Key Points
-
The lining of the digestive tract is exposed to an extraordinarily broad range of chemicals and organisms
-
The gut continuously monitors the composition of its contents to optimize digestion and absorption, and to ward off threats to its integrity
-
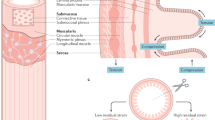
The gut has numerous sensors that detect nutrients, distension, symbiotic and pathogenic microorganisms, toxins and other components of its luminal contents
-
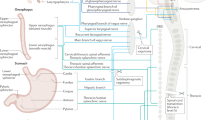
For the digestive system to react appropriately to its environment, the sensory information is communicated to extensive endocrine, neural, immune and nonimmune tissue defence systems
-
More than 20 receptors that face the luminal contents are potential therapeutic targets for diabetes, obesity and digestive disorders; restricting orally active therapeutics to the lumen could reduce off-target actions
Abstract
The gastrointestinal tract presents the largest and most vulnerable surface to the outside world. Simultaneously, it must be accessible and permeable to nutrients and must defend against pathogens and potentially injurious chemicals. Integrated responses to these challenges require the gut to sense its environment, which it does through a range of detection systems for specific chemical entities, pathogenic organisms and their products (including toxins), as well as physicochemical properties of its contents. Sensory information is then communicated to four major effector systems: the enteroendocrine hormonal signalling system; the innervation of the gut, both intrinsic and extrinsic; the gut immune system; and the local tissue defence system. Extensive endocrine–neuro–immune–organ-defence interactions are demonstrable, but under-investigated. A major challenge is to develop a comprehensive understanding of the integrated responses of the gut to the sensory information it receives. A major therapeutic opportunity exists to develop agents that target the receptors facing the gut lumen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ferraris, R. P., Lee, P. P. & Diamond, J. M. Origin of regional and species differences in intestinal glucose uptake. Am. J. Physiol. 257, G689–G697 (1989).
MacDonald, T. T. & Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 307, 1920–1925 (2005).
Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 8, 411–420 (2008).
Engelstoft, M. S., Egerod, K. L., Holst, B. & Schwartz, T. W. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metab. 8, 447–449 (2008).
Rehfeld, J. F. A centenary of gastrointestinal endocrinology. Horm. Metab. Res. 36, 735–741 (2004).
Janssen, S. & Depoortere, I. Nutrient sensing in the gut: new roads to therapeutics? Trends Endocrinol. Metab. 24, 92–100 (2013).
Wu, S. V. et al. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc. Natl Acad. Sci. USA 99, 2392–2397 (2002).
Rozengurt, E. & Sternini, C. Taste receptor signaling in the mammalian gut. Curr. Opin. Pharm. 7, 557–562 (2007).
Young, R. L. Sensing via intestinal sweet taste pathways. Front. Neurosci. 5, 1–13 (2011).
Sutherland, K., Young, R. L., Cooper, N. J., Horowitz, M. & Blackshaw, L. A. Phenotypic characterization of taste cells of the mouse small intestine. Am. J. Physiol. 292, G1420–G1428 (2007).
Huang, A. L. et al. The cells and logic for mammalian sour taste detection. Nature 442, 934–938 (2006).
Chaudhari, N. & Roper, S. D. The cell biology of taste. J. Cell Biol. 190, 285–296 (2010).
Margolskee, R. F. et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl Acad. Sci. USA 104, 15075–15080 (2007).
Geraedts, M. C. P. et al. Transformation of postingestive glucose responses after deletion of sweet taste receptor subunits or gastric bypass surgery. Am. J. Physiol. 303, E464–E474 (2012).
Brown, R. J., Walter, M. & Rother, K. I. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care 32, 2184–2186 (2009).
Gerspach, A. C., Steinert, R. E., Schönenberger, L., Graber-Maier, A. & Beglinger, C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am. J. Physiol. 301, E317–E325 (2011).
Drucker, D. J. & Nauck, M. A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705 (2006).
Reimann, F. et al. Glucose sensing in L cells: a primary cell study. Cell Metab. 8, 532–539 (2008).
Gorboulev, V. et al. Na+-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61, 187–196 (2012).
Diakogiannaki, E., Gribble, F. & Reimann, F. Nutrient detection by incretin hormone secreting cells. Physiol. Behav. 106, 387–393 (2012).
Brubaker, P. L., Izzo, A. A., Hill, M. & Drucker, D. J. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am. J. Physiol. 272, E1050–E1058 (1997).
Hsieh, J. et al. Glucagon-like peptide-2 increase intestinal lipid absorption and chylomicron production via CD36. Gastroenterology 137, 997–1005 (2009).
Shirazi-Beechey, S. P., Moran, A. W., Batchelor, D. J., Daly, K. & Al-Rammahi, M. Influences of food constituents on gut health glucose sensing and signalling; regulation of intestinal glucose transport. Proc. Nutr. Soc. 70, 185–193 (2011).
Iwatsuki, K. et al. Detecting sweet and umami tastes in the gastrointestinal tract. Acta Physiol. 204, 169–177 (2012).
Hass, N., Schwarzenbacher, K. & Breer, H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res. 339, 493–504 (2010).
Iwatsuki, K. & Uneyama, H. Sense of taste in the gastrointestinal tract. J. Pharmacol. Sci. 118, 123–128 (2012).
Daly, K. et al. Sensing of amino acids by the gut-expressed taste receptor T1R1–T1R3 stimulates CCK secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G271–G282 (2013).
Janssen, S. et al. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl Acad. Sci. USA 108, 2094–2099 (2011).
Hunt, J. N. & Stubbs, D. F. The volume and energy content of meals as determinants of gastric emptying. J. Physiol. 245, 209–225 (1975).
Edfalk, S., Steneberg, P. & Edlund, H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57, 2280–2287 (2008).
Strader, A. D. & Woods, S. C. Gastrointestinal hormones and food intake. Gastroenterology 128, 175–191 (2005).
Mawe, G. M. Nerves and hormones interact to control gallbladder function. News Physiol. Sci. 13, 84–90 (1998).
Furness, J. B. The Enteric Nervous System (Blackwell, 2006).
Wang, Y. et al. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G528–G537 (2011).
Little, T. J. et al. The release of GLP-1 and ghrelin, but not GIP and CCK, by glucose is dependent upon the length of small intestine exposed. Am. J. Physiol. 291, E647–E655 (2006).
Xiong, Y. et al. Activation of FFA1 mediates GLP-1 secretion in mice. Evidence for allosterism at FFA1. Mol. Cell. Endocrinol. 369, 119–129 (2013).
Poreba, M. A. et al. Role of fatty acid transport protein 4 in oleic acid-induced glucagon-like peptide-1 secretion from murine intestinal L cells. Am. J. Physiol. Endocrinol. Metab. 303, E899–E807 (2012).
Tolhurst, G., Reimann, F. & Gribble, F. M. in Appetite Control, Handbook of Experimental Pharmacology (ed. Joost, H.-G.) 309–335 (Springer-Verlag, 2012).
Karaki, S.-I. et al. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 324, 353–360 (2006).
Chu, Z. L. et al. A role of intestinal endocrine cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology 149, 2038–2047 (2008).
Cox, H. M. et al. Peptide YY is critical for acylethanolamine receptor Gpr119-induced activation of gastrointestinal mucosal responses. Cell Metab. 11, 532–542 (2010).
Lin, H. C., Zhao, X.-T., Wang, L. & Wong, H. Fat-induced ileal brake in the dog depends on peptide YY. Gastroenterology 110, 1491–1495 (1996).
Holst, J. J. The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 (2007).
Chaudhri, O. B., Field, B. C. T. & Bloom, S. R. Gastrointestinal satiety signals. Int. J. Obesity 32, S28–S31 (2008).
Abbott, C. R. et al. The inhibitory effects of peripheral administration of peptide YY3–36 and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 1044, 127–131 (2005).
Ogawa, N. et al. Intestinal fatty acid infusion modulates food preference as well as calorie intake via the vagal nerve and mid-brain-hypothalamic neural pathways in rats. Metabolism 61, 1312–1320 (2012).
Raybould, H. E. Mechanisms of CCK signaling from gut to brain. Curr. Opin. Pharm. 7, 570–574 (2007).
Cordier-Bussat, M. et al. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes 47, 1038–1045 (1998).
Haid, D., Jordan-Biegger, C., Widmayer, P. & Breer, H. Receptors responsive to protein breakdown products in G-cells and D-cells of mouse, swine and human. Front. Physiol. 3, 1–15 (2012).
Haid, D., Widmayer, P. & Breer, H. Nutrient sensing receptors in gastic endocrine cells. J. Mol. Hist. 42, 355–364 (2011).
Drucker, D. J., Ehrlich, P., Asa, S. L. & Brubaker, P. L. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc. Natl Acad. Sci. USA 93, 7911–7916 (1996).
Sigalet, D. L. et al. Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am. J. Physiol. 293, G211–G221 (2007).
Rowland, K. J. & Brubaker, P. L. The “cryptic” mechanism of action of glucagon-like peptide-2. Am. J. Physiol. 301, G1–G8 (2011).
Yusta, B., Holland, D., Waschek, J. A. & Drucker, D. J. Intestinotrophic glucagon-like peptide-2 (GLP-2) activates intestinal gene expression and growth factor-dependent pathways independent of the vasoactive intestinal peptide gene in mice. Endocrinology 153, 2623–2632 (2012).
Cani, P. D. et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103 (2009).
Lee, S.-J. et al. Disruption of the murine Glp2r impairs paneth cell function and increases susceptibility to small bowel enteritis. Endocrinology 153, 1141–1151 (2012).
Bremholm, L., Hornum, M., Henriksen, B. M., Larsen, S. & Holst, J. J. Glucagon-like peptide-2 increases mesenteric blood flow in humans. Scand. J. Gastroenterol. 44, 314–319 (2009).
Bogunovic, M. et al. Enteroendocrine cells express functional toll-like receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1770–G1783 (2007).
Palazzo, M. et al. Activation of enteroendocrine cells via TLRs induces hormone, chemokine and defensin secretion. J. Immunol. 178, 4296–4303 (2007).
Selleri, S. et al. Induction of pro-inflammatory programs in enteroendocrine cells by the toll-like receptor agonists flagellin and bacterial LPS. Int. Immunol. 20, 961–970 (2008).
Holzer, P. TRP channels in the digestive system. Curr. Pharm. Biotechnol. 12, 24–34 (2011).
Breer, H., Eberle, J., Frick, C., Haid, D. & Widmayer, P. Gastrointestinal chemosensation: chemosensory cells in the alimentary tract. Histochem. Cell Biol. 138, 13–24 (2012).
Franz, C., Baser, K. H. C. & Windisch, W. Essential oils and aromatic plants in animal feeding–A European perspective. Flavour Fragr. J. 25, 327–340 (2010).
Venkatachalam, K. & Montell, C. TRP channels. Annu. Rev. Biochem. 76, 387–417 (2007).
Purhonen, A. K., Louhivuori, L. M., Kiehne, K., Akerman, K. E. O. & Herzig, K. H. TRPA1 channel activation induces cholecystokinin release via extracellular calcium. FEBS Lett. 582, 229–232 (2008).
Nozawa, K. et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. USA 106, 3408–3413 (2009).
Liman, E. R. TRPM5 and taste transduction. Handb. Exp. Pharmacol. 179, 286–296 (2007).
Damak, S. et al. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem. Senses 31, 253–264 (2006).
Braun, T., Voland, P., Kunz, L., Prinz, C. & Gratzl, M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology 132, 1890–1901 (2007).
Kaji, I., Karaki, S.-I. & Kuwahara, A. Effects of luminal thymol on epithelial transport in human and rat colon. Am. J. Physiol. 300, G1132–G1143 (2011).
Russell-Jones, D. et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (Duration-4). Diabetes Care 35, 252–258 (2012).
Katz, L. B. et al. Effects of JNJ-38431055, a novel GPR119 receptor agonist, in randomized, double-blind, placebo-controlled studies in subjects with type 2 diabetes. Diabetes Obes. Metab. 14, 709–716 (2012).
Stearns, A. T., Balakrishnan, A., Rhoads, D. B. & Tavakkolizadeh, A. Rapid upregulation of sodium-glucosetransporter SGLT1 in response to intestinal sweet taste stimulation. Ann. Surg. 251, 865–871 (2010).
Osswald, C. et al. Mice without the regulator gene Rsc1A1 exhibit increased Na+-D-Glucose cotransport in small intestine and develop obesity. Mol. Cell. Biol. 25, 78–87 (2005).
Jeppsesen, P. B. et al. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 60, 902–914 (2011).
Cervero, F. Sensory innervation of the viscera: Peripheral basis of visceral pain. Physiol. Rev. 74, 95–138 (1994).
Brookes, S. J. H., Spencer, N. J., Costa, M. & Zagorodnyuk, V. P. Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastroenterol. Hepatol. 10, 286–296 (2013).
Berthoud, H. R., Kressel, M., Raybould, H. E. & Neuhuber, W. L. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI tracing. Anat. Embryol. 191, 203–212 (1995).
Bucinskaite, V. et al. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol. Motil. 21, 978–e78 (2009).
Andrews, P. L. R. et al. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can. J. Physiol. Pharmacol. 68, 325–345 (1990).
Date, Y. et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123, 1120–1128 (2002).
le Roux, C. W. et al. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J. Clin. Endocrinol. Metab. 90, 4521–4524 (2005).
Hao, S., Sternini, C. & Raybould, H. E. Role of CCK1 and Y2 receptors in activation of hindbrain neurons induced by intragastric administration of bitter taste receptor ligands. Am. J. Physiol. 294, R33–R38 (2008).
Perez-Burgos, A. et al. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am. J. Physiol. 304, G211–G220 (2013).
Hosoi, T., Okuma, Y., Matsuda, T. & Nomura, Y. Novel pathway for LPS-induced afferent vagus nerve activation: Possible role of nodose ganglion. Autonom. Neurosci. 120, 104–107 (2005).
Berthoud, H. R., Patterson, L. M., Neumann, F. & Neuhuber, W. L. Distribution and structure of vagal afferent intraganglionic laminar endings IGLEs in the rat gastrointestinal tract. Anat. Embryol. 195, 183–191 (1997).
Castelucci, P., Robbins, H. L. & Furness, J. B. P2X2 purine receptor immunoreactivity of intraganglionic laminar endings in the mouse gastrointestinal tract. Cell Tissue Res. 312, 167–174 (2003).
Zagorodnyuk, V. P., Chen, B. N. & Brookes, S. J. H. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J. Physiol. 534, 255–268 (2001).
Lynn, P. A., Olsson, C., Zagorodnyuk, V., Costa, M. & Brookes, S. J. H. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology 125, 786–794 (2003).
Powley, T. L. & Phillips, R. J. Vagal intramuscular array afferents form complexes with interstitial cells of cajal in gastrointestinal smooth muscle: analogues of muscle spindle organs? Neuroscience 186, 188–200 (2011).
Hughes, P. A., Brierley, S. M. & Martin, C. M. TRPV1-expressing sensory fibres and IBS: links with immune function. Gut 58, 465–466 (2009).
Furness, J. B., Papka, R. E., Della, N. G., Costa, M. & Eskay, R. L. Substance P-like immunoreactivity in nerves associated with the vascular system in guinea-pigs. Neuroscience 7, 447–459 (1982).
Holzer, P. Role of visceral afferent neurons in mucosal inflammation and defense. Curr. Opin. Pharm. 7, 563–569 (2007).
Ness, T. J. & Gebhart, G. F. Visceral pain: a review of experimental studies. Pain 41, 167–234 (1990).
Feng, B. et al. Long-term sensitization of mechanosensitive and -insensitive afferents in mice with persistent colorectal hypersensitivity. Am. J. Physiol. 302, G676–G683 (2012).
Ray, B. S. & Neill, C. L. Abdominal visceral sensation in man. Ann. Surg. 126, 709–724 (1947).
Bingham, J. R., Ingelfinger, F. J. & Smithwick, R. H. The effect of sympathectomy on abdominal pain in man. Gastroenterology 15, 18–31 (1950).
Furness, J. B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294 (2012).
Szurszewski, J. H. & Miller, S. M. in Physiology of the Gastrointestinal Tract (ed. Johnson, L. R.) 795–877 (Raven Press, 1994).
Edholm, T. et al. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol. Motil. 22, 1191–e315 (2010).
Pocai, A. Unraveling oxyntomodulin, GLP1's enigmatic brother. J. Endocrinol. 215, 335–346 (2012).
Gershon, M. D. & Kirchgessner, A. L. Identification, characterization and projections of intrinsic primary afferent neurones of the submucosal plexus: Activity-induced expression of c-fos immunoreactivity. J. Auton. Nerv. Syst. 33, 185–187 (1991).
Kunze, W. A. A., Bornstein, J. C. & Furness, J. B. Identification of sensory nerve cells in a peripheral organ, the intestine of a mammal. Neuroscience 66, 1–4 (1995).
Furness, J. B., Jones, C., Nurgali, K. & Clerc, N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog. Neurobiol. 72, 143–164 (2004).
Kunze, W. A. A., Furness, J. B., Bertrand, P. P. & Bornstein, J. C. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. J. Physiol. 506, 827–842 (1998).
Smith, T. K., Spencer, N. J., Hennig, G. W. & Dickson, E. J. Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterol. Motil. 19, 869–878 (2007).
Mazzuoli, G. & Schemann, M. Mechanosensitive enteric neurons in the myenteric plexus of the mouse intestine. PLoS ONE 7, e39887 (2012).
Beyak, M. J. Visceral afferents—Determinants and modulation of excitability. Autonom. Neurosci. 153, 69–78 (2010).
Camilleri, M. Review article: new receptor targets for medical therapy in irritable bowel syndrome. Aliment. Pharmacol. Ther. 31, 35–46 (2009).
Blackshaw, L. A., Page, A. J. & Young, R. L. Metabotropic glutamate receptors as novel therapeutic targets on visceral sensory pathways. Front. Neurosci. 5, 1–7 (2011).
Page, A. J., Symonds, E., Peiris, M., Blackshaw, L. A. & Young, R. L. Peripheral neural targets in obesity. Br. J. Pharmacol. 166, 1537–1558 (2012).
Garcia, J. M., Friend, J. & Allen, S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Support Care Cancer 21, 129–137 (2013).
Fagarasan, S., Kawamoto, S., Kanagawa, O. & Suzuki, K. Adaptive immune regulation in the gut: T cell–dependent and T cell–independent IgA synthesis. Annu. Rev. Immunol. 28, 243–273 (2010).
Backhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A. & Gordon, J. I. Host–bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005).
Cerf-Bensussan, N. & Gaboriau-Routhiau, V. The immune system and the gut microbiota: friends or foes? Nat. Rev. Immunol. 10, 735–744 (2010).
Garrett, W. S., Gordon, J. I. & Glimcher, L. H. Homeostasis and inflammation in the intestine. Cell 140, 859–870 (2010).
Treiner, E. et al. Selection of evolutionarily conserved mucosal-associated invariant T cell by MR1. Nature 422, 164–171 (2003).
Klaasen, H. L. B. M. et al. Apathogenic, intestinal, segmented, filamentous bacteria stimulate the mucosal immune system of mice. Infect. Immun. 61, 303–306 (1993).
Mazmanian, S. K., Liu, C. H., Tzianabos, A. O. & Kasper, D. L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118 (2005).
Bäckhed, F. et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl Acad. Sci. USA 101, 15718–15723 (2004).
Neutra, M. R., Mantis, N. J. & Kraehenbuhl, J. P. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Rev. Immunol. 2, 1004–1009 (2001).
Mowat, A. M. Anatomical basis of tolerence and immunity to intestinal antigens. Nat. Rev. Immunol. 3, 331–341 (2003).
Kyd, J. M. & Cripps, A. W. Functional differences between M cells and enterocytes in sampling luminal antigens. Vaccine 26, 6221–6224 (2008).
Kjer-Nielsen, L. et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723 (2012).
Chua, W.-J. & Hansen, T. H. Vitamins prime immunity. Nature 491, 680–681 (2012).
Gold, M. C. & Lewinsohn, D. M. Co dependents: MR1 restricted MAIT cells and their antimicrobial function. Nat. Rev. Microbiol. 11, 14–19 (2013).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Dann, S. M. & Eckmann, L. Innate immune defenses in the intestinal tract. Curr. Opin. Gastroenterol. 23, 115–120 (2007).
Ouellette, A. J. Paneth cells and innate mucosal immunity. Curr. Opin. Gastroenterol. 26, 547–553 (2010).
Vaishnava, S., Behrendt, C. L., Ismail, A. S., Eckmann, L. & Hooper, L. V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host–microbial interface. Proc. Natl Acad. Sci. USA 105, 20858–20863 (2008).
Bevins, C. L. & Salzman, N. H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 9, 356–368 (2011).
Abraham, C. & Cho, J. H. Inflammatory bowel disease. N. Engl. J. Med. 361, 2066–2078 (2009).
Buchman, A. L., Katz, S., Fang, J. C., Bernstein, C. N. & Abou-Assi, S. G. Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn's disease. Inflamm. Bowel Dis. 16, 962–973 (2010).
Rubio-Aliaga, I. & Daniel, H. Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica 38, 1022–1042 (2008).
Smith, D. E., Clemencon, B. & Hediger, M. A. Proton-coupled oligopeptide transporter family SLC15: Physiological, pharmacological and pathological implications. Mol. Asp. Med. 34, 323–336 (2013).
Hagenbuch, B. & Gui, C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 38, 778–801 (2008).
Thelen, K. & Dressman, J. B. Cytochrome P450-mediated metabolism in the human gut wall. J. Pharm. Pharmacol. 61, 541–558 (2009).
Birlouez-Aragon, I. et al. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am. J. Clin. Nutr. 91, 1220–1226 (2010).
Chen, P., Zhao, J. & Gregersen, H. Up-regulated expression of advanced glycation end-products and their receptor in the small intestine and colon of diabetic rats. Dig. Dis. Sci. 57, 48–57 (2012).
Zong, H. et al. Homodimerization is essential for the receptor for advanced glycation end products (RAGE)-mediated signal transduction. J. Biol. Chem. 285, 23137–23146 (2010).
Jeyabal, P. V. S., Kumar, R., Gangula, P. R. R., Micci, M.-A. & Pasricha, P. J. Inhibitors of advanced glycation end-products prevent loss of enteric neuronal nitric oxide synthase in diabetic rats. Neurogastroenterol. Motil. 20, 253–261 (2008).
Sanger, G. J. & Andrews, P. L. R. Treatment of nausea and vomiting: Gaps in our knowledge. Autonom. Neurosci. 129, 3–16 (2006).
Bertrand, P. P., Kunze, W. A. A., Furness, J. B. & Bornstein, J. C. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience 101, 459–469 (2000).
Gershon, M. D. Nerves, reflexes, and the enteric nervous system. J. Clin. Gastroenterol. 38, S184–S193 (2005).
Lee, J., Yamamoto, T., Hayashi, S., Kuramoto, H. & Kadowaki, M. Enhancement of CGRP sensory afferent innervation in the gut during the development of food allergy in an experimental murine model. Biochem. Biophys. Res. Commun. 430, 895–900 (2013).
Lundgren, O. et al. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 287, 491–495 (2000).
Field, M. Intestinal ion transport and the pathophysiology of diarrhea. J. Clin. Invest. 111, 931–943 (2003).
Mawe, G. M., Strong, D. S. & Sharkey, K. A. Plasticity of enteric nerve functions in the inflamed and postinflamed gut. Neurogastroenterol. Motil. 21, 481–491 (2009).
Feng, B., La, J. H., Schwartz, E. S. & Gebhart, G. F. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1085–G1098 (2012).
Matteoli, G. & Boeckxstaens, G. E. The vagal innervation of the gut and immune homeostasis. Gut 62, 1214–1222 (2013).
Thomas, C. et al. TGR5-Mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10, 167–177 (2009).
Sigalet, D. L., Wallace, L., De Heuval, E. & Sharkey, K. A. The effects of glucagon-like peptide 2 on enteric neurons in intestinal inflammation. Neurogastroenterol. Motil. 22, 1318–e350 (2010).
Hyland, N. P., Sjöberg, F., Tough, I. R., Herzog, H. & Cox, H. M. Functional consequences of neuropeptide Y Y2 receptor knockout and Y2 antagonism in mouse and human colonic tissues. Br. J. Pharmacol. 139, 863–871 (2003).
Cox, H. M. Peptide YY: A neuroendocrine neighbor of note. Peptides 28, 345–351 (2007).
Kosinski, J. R. et al. The glucagon receptor is involved in mediating the body weight-lowering effects of oxyntomodulin. Obesity 20, 1566–1571 (2012).
Brubaker, P. L. & Anini, Y. Direct and indirect mechanisms regulating secretion of glucagon-like peptide-1 and glucagon-like peptide-2. Can. J. Physiol. Pharmacol. 81, 1005–1012 (2003).
Sandoval, D., Dunki-Jacobs, A., Sorrell, J., Seeley, R. J. & D'Alessio, D. D. Impact of intestinal electrical stimulation on nutrient-induced GLP-1 secretion in vivo. Neurogastroenterol. Motil. http://dx.doi.org/10.1111/nmo.12152.
Stengel, A., Goebel, M., Wang, L. & Taché, Y. Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: Role as regulators of food intake and body weight. Peptides 31, 357–369 (2010).
Brubaker, P. L. A beautiful cell (or two or three?). Endocrinology 153, 2945–2948 (2012).
Acknowledgements
Work from the University of Melbourne laboratory is supported by the National Health and Medical Research Council of Australia. We are grateful for insightful comments of J. Brock, H. Cox, J. Keast, A. Lomax and R. Young.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Furness, J., Rivera, L., Cho, HJ. et al. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol 10, 729–740 (2013). https://doi.org/10.1038/nrgastro.2013.180
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2013.180
This article is cited by
-
Immunoglobulin superfamily member 3 is required for the vagal neural crest cell migration and enteric neuronal network organization
Scientific Reports (2023)
-
Gut-Brain Coupling and Multilevel Physiological Response to Biofeedback Relaxation After a Stressful Task Under Virtual Reality Immersion: A Pilot Study
Applied Psychophysiology and Biofeedback (2023)
-
Semantic clustering analysis of E3-ubiquitin ligases in gastrointestinal tract defines genes ontology clusters with tissue expression patterns
BMC Gastroenterology (2022)
-
Intestinal cellular heterogeneity and disease development revealed by single-cell technology
Cell Regeneration (2022)
-
Using zebrafish to understand reciprocal interactions between the nervous and immune systems and the microbial world
Journal of Neuroinflammation (2022)