Abstract
Aspirin is being used as an effective analgesic and anti-inflammatory agent at doses >325 mg daily. At low doses (75–325 mg daily), aspirin is the key antiplatelet drug in the pharmacological prevention of cardiovascular diseases. Topical and systemic effects of aspirin in the gastrointestinal mucosa are associated with mucosal damage in the upper and lower gastrointestinal tract. The risk of upper gastrointestinal bleeding with aspirin is increased with old age, male sex, ulcer history and concomitant medication with NSAIDs, cyclooxygenase 2 selective inhibitors, corticosteroids or other antithrombotic agents. In some patients, the cardiovascular benefits of low-dose aspirin might be overcome by the risk of gastrointestinal complications, but withdrawal of aspirin therapy can precipitate a cardiovascular event. These patients will need concomitant therapy with antisecretory agents, especially PPIs, to reduce the gastrointestinal risk. Eradication of Helicobacter pylori infection might be an additional option in patients with a history of ulcer. Furthermore, there is growing evidence that long-term use of aspirin decreases the risk of colorectal cancer, even at low doses. As aspirin is one of the most prescribed drugs worldwide and its clinical impact is huge, physicians need to consider the benefits and harms for each individual patient in order to maximize the benefits of aspirin.
Key Points
-
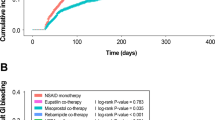
Low-dose aspirin prevents cardiovascular morbidity and mortality in at-risk patients with a history of cardiovascular disease, but can increase the risk of upper and lower gastrointestinal complications
-
Enteric-coated or buffered aspirin preparations are also associated with a risk of gastrointestinal complications; nonaspirin antiplatelet therapy also increases the risk of upper gastrointestinal bleeding
-
Aspirin-induced damage to the gastrointestinal tract can cause clinical bleeding in some patients, especially in those with risk factors, including age >70 years or a history of peptic ulcer
-
Aspirin-induced gastrointestinal damage can be reduced by the use of co-therapy with antisecretory agents, especially PPIs
-
Although infection with Helicobacter pylori might be a risk factor for aspirin-induced ulcer bleeding, the effect of H. pylori eradication on gastrointestinal outcomes in at-risk patients requires further investigation
-
The incidence of mortality from cardiovascular disease far exceeds that associated with gastrointestinal hemorrhage; however, the benefits of aspirin may be reduced in patients with a high risk of gastrointestinal complications
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others

References
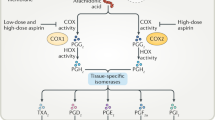
Simmons, D. L., Botting, R. M. & Hla, T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56, 387–437 (2004).
Loll, P. J., Picot, D. & Garavoto, R. M. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat. Struct. Biol. 2, 637–643 (1995).
Oullet, M., Riendeau, D. & Percival, M. D. A high level of cyclooxygenase-2 inhibitor selectivity is associated with a reduced interference of platelet cyclooxygenase-1 inactivation by aspirin. Proc. Natl Acad. Sci. USA 98, 14583–14588 (2001).
Kim, C. & Beckles, G. L. Cardiovascular disease risk reduction in the Behavioral Risk Factor Surveillance System. Am. J. Prev. Med. 27, 1–7 (2004).
Esmatjes, E., Castell, C., Franch, J., Puigoriol, E. & Hernandez, R. Acetylsalicylic acid consumption in patients with diabetes mellitus [Spanish]. Med. Clin. (Barc.) 122, 96–98 (2003).
Wallace, J. L. & Chin, B. C. Inflammatory mediators in gastrointestinal defense and injury. Proc. Soc. Exp. Biol. Med. 214, 192–203 (1997).
Silen, W. & Ito, S. Mechanisms for rapid re-epithelialization of the gastric mucosal surface. Annu. Rev. Physiol. 47, 217–229 (1985).
Konturek, S. J., Dembinski, A., Warzecha, Z., Brzozowski, T. & Gregory, H. Role of epidermal growth factor in healing of chronic gastroduodenal ulcers in rats. Gastroenterology 94, 1300–1307 (1988).
Tarnawski, A., Szabo, I. L., Husain, S. S. & Soreghan, B. Regeneration of gastric mucosa during ulcer healing is triggered by growth factors and signal transduction pathways. J. Physiol. Paris 95, 337–344 (2001).
Ma, L. et al. Platelets modulate gastric ulcer healing: role of endostatin and vascular endothelial growth factor release. Proc. Natl Acad. Sci. USA 98, 6470–6475 (2001).
Reuter, B. K., Cirino, G. & Wallace, J. L. Markedly reduced intestinal toxicity of a diclofenac derivative. Life Sci. 55, PL1–PL8 (1994).
Capone, M. L. et al. Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects. Circulation 109, 1468–1471 (2004).
Capone, M. L. et al. Pharmacodynamic of cyclooxygenase inhibitors in humans. Prostaglandins Other Lipid Mediat. 82, 85–94 (2007).
Tomisato, W. et al. Role of direct cytotoxic effects of NSAIDs in the induction of gastric lesions. Biochem. Pharmacol. 67, 575–585 (2004).
Hingson, D. J. & Ito, S. Effect of aspirin and related compounds on the fine structure of mouse gastric mucosa. Gastroenterology 61, 156–177 (1971).
Geall, M. G., Phillips, S. F. & Summerskill, W. H. Profile of gastric potential difference in man. Effects of aspirin, alcohol, bile, and endogenous acid. Gastroenterology 58, 437–443 (1970).
Baskin, W. N., Ivey, K. J., Krause, W. J., Jeffrey, G. E. & Gemmell, R. T. Aspirin-induced ultrastructural changes in human gastric mucosa: correlation with potential difference. Ann. Intern. Med. 85, 299–303 (1976).
O'Laughlin, J. C., Hoftiezer, J. W. & Ivey, K. J. Effect of aspirin on the human stomach in normals: endoscopic comparison of damage produced one hour, 24 hours, and 2 weeks after administration. Scand. J. Gastroenterol. Suppl. 67, 211–214 (1981).
Derry, S. & Loke, Y. K. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ 321, 1183–1187 (2000).
Sorensen, H. T. et al. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am. J. Gastroenterol. 95, 2218–2224 (2000).
Kelly, J. P. et al. Risk of aspirin-associated major upper-gastrointestinal bleeding with enteric-coated or buffered product. Lancet 348, 1413–1416 (1996).
Chowdhury, A. et al. Gastro-duodenal mucosal changes associated with low-dose aspirin therapy: a prospective, endoscopic study. Indian J. Gastroenterol. 20, 227–229 (2001).
Garcia Rodriguez, L. A., Hernandez-Diaz, S. & de Abajo, F. J. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiologic studies. Br. J. Clin. Pharmacol. 52, 563–571 (2001).
Lichtenberger, L. M. Where is the evidence that cyclooxygenase inhibition is the primary cause of nonsteroidal anti-inflammatory drug (NSAID)-induced gastrointestinal injury? Topical injury revisited. Biochem. Pharmacol. 61, 631–637 (2001).
Rainsford, K. D. Profile and mechanisms of gastrointestinal and other side effects of nonsteroidal anti-inflammatory drugs (NSAIDs). Am. J. Med. 107, 27S–35S (1999).
Bjarnason, I., Scarpignato, C., Takeuchi, K. & Rainsford, K. D. Determinants of the short term gastric damage caused by NSAIDs in man. Aliment. Pharmacol. Ther. 26, 95–106 (2007).
Darling, R. L. et al. The effects of aspirin on gastric mucosal integrity, surface hydrophobicity, and prostaglandin metabolism in cyclooxygenase knockout mice. Gastroenterology 127, 94–104 (2004).
Lichtenberger, L. M., Richards, J. E. & Hills, B. A. Effect of 16,16-dimethyl prostaglandin E2 on the surface hydrophobicity of aspirin-treated canine gastric mucosa. Gastroenterology 88, 308–314 (1985).
Oh, K. W., Qian, T., Brenner, D. A. & Lemasters, J. J. Salicylate enhances necrosis and apoptosis mediated by the mitochondrial permeability transition. Toxicol. Sci. 73, 44–52 (2003).
Kamada, G. et al. Evaluation of gastric mucosal blood flow using contrast enhanced ultrasonography during low dose aspirin therapy and during NSAID and low dose aspirin combined therapy [abstract M1114]. Gastroenterology 134 (Suppl. 1), A-341 (2008).
Odashima, M. et al. Attenuation of gastric mucosal inflammation induced by aspirin through inhibition of selective type III phosphodiesterase in rats. Dig. Dis. Sci. 52, 1355–1359 (2007).
Mahmud, T., Scott, D. L. & Bjarnason, I. A unifying hypothesis for the mechanism of NSAID related gastrointestinal toxicity. Ann. Rheum. Dis. 55, 211–213 (1996).
Konturek, P. C., Kania, J., Hahn, E. G. & Konturek, J. W. Ascorbic acid attenuates aspirin induced gastric damage: role of inducible nitric oxide synthase. J. Physiol. Pharmacol. 57 (Suppl. 5), 125–136 (2006).
Davies, N. M. & Wallace, J. L. Nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: new insights into an old problem. J. Gastroenterol. 32, 127–133 (1997).
Smecuol, E. et al. Low-dose aspirin affects the small bowel mucosa: results of a pilot study with a multidimensional assessment. Clin. Gastroenterol. Hepatol. 7, 524–529 (2009).
Goldstein, J. L. et al. Video capsule endoscopy to prospectively assesses small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin. Gastroenterol. Hepatol. 3, 133–141 (2005).
Massó González, E. L., Patrignani, P., Tacconelli, S. & Rodríguez, L. A. Variability of risk of upper gastrointestinal bleeding among nonsteroidal anti-inflammatory drugs. Arthritis Rheum. 62, 1592–1601 (2010).
Tanaka, A., Araki, H., Komoike, Y., Hase, S. & Takeuchi, K. Inhibition of both COX-1 and COX-2 is required for development of gastric damage in response to nonsteroidal antiinflammatory drugs. J. Physiol. Paris 95, 21–27 (2001).
Campbell, C. L., Smyth, S., Montalescot, G. & Steinhubl, S. R. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA 297, 2018–2024 (2007).
Cohen, M. M. & MacDonald, W. C. Mechanism of aspirin injury to human gastroduodenal mucosa. Prostaglandins Leukot. Med. 9, 241–255 (1982).
Hogan, D. L., Ainsworth, M. A. & Isenberg, J. I. Review article: gastroduodenal bicarbonate secretion. Aliment. Pharmacol. Ther. 8, 475–488 (1994).
Johansson, C., Aly, A., Befrits, R., Smedfors, B. & Uribe, A. Protection of the gastroduodenal mucosa by prostaglandins. Scand. J. Gastroenterol. Suppl. 110, 41–48 (1985).
Cole, A. T. et al. Protection of human gastric mucosa against aspirin-enteric coating or dose reduction? Aliment. Pharmacol. Ther. 13, 187–193 (1999).
Arai, I., Hirose, H., Muramatsu, M., Okuyama, S. & Aihara, H. Possible involvement of non-steroidal anti-inflammatory drugs in vagal-mediated gastric acid secretion in rats. Jpn J. Pharmacol. 37, 91–99 (1985).
Jaramillo, E. et al. The effect of arachidonic acid and its metabolites on acid production in isolated human parietal cells. Scand. J. Gastroenterol. 24, 1231–1237 (1989).
Leivonen, M., Sipponen, P. & Kivilaakso, E. Gastric changes in coronary-operated patients with low-dose aspirin. Scand. J. Gastroenterol. 27, 912–916 (1992).
Niv, Y. et al. Endoscopy in asymptomatic minidose aspirin consumers. Dig. Dis. Sci. 50, 78–80 (2005).
Yeomans, N. D. et al. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment. Pharmacol. Ther. 22, 795–801 (2005).
Sopena, F. & Lanas, A. How to advise aspirin use in patients who need NSAIDs. Curr. Pharm. Des. 13, 2248–2260 (2007).
Capone, M. L. et al. Pharmacodynamic interaction of naproxen with low-dose aspirin in healthy subjects. J. Am. Coll. Cardiol. 45, 1295–1301 (2005).
Patrono, C., Garcia Rodriguez, L. A., Landolfi, R. & Baigent, C. Low-dose aspirin for the prevention of atherothrombosis. N. Engl. J. Med. 353, 2373–2383 (2005).
Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324, 71–86 (2002).
Maulaz, A. B., Bezerra, D. C. & Michel, P. Effect of discontinuing aspirin therapy on the risk of brain ischemic stroke. Arch. Neurol. 62, 1217–1220 (2005).
Berger, J. S., Brown, D. L. & Becker, R. C. Low-dose aspirin in patients with stable cardiovascular disease: a meta-analysis. Am. J. Med. 121, 43–49 (2008).
Dalen, J. E. Aspirin to prevent heart attack and stroke: what's the right dose? Am. J. Med. 119, 198–202 (2006).
Lanas, A. Gastrointestinal bleeding associated with low-dose aspirin use: relevance and management in clinical practice. Expert Opin. Drug Saf. 10, 45–54 (2011).
Baigent, C. et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Antithrombotic Trialists' (ATT) Collaboration. Lancet 373, 1849–1860 (2009).
Ridker, P. M., MacFadyen, J., Libby, P. & Glynn, R. J. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER). Am. J. Cardiol. 106, 204–209 (2010).
Yeomans, N. et al. Efficacy of esomeprazole (20 mg once daily) for reducing the risk of gastroduodenal ulcers associated with continuous use of low-dose aspirin. Am. J. Gastroenterol. 103, 2465–2473 (2008).
Scheiman, J. M. et al. Prevention of low-dose acetylsalicylic acidassociated gastric/duodenal ulcers with esomeprazole 20 mg and 40 mg once daily in patients at increased risk of ulcer development: a randomized controlled trial (OBERON). Gastroenterology 136, A-70 (2009).
Lanas, A. Nonsteroidal anti-inflammatory drugs and cyclooxygenase inhibition in the gastrointestinal tract: a trip from peptic ulcer to colon cancer. Am. J. Med. Sci. 338, 96–106 (2009).
Hayden, M., Pignone, M., Phillips, C. & Mulrow, C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the US Preventive Services Task Force. Ann. Intern. Med. 136, 161–172 (2002).
McQuaid, K. R. & Laine, L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am. J. Med. 119, 624–638 (2006).
Juul-Moller, S. et al. Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. The Swedish Angina Pectoris Aspirin Trial (SAPAT) Group. Lancet 340, 1421–1425 (1992).
The SALT Collaborative Group. Swedish aspirin low-dose trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. Lancet 338, 1345–1349 (1991).
Lanas, A. et al. A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use. Am. J. Gastroenterol. 100, 1685–1693 (2005).
Fortun, P. J. & Hawkey, C. J. Nonsteroidal antiinfl ammatory drugs and the small intestine. Curr. Opin. Gastroenterol. 21, 169–175 (2005).
Bjarnason, I., Williams, P., Smethurst, P., Peters, T. J. & Levi, A. J. Effect of non-steroidal anti-inflammatory drugs and prostaglandins on the permeability of the human small intestine. Gut 27, 1292–1299 (1986).
Bjarnason, I. et al. Glucose and citrate reduce the permeability changes caused by indomethacin in humans. Gastroenterology 102, 1546–1550 (1992).
Moore, R. A., Derry, S. & McQuay, H. J. Faecal blood loss with aspirin, nonsteroidal anti-inflammatory drugs and cyclo-oxygenase-2 selective inhibitors: systematic review of randomized trials using autologous chromium-labelled erythrocytes. Arthritis Res. Ther. 10, R7 (2008).
Lanas, A. & Scheiman, J. Low-dose aspirin and upper gastrointestinal damage: epidemiology, prevention and treatment. Curr. Med. Res. Opin. 23, 163–173 (2007).
Hernandez-Diaz, S. & Garcia Rodriguez, L. A. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 4, 22 (2006).
Bhatt, D. L. et al. American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 52, 1502–1517 (2008).
Harrington, R. A. et al. American College of Chest Physicians. Antithrombotic therapy for non-ST-segment elevation acute coronary syndromes: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 133 (Suppl. 6), 670S–707S (2008).
CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 348, 1329–1339 (1996).
Ibáñez, L. et al. Upper gastrointestinal bleeding associated with antiplatelet drugs. Aliment. Pharmacol. Ther. 23, 235–242 (2006).
Lanas, A. et al. Risk of upper gastrointestinal ulcer bleeding associated with selective COX-2 inhibitors, traditional non-aspirin NSAIDs, aspirin, and combinations. Gut 55, 1731–1738 (2006).
Chan, F. K. et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N. Engl. J. Med. 352, 238–244 (2005).
Lanas, A. et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with NSAIDs and anti-platelet agents. Am. J. Gastroenterol. 102, 507–515 (2007).
Lanas, A. et al. Nitrovasodilators, low dose aspirin, other nonsteroidal antiinflammatory drugs, and the risk of upper gastrointestinal bleeding. N. Engl. J. Med. 343, 834–839 (2000).
Taha, A. S., McCloskey, C., Prasad, R. & Bezlyak, V. Famotidine for the prevention of peptic ulcers and oesophagitis in patients taking low-dose aspirin (FAMOUS): a phase III, randomised, double-blind, placebo-controlled trial. Lancet 374, 119–125 (2009).
Nema, H. & Kato, M. Comparative study of therapeutic effects of PPI and H2RA on ulcers during continuous aspirin therapy. World J. Gastroenterol. 16, 5342–5346 (2010).
Ng, F. H. et al. Famotidine is inferior to pantoprazole in preventing recurrence of aspirin-related peptic ulcers or erosions. Gastroenterology 138, 82–88 (2010).
Ho, P. M. et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. J. Am. Med. Assoc. 301, 937–944 (2009).
Laine, L. & Hennekens, C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am. J. Gastroenterol. 105, 34–41 (2010).
Juurlink, D. N. et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 180, 713–718 (2009).
Gilard, M. et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J. Am. Coll. Cardiol. 51, 256–260 (2008).
Sibbing, D. et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb. Haemost. 101, 714–719 (2009).
Small, D. S. et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J. Clin. Pharmacol. 48, 475–484 (2008).
Bhatt, D. L. et al. The COGENT Investigators. Clopidogrel with or without omeprazole in coronary artery disease. N. Engl. J. Med. 363, 1909–1917 (2010).
FDA Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC) [online], (2009).
EMEA Public statement on possible interaction between clopidogrel and proton pump inhibitors [online], (2009).
Lanas, A. et al. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low dose aspirin. Aliment. Pharmacol. Ther. 16, 779–786 (2002).
Chan, F. K. et al. Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxen. N. Engl. J. Med. 344, 967–973 (2001).
Lai, K. C. et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N. Engl. J. Med. 346, 2033–2038 (2002).
Fletcher, E. H. et al. Systematic review: Helicobacter pylori and the risk of upper gastrointestinal bleeding risk in patients taking aspirin. Aliment. Pharmacol. Ther. 32, 831–839 (2010).
Lanas, A. & Hunt, R. Prevention of anti-inflammatory drug-induced gastrointestinal damage: benefits and risks of therapeutic strategies. Ann. Med. 38, 415–428 (2006).
Gao, R. & Li, X. Risk assessment and aspirin use in Asian and Western populations. Vasc. Health Risk Manag. 6, 943–956 (2010).
Lanas, A. et al. First Spanish consensus on peptic ulcer bleeding management. Consenso sobre Hemorragia Digestiva por Úlcera Péptica [Spanish]. Med. Clin. (Barc.) 135, 608–616 (2010).
Sung, J. Y. et al. Continuation of low dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann. Intern. Med. 152, 1–9 (2010).
Brzozowska, I. et al. Role of prostaglandins, nitric oxide, sensory nerves and gastrin in acceleration of ulcer healing by melatonin and its precursor, L-tryptophan. J. Pineal Res. 32, 149–162 (2002).
Konturek, S. J., Konturek, P. C. & Brzozowski, T. Melatonin in gastroprotection against stress-induced acute gastric lesions and in healing of chronic gastric ulcers. J. Physiol. Pharmacol. 57 (Suppl. 5), 51–66 (2006).
Konturek, P. C. et al. Role of melatonin in mucosal gastroprotection against aspirin-induced gastric lesions in humans. J. Pineal Res. 48, 318–323 (2010).
Pilotto, A., Franceschi, M., Maggi, S., Addante, F. & Sancarlo, D. Optimal management of peptic ulcer disease in the elderly. Drugs Aging 27, 545–558 (2010).
Chan, A. T. et al. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology 134, 21–28 (2008).
Rothwell, P. M. et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 376, 1741–1750 (2010).
Cole, B. F. et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J. Natl Cancer Inst. 101, 256–266 (2009).
Meyer, B. J. Antithrombotic drugs: insights from cardiology. Cerebrovasc. Dis. 8, 19–27 (1998).
Patrono, C. in Platelets in thrombotic and non thrombotic disorders: pathophysiology, pharmacology and therapeutics 919–928 (Cambridge University Press, 2002).
Hankey, G. J. & Eikelboom, J. W. Aspirin resistance. Lancet 367, 606–617 (2006).
Wallace, J. L. & Ma, L. Inflammatory mediators in gastrointestinal defence and injury. Exp. Biol. Med. (Maywood) 226, 1003–1015 (2001).
Wallace, J. L. & Miller, M. J. Nitric oxide in mucosal defence: A little goes a long way. Gastroenterology 119, 512–520 (2000).
Fiorucci, S. et al. Gastrointestinal safety of NO-aspirin (NCX 4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterology 124, 600–607 (2003).
Goddard, P. J. & Lichtenberger, L. M. In vitro recovery of canine gastric mucosal surface hydrophobicity and potential difference after aspirin damage. Dig. Dis. Sci. 40, 1357–1359 (1995).
Cryer, B. et al. Low-dose aspirin-induced ulceration is attenuated by aspirin-phosphatidylcholine: a randomized clinical trial. Am. J. Gastroenterol. 106, 272–277 (2011).
Giustarini, D., Del Soldato, P., Sparatore, A. & Rossi, R. Modulation of thiol homeostasis induced by H2S-releasing aspirin. Free Radic. Biol. Med. 48, 1263–1272 (2010).
Acknowledgements
The authors acknowledge the support of grants PI08 and P1301 from Instituto de Salud Carlos III and grant B01 from the Government of Aragón.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
A. Lanas is a consultant for AstraZeneca, Bayer, Nicox and Pfizer. A. Lanas has received grant/research support from AstraZeneca and has been a speaker for Pfizer. A. Lanas is involved in the adjudication committee of the ARRIVE trial conducted by Bayer. C. Sostres declares no competing interests.
Rights and permissions
About this article
Cite this article
Sostres, C., Lanas, A. Gastrointestinal effects of aspirin. Nat Rev Gastroenterol Hepatol 8, 385–394 (2011). https://doi.org/10.1038/nrgastro.2011.97
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2011.97
This article is cited by
-
Monolithic-to-focal evolving biointerfaces in tissue regeneration and bioelectronics
Nature Chemical Engineering (2024)
-
Unveiling the Influence of Glutathione in Suppressing the Conversion of Aspirin to Salicylic Acid: A Fluorescence and DFT Study
Journal of Fluorescence (2024)
-
The role of non-steroidal anti-inflammatory drugs as adjuncts to periodontal treatment and in periodontal regeneration
Journal of Translational Medicine (2023)
-
Synthesis, and docking studies of arylhydrazone compounds and evaluation of their platelet aggregation inhibitory effect and cytotoxicity
Medicinal Chemistry Research (2022)
-
Pharmacokinetic and Pharmacodynamic Profile of a Novel Phospholipid Aspirin Formulation
Clinical Pharmacokinetics (2022)