage liver disease: a systematic review and meta-analysis
Stem Cell Research & Therapy (2021)
-
Cell Therapy and Bioengineering in Experimental Liver Regenerative Medicine: In Vivo Injury Models and Grafting Strategies
Current Transplantation Reports (2021)