Abstract
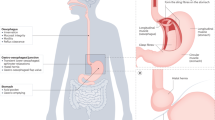
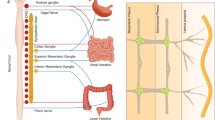
Neurogastroenterology is defined as neurology of the gastrointestinal tract, liver, gallbladder and pancreas and encompasses control of digestion through the enteric nervous system (ENS), the central nervous system (CNS) and integrative centers in sympathetic ganglia. This Review provides a broad overview of the field of neurogastroenterology, with a focus on the roles of the ENS in the control of the musculature of the gastrointestinal tract and transmucosal fluid movement. Digestion is controlled through the integration of multiple signals from the ENS and CNS; neural signals also pass between distinct gut regions to coordinate digestive activity. Moreover, neural and endocrine control of digestion is closely coordinated. Interestingly, the extent to which the ENS or CNS controls digestion differs considerably along the digestive tract. The importance of the ENS is emphasized by the life-threatening effects of certain ENS neuropathies, including Hirschsprung disease and Chagas disease. Other ENS disorders, such as esophageal achalasia and gastroparesis, cause varying degrees of dysfunction. The neurons in enteric reflex pathways use a wide range of chemical messengers that signal through an even wider range of receptors. These receptors provide many actual and potential targets for modifying digestive function.
Key Points
-
The enteric nervous system (ENS) is an extensive reflex control system for digestive function that works with the central nervous system (CNS) and neural pathways that pass through sympathetic ganglia
-
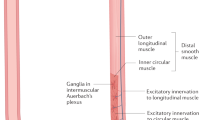
The ENS of the small intestine and colon has complete reflex pathways that control patterns of contractile activity, local blood flow and transmucosal movement of fluids
-
The CNS has essential roles in control of esophageal, stomach and colorectal functions
-
Control of transmucosal fluid movement by the ENS and CNS is closely integrated
-
The ENS interacts with both the gut endocrine and immune systems and has roles in modifying nutrient absorption and maintaining the mucosal barrier
-
Enteric neuropathies in which control of muscle contractile activity or neural control of transmucosal fluid movement fail are life-threatening
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bayliss, W. M. & Starling, E. H. The movements and innervation of the small intestine. J. Physiol. 24, 99–143 (1899).
Furness, J. B. The Enteric Nervous System (Blackwell, Oxford, 2006).
Brehmer, A. Structure of enteric neurons. Adv. Anat. 186, 1–94 (2006).
Brehmer, A., Rupprecht, H. & Neuhuber, W. Two submucosal nerve plexus in human intestines. Histochem. Cell Biol. 133, 149–161 (2010).
Toumi, F. et al. Human submucosal neurones regulate intestinal epithelial cell proliferation: evidence from a novel co-culture model. Neurogastroenterol. Motil. 15, 239–242 (2003).
Savidge, T. C. et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 132, 1344–1358 (2007).
Swenson, O. Hirschsprung's disease: a review. Pediatrics 109, 914–918 (2002).
Matsuda, N. M., Miller, S. M. & Evora, P. R. B. The chronic gastrointestinal manifestations of Chagas disease. Clinics 64, 1219–1224 (2009).
De Giorgio, R. et al. Inflammatory neuropathies of the enteric nervous system. Gastroenterology 126, 1872–1883 (2004).
Knowles, C. H. et al. The London classification of gastrointestinal neuromuscular pathology: Report on behalf of the Gastro 2009 International Working Group. Gut 59, 882–887 (2010).
Di Nardo, G. et al. Review article: molecular, pathological and therapeutic features of human enteric neuropathies. Aliment. Pharmacol. Ther. 28, 25–45 (2008).
Lundgren, O. Enteric nerves and diarrhoea. Pharmacol. Toxicol. 90, 109–120 (2002).
Podewils, L. J., Mintz, E. D., Nataro, J. P. & Parashar, U. D. Acute, infectious diarrhea among children in developing countries. Semin. Pediatr. Infect. Dis. 15, 155–168 (2004).
Hasler, W. L. in Gastroenterology (ed. Yamada, T.) 195–219 (Lippincott Williams & Wilkins, Philadelphia, 2003).
Schubert, M. L. & Peura, D. A. Control of gastric acid secretion in health and disease. Gastroenterology 134, 1842–1860 (2008).
de Groat, W. C. et al. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J. Auton. Nerv. Syst. 3, 135–160 (1981).
Jean, A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol. Rev. 81, 929–969 (2001).
Bieger, D. & Hopkins, D. A. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J. Comp. Neurol. 262, 546–562 (1987).
Cannon, W. B. Oesophageal peristalsis after bilateral vagotomy. Am. J. Physiol. 19, 436–444 (1907).
Wörl, J., Mayer, B. & Neuhuber, W. L. Spatial relationships of enteric nerve fibers to vagal motor terminals and the sarcolemma in motor endplates of the rat esophagus: a confocal laser scanning and electron-microscopic study. Cell Tissue Res. 287, 113–118 (1997).
Izumi, N., Matsuyama, H., Ko, M., Shimizu, Y. & Takewaki, T. Role of intrinsic nitrergic neurones on vagally mediated striated muscle contractions in the hamster oesophagus. J. Physiol. 551, 287–294 (2003).
Spechler, S. J., Fitzgerald, R. C., Prasad, G. A. & Wang, K. G. History, molecular mechanisms, and endoscopic treatment of Barrett's esophagus. Gastroenterology 138, 854–869 (2010).
Reynolds, R. P. E., El-Sharkawy, T. Y. & Diamant, N. E. Lower esophageal sphincter function in the cat: role of central innervation assessed by transient vagal blockade. Am. J. Physiol. 246, G666–G674 (1984).
Rivera, L. R., Poole, D. P., Thacker, M. & Furness, J. B. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol. Motil. 23, 980–988 (2011).
Diamant, N. E. & Akin, A. Effect of gastric contraction of the lower esophageal sphincter. Gastroenterology 63, 38–44 (1972).
Franzi, S. J., Martin, C. J., Cox, M. R. & Dent, J. Response of canine lower esophageal sphincter to gastric distension. Am. J. Physiol. 259, G380–G385 (1990).
Dent, J. From 1906 to 2006—a century of major evolution of understanding of gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 24, 1269–1281 (2006).
Sanders, K. M., Koh, S. D. & Ward, S. M. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu. Rev. Physiol. 68, 307–343 (2006).
Cannon, W. B. Peristalsis, segmentation and the myenteric reflex. Am. J. Physiol. 30, 114–128 (1912).
Kelly, K. A., Code, C. F. & Elveback, L. R. Patterns of canine gastric electrical activity. Am. J. Physiol. 217, 461–470 (1969).
Hasler, W. L. in Gastroenterology (ed. Yamada, T.) 220–247 (Lippincott Williams & Wilkins, Philadelphia, USA, 2003).
Furness, J. B., Jones, C., Nurgali, K. & Clerc, N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog. Neurobiol. 72, 143–164 (2004).
Kirchgessner, A. L., Tamir, H. & Gershon, M. D. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J. Neurosci. 12, 235–248 (1992).
Bertrand, P. P., Kunze, W. A. A., Bornstein, J. C., Furness, J. B. & Smith, M. L. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am. J. Physiol. 273, G422–G435 (1997).
Kunze, W. A. A., Furness, J. B., Bertrand, P. P. & Bornstein, J. C. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. J. Physiol. 506, 827–842 (1998).
Furness, J. B., Kunze, W. A. A., Bertrand, P. P., Clerc, N. & Bornstein, J. C. Intrinsic primary afferent neurons of the intestine. Prog. Neurobiol. 54, 1–18 (1998).
Spencer, N. J. & Smith, T. K. Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J. Physiol. 558, 577–596 (2004).
Spencer, N. J., Dickson, E. J., Hennig, G. W. & Smith, T. K. Sensory elements within the circular muscle are essential for mechanotransduction of ongoing peristaltic reflex activity in guinea-pig distal colon. J. Physiol. 576, 519–531 (2006).
Mazzuoli, G. & Schemann, M. Multifunctional rapidly adapting mechanosensitive enteric neurons (RAMEN) in the myenteric plexus of the guinea pig ileum. J. Physiol. 587, 4681–4693 (2009).
Hendriks, R., Bornstein, J. C. & Furness, J. B. An electrophysiological study of the projections of putative sensory neurons within the myenteric plexus of the guinea-pig ileum. Neurosci. Lett. 110, 286–290 (1990).
Lawson S. N. in Peripheral Neuropathy (eds Dyck, P. J. & Thomas, P. K.) 163–202 (Elsevier, Philadelphia, PA, 2005).
Kunze, W. A. A. & Furness, J. B. The enteric nervous system and regulation of intestinal motility. Annu. Rev. Physiol. 61, 117–142 (1999).
Weidmann, S., Schrödl, F., Neuhuber, W. & Brehmer, A. Quantitative estimation of putative primary afferent neurons in the myenteric plexus of human small intestine. Histochem. Cell Biol. 128, 399–407 (2007).
Chiocchetti, R. et al. Characterisation of neurons expressing calbindin immunoreactivity in the ileum of the unweaned and mature sheep. Cell Tissue Res. 318, 289–303 (2004).
Gwynne, R. M., Thomas, E. A., Goh, S. M., Sjövall, H. & Bornstein, J. C. Segmentation induced by intraluminal fatty acid in isolated guinea-pig duodenum and jejunum. J. Physiol. 556, 557–569 (2004).
Thomas, E. A. & Bornstein, J. C. Inhibitory cotransmission or after-hyperpolarizing potentials can regulate firing in recurrent networks with excitatory metabotropic transmission. Neuroscience 120, 333–352 (2003).
Ferens, D., Baell, J., Lessene, G., Smith, J. E. & Furness, J. B. Effects of modulators of Ca2+-activated, intermediate-conductance potassium channels on motility of the rat small intestine, in vivo. Neurogastroenterol. Motil. 19, 383–389 (2007).
Wingate, D. L. Backwards and forwards with the migrating complex. Dig. Dis. Sci. 26, 641–666 (1981).
Sarna, S., Stoddard, C., Belbeck, L. & McWade, D. Intrinsic nervous control of migrating myoelectric complexes. Am. J. Physiol. 241, G16–G23 (1981).
El Sharkawy, T. Y., Markus, H. & Diamant, N. E. Neural control of the intestinal migrating myoelectric complex. A pharmacological analysis. Can. J. Physiol. Pharmacol. 60, 794–804 (1982).
Ehrlein, H. J. Retroperistaltism and duodenogastric reflux in dogs. Scand. J. Gastroenterol. 16 (Suppl.), 29–32 (1981).
Lang, I. M., Sarna, S. K. & Shaker, R. Gastrointestinal motor and myoelectric correlates of motion sickness. Am. J. Physiol. 277, G642–G652 (1999).
Vallance, B. A., Blennerhassett, P. A. & Collins, S. M. Increased intestinal muscle contractility and worm expulsion in nematode-infected mice. Am. J. Physiol. 272, G321–G327 (1997).
Field, M. Intestinal ion transport and the pathophysiology of diarrhea. J. Clin. Invest. 111, 931–943 (2003).
Mellander, A., Järbur, K. & Sjövall, H. Pressure and frequency dependent linkage between motility and epithelial secretion in human proximal small intestine. Gut 46, 376–384 (2000).
Lynch, A. C. & Frizelle, F. A. Colorectal motility and defecation after spinal cord injury in humans. Prog. Brain Res. 152, 335–343 (2006).
Shimizu, Y. et al. Evidence that stimulation of ghrelin receptors in the spinal cord initiates propulsive activity in the colon of the rat. J. Physiol. 576, 329–338 (2006).
Lynch, A. C., Antony, A., Dobbs, B. R. & Frizelle, F. A. Bowel dysfunction following spinal cord injury. Spinal Cord 39, 193–203 (2001).
Ferens, D. M. et al. Stimulation of defecation in spinal cord-injured rats by a centrally acting ghrelin receptor agonist. Spinal Cord 49, 1036–1041 (2011).
Wright, E. M. & Loo, D. D. F. Coupling between Na+, sugar, and water transport across the intestine. Ann. NY Acad. Sci. 915, 54–66 (2000).
Sjövall, H. et al. Intestinal fluid and electrolyte transport in man during reduced circulating blood volume. Gut 27, 913–918 (1986).
Young, R. L. Sensing via intestinal sweet taste pathways. Front. Neurosci. 5, 1–13 (2011).
Shirazi-Beechey, S. P., Moran, A. W., Batchelor, D. J., Daly, K. & Al-Rammahi, M. Influences of food constituents on gut health glucose sensing and signalling; regulation of intestinal glucose transport. Proc. Nutr. Soc. 70, 185–193 (2011).
Sigalet, D. L. et al. Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am. J. Physiol. 293, G211–G221 (2007).
Cox, H. M. Peptide YY: a neuroendocrine neighbor of note. Peptides 28, 345–351 (2007).
Estall J. L. & Drucker D. J. Glucagon-like peptide-2. Annu. Rev. Nutr. 26, 391–411 (2006).
Gwynne, R. M., Ellis, M., Sjövall, H. & Bornstein, J. C. Cholera toxin induces sustained hyperexcitability in submucosal secretomotor neurons in guinea pig jejunum. Gastroenterology 136, 299–308 (2009).
Malagelada, J.-R., Rees, W. D. W., Mazzotta, L. J. & Go, V. L. W. Gastric motor abnormalities in diabetic and postvagotomy gastroparesis: effect of metoclopramide and bethanechol. Gastroenterology 78, 286–293 (1980).
Camilleri, M. Alvimopan, a selective peripherally acting μ-opioid antagonist. Neurogastroenterol. Motil. 17, 157–165 (2005).
Sanger, G. J. & Hicks, G. A. Drugs targeting functional bowel disorders: insights from animal studies. Curr. Opin. Pharmacol. 2, 678–683 (2002).
Camilleri, M. et al. Pharmacological and pharmacokinetic aspects of functional gastrointestinal disorders. Gastroenterology 130, 1421–1434 (2006).
De Giorgio, R., Barbara, G., Furness, J. B. & Tonini, M. Novel therapeutic targets for enteric nervous system disorders. Trends Pharmacol. Sci. 28, 473–481 (2007).
Sanger, G. J. & Alpers, D. H. Development of drugs for gastrointestinal motor disorders: translating science to clinical need. Neurogastroenterol. Motil. 20, 177–184 (2008).
Rehfeld, J. F. A centenary of gastrointestinal endocrinology. Horm. Metab. Res. 36, 735–741 (2004).
Raybould, H. E. Mechanisms of CCK signaling from gut to brain. Curr. Opin. Pharmacol. 7, 570–574 (2007).
Brookes, S. J. H., Steele, P. A. & Costa, M. Identification and immunohistochemistry of cholinergic and non-cholinergic circular muscle motor neurons in the guinea-pig small intestine. Neuroscience 42, 863–878 (1991).
Holzer, P. & Holzer Petsche, U. Tachykinins in the gut. Part 1. Expression, release and motor function. Pharmacol. Ther. 73, 173–217 (1997).
Grider, J. R. Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J. Pharmacol. Exp. Ther. 307, 460–467 (2003).
Fahrenkrug, J. et al. Nervous release of vasoactive intestinal polypeptide in the gastrointestinal tract of cats: possible physiological implications. J. Physiol. 284, 291–305 (1978).
Costa, M. et al. Projections and chemical coding of neurons with immunoreactivity for nitric oxide synthase in the guinea-pig small intestine. Neurosci. Lett. 148, 121–125 (1992).
Sanders, K. M. & Ward, S. M. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am. J. Physiol. 262, G379–G392 (1992).
Xue, L. et al. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc. Natl Acad. Sci. USA 97, 1851–1855 (2000).
Brookes, S. J. H., Steele, P. A. & Costa, M. Calretinin immunoreactivity in cholinergic motor neurones, interneurones and vasomotor neurones in the guinea-pig small intestine. Cell Tissue Res. 263, 471–481 (1991).
Young, H. M., Furness, J. B. & Povey, J. M. Analysis of connections between nitric oxide synthase neurons in the myenteric plexus of the guinea-pig small intestine. J. Neurocytol. 24, 257–263 (1995).
Brookes, S. J. H. Classes of enteric nerve cells in the guinea-pig small intestine. Anat. Rec. 262, 58–70 (2001).
Furness, J. B. & Costa, M. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their projections in the guinea-pig small intestine. Neuroscience 7, 341–349 (1982).
Monro, R. L., Bertrand, P. P. & Bornstein, J. C. ATP and 5-HT are the principal neurotransmitters in the descending excitatory reflex pathway of the guinea-pig ileum. Neurogastroenterol. Motil. 14, 255–264 (2002).
Gwynne, R. M. & Bornstein, J. C. Synaptic transmission at functionally identified synapses in the enteric nervous system: roles for both ionotropic and metabotropic receptors. Curr. Neuropharmacol. 5, 1–17 (2007).
Portbury, A. L. et al. Cholinergic, somatostatin-immunoreactive interneurons in the guinea pig intestine: morphology, ultrastructure, connections and projections. J. Anat. 187, 303–321 (1995).
Li, Z. S. & Furness, J. B. Immunohistochemical localization of cholinergic markers in putative intrinsic primary afferent neurons of the guinea-pig small intestine. Cell Tissue Res. 294, 35–43 (1998).
Johnson, P. J. & Bornstein, J. C. Neurokinin-1 and -3 receptor blockade inhibits slow excitatory synaptic transmission in myenteric neurons and reveals slow inhibitory input. Neuroscience 126, 137–147 (2004).
Surprenant, A. Slow excitatory synaptic potentials recorded from neurones of guinea-pig submucous plexus. J. Physiol. 351, 343–361 (1984).
Monro, R. L., Bertrand, P. P. & Bornstein, J. C. ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J. Physiol. 556, 571–584 (2004).
Cassuto, J., Fahrenkrug, J., Jodal, M., Tuttle, R. & Lundgren, O. The release of vasoactive intestinal polypeptide from the cat small intestine exposed to cholera toxin. Gut 22, 958–963 (1981).
Banks, M. R., Farthing, M. J. G., Robberecht, P. & Burleigh, D. E. Antisecretory actions of a novel vasoactive intestinal polypeptide (VIP) antagonist in human and rat small intestine. Br. J. Pharmacol. 144, 994–1001 (2005).
Keast, J. R., Furness, J. B. & Costa, M. Investigations of nerve populations influencing ion transport that can be stimulated electrically, by serotonin and by a nicotinic agonist. Naunyn-Schmiedeberg's Arch. Pharmacol. 331, 260–266 (1985).
Holst, J. J. et al. GRP nerves in pig antrum: role of GRP in vagal control of gastrin secretion. Am. J. Physiol. 253, G643–G649 (1987).
Weigert, N. et al. Role of vagal fibers and bombesin/gastrin-releasing peptide-neurons in distension-induced gastrin release in rats. Regul. Pept. 69, 33–40 (1997).
Nilsson, G., Simon, J., Yalow, R. S. & Berson, S. A. Plasma gastrin and gastric acid responses to sham feeding and feeding in dogs. Gastroenterology 63, 51–59 (1972).
Feldman, M., Richardson, C. T., Taylor, I. L. & Walsh, J. H. Effect of atropine on vagal release of gastrin and pancreatic polypeptide. J. Clin. Invest. 63, 294–298 (1979).
Finkleman, B. On the nature of inhibition in the intestine. J. Physiol. 70, 145–157 (1930).
Macrae, L. M., Furness, J. B. & Costa, M. Distribution of subgroups of noradrenaline neurons in the coeliac ganglion of the guinea-pig. Cell Tissue Res. 244, 173–180 (1986).
Costa, M. & Furness, J. B. Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience 13, 911–919 (1984).
Dresel, P. & Wallentin, L. Effects of sympathetic vasoconstrictor fibres, noradrenaline and vasopresin on the intestinal vascular resistance during constant blood flow or blood pressure. Acta Physiol. Scand. 66, 427–436 (1966).
Furness, J. B. The adrenergic innervation of the vessels supplying and draining the gastrointestinal tract. Z. Zellforsch. Mikrosk. Anat. 113, 67–82 (1971).
Furness, J. B. et al. Distribution, pathways and reactions to drug treatment of nerves with neuropeptide Y- and pancreatic polypeptide-like immunoreactivity in the guinea-pig digestive tract. Cell Tissue Res. 234, 71–92 (1983).
Crowcroft, P. J., Holman, M. E. & Szurszewski, J. H. Excitatory input from the distal colon to the inferior mesenteric ganglion in the guinea-pig. J. Physiol. 219, 443–461 (1971).
Dalsgaard, C. J. et al. Origin of peptide-containing fibers in the inferior mesenteric ganglion of the guinea-pig: immunohistochemical studies with antisera to substance P, enkephalin, vasoactive intestinal polypeptide, cholecystokinin and bombesin. Neuroscience 9, 191–211 (1983).
Love, J. A. & Szurszewski, J. H. The electrophysiological effects of vasoactive intestinal peptide in the guinea-pig inferior mesenteric ganglion. J. Physiol. 394, 67–84 (1987).
Acknowledgements
Work from the author's laboratory is supported by the National Health and Medical Research Council of Australia. I thank Dr Alan Lomax and Dr Daniel Poole for their helpful comments and Dr Trung Nguyen for assistance with the figures.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Furness, J. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9, 286–294 (2012). https://doi.org/10.1038/nrgastro.2012.32
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2012.32
This article is cited by
-
GABAergic and glutamatergic inputs to the medulla oblongata and locus coeruleus noradrenergic pathways are critical for seizures and postictal antinociception neuromodulation
Scientific Reports (2024)
-
A flexible, thin-film microchannel electrode array device for selective subdiaphragmatic vagus nerve recording
Microsystems & Nanoengineering (2024)
-
Herbal medicine and gut microbiota: exploring untapped therapeutic potential in neurodegenerative disease management
Archives of Pharmacal Research (2024)
-
The crosstalk between enteric nervous system and immune system in intestinal development, homeostasis and diseases
Science China Life Sciences (2024)
-
Effects of natural products on functional constipation: analysis of active ingredient and mechanism
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)