Abstract
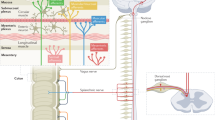
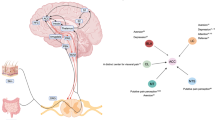
A subset of patients with IBS have visceral hypersensitivity and/or somatic hypersensitivity. Visceral hypersensitivity might have use as a clinical marker of IBS and could account for symptoms of urgency for bowel movements, bloating and abdominal pain. The mechanisms that lead to chronic visceral hypersensitivity in patients who have IBS are unclear. However, several working models may be considered, including: nociceptive input from the colon that leads to hypersensitivity; increased intestinal permeability that induces a visceral nociceptive drive; and alterations in the expression of microRNAs in gastrointestinal tissue that might be delivered via blood microvesicles to other target organs, such as the peripheral and/or central nervous system. As such, the chronic visceral hypersensitivity that is present in a subset of patients with IBS might be maintained by both peripheral and central phenomena. The theories underlying the development of chronic visceral hypersensitivity in patients with IBS are supported by findings from new animal models in which hypersensitivity follows transient inflammation of the colon. The presence of somatic hypersensitivity and an alteration in the neuroendocrine system in some patients who have IBS suggests that multisystemic factors are involved in the overall disorder. Thus, IBS is similar to other chronic pain disorders, such as fibromyalgia, chronic regional pain disorder and temporomandibular joint disorder, as chronic nociceptive mechanisms are activated in all of these disorders.
Key Points
-
Visceral and somatic hypersensitivity are present in some patients with functional gastrointestinal disorders
-
Injury to visceral afferents is the most common underlying cause of visceral hypersensitivity that is maintained by either peripheral and/or central nervous system mechanisms
-
Animal models of hypersensitivity have been used to examine the neural mechanisms of hypersensitivity following inflammatory injury, such as alterations in the N-methyl, D-aspartate receptor, dorsal horn neurons or c-Fos
-
Increased intestinal permeability might lead to hypersensitivity and abdominal pain in patients with functional gastrointestinal disorders
-
Functional gastrointestinal disorders are similar to other chronic pain disorders in which persistent nociceptive mechanisms are activated
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Mayer, E. A. & Gebhart, G. F. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology 107, 271–293 (1994).
Mayer, E. A. et al. Functional GI disorders: from animal models to drug development. Gut 57, 384–404 (2008).
Bouin, M., Meunier, P., Riberdy-Poitras, M. & Poitras, P. Pain hypersensitivity in patients with functional gastrointestinal disorders: a gastrointestinal-specific defect or a general systemic condition? Dig. Dis. Sci. 46, 2542–2548 (2001).
Zhou, Q., Fillingim, R. B., Riley, J. L., Malarkey, W. B. & Verne, G. N. Central and peripherial hypersensitivity in the irritable bowel syndrome. Pain 148, 454–461 (2010).
Piché, M., Arsenault, M., Poitras, P., Rainville, P. & Bouin, M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain 148, 49–58 (2010).
Naliboff, B. D. et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology 131, 352–365 (2006).
Spiller, R. C. Postinfectious irritable bowel syndrome. Gastroenterology 124, 1662–1671 (2003).
Mearin, F. et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology 129, 98–104 (2005).
Dupont, A. W. Post-infectious irritable bowel syndrome. Curr. Gastroenterol. Rep. 9, 378–384 (2007).
Camilleri, M. & Gorman, H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol. Motil. 19, 545–552 (2007).
Zhou, Q., Souba, W. W., Croce, C. & Verne, G. N. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 59, 775–784 (2010).
Su, X. & Gebhart, G. F. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J. Neurophysiol. 80, 2632–2644 (1998).
Gebhart, G. F. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G834–G838 (2000).
Traub, R. J. et al. A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology 135, 2075–2083 (2008).
Zhou, Q., Caudle, R. M., Price, D. D. & Verne, G. N. Visceral and somatic hypersensitivity in a subset of rats following TNBS-Induced colitis. Pain 134, 9–15 (2008).
Al-Chaer, E. D., Kawasaki, M. & Pasricha, P. J. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 119, 1276–1285 (2000).
Mayer, E. A. & Collins, S. M. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology 122, 2032–2048 (2002).
Gwee, K. A. et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet 347, 150–153 (1996).
McKendrick, M. W. & Read, N. W. Irritable bowel syndrome—post salmonella infection. J. Infect. 29, 1–3 (1994).
Accarino, A. M., Azpiroz, F. & Malagelada, J. R. Selective dysfunction of mechanosensitive intestinal afferents in irritable bowel syndrome. Gastroenterology 108, 636–643 (1995).
Whitehead, W. E. et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology 98, 1187–1192 (1990).
Zighelboim, J., Talley, N. J., Phillips. S. F., Harmsen, W. S. & Zinsmeister, A. R. Visceral perception in irritable bowel syndrome. Rectal and gastric responses to distension and serotonin type 3 antagonism. Dig. Dis. Sci. 40, 819–827 (1995).
Zhou, Q., Price, D. D., Caudle, R. M. & Verne, G. N. Spinal NMDA NR1 subunit expression following transient TNBS colitis. Brain Res. 1279, 109–120 (2009).
Price, D. D. et al. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. Neuroimage 47, 995–1001 (2009).
Chang, L., Mayer, E. A., Johnson, T., FitzGerald, L. Z. & Naliboff, B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain 84, 297–307 (2000).
Moore, P. A., Duncan, G. H., Scott, D. S., Gregg, J. M. & Ghia, J. N. The submaximal effort tourniquet test: its use in evaluating experimental and chronic pain. Pain 6, 375–382 (1979).
Carli, G., Suman, A. L., Biasi, G. & Marcolongo, R. Reactivity to superficial and deep stimuli in patients with chronic musculoskeletal pain. Pain 100, 259–269 (2002).
Fillingim, R. B., Maixner, W., Kincaid, S., Sigurdsson, A. & Harris, M. B. Pain sensitivity in patients with temporomandibular disorders: relationship to clinical and psychosocial factors. Clin. J. Pain 12, 260–269 (1996).
Ness, T. J., Powell-Boone, T., Cannon, R., Lloyd, L. K. & Fillingim, R. B. Psychological evidence of hypersensitivity in subjects with interstitial cystitis. J. Urol. 173, 1983–1987 (2005).
Posserud, I. et al. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut 53, 1102–1108 (2004).
Chang, L. et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterolol. Motil. 21, 149–159 (2009).
Gracely, R. H., Lynch, S. A. & Bennett, G. J. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain 51, 175–194 (1992).
Lembo, T. et al. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology 107, 1686–1696 (1994).
Sabate, J. M., Coffin, B., Jian, R., Le Bars, D. & Bouhassira, D. Rectal sensitivity assessed by a reflexologic technique: further evidence for two types of mechanoreceptors. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G692–G699 (2000).
Verne, G. N., Robinson, M. E., Vase, L. & Price, D. D. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain 105, 223–230 (2003).
Zhou, Q., Price, D. D. & Verne, G. N. Reversal of visceral and somatic hypersensitivity in a subset of hypersensitive rats by intracolonic lidocaine. Pain 139, 218–224 (2008).
Coffin, B., Bouhassira, D., Sabaté, J. M., Barbe, L. & Jian, R. Alteration of the spinal modulation of nociceptive processing in patients with irritable bowel syndrome. Gut 53, 1465–1470 (2004).
Zhou, Q., Price, D. D., Callam, C. S., Woodruff, M. A. & Verne, G. N. Effects of the N-methyl-D-aspartate receptor on temporal summation of second pain (wind-up) in irritable bowel syndrome. J. Pain 12, 297–303 (2011).
Zhang, R. et al. Elevated expression of c-fos in central nervous system correlates with visceral hypersensitivity in irritable bowel syndrome (IBS): a target for IBS treatment. Int. J. Colorectal Dis. doi:10.1007/s00384-011-1153-4.
Myers, B. & Greenwood-Van Meerveld, B. Divergent effects of amygdala glucocorticoid and minealcorticoid receptors in the regulation of visceal and somatic pain. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G295–G303 (2010).
Bravo, J. A., Dinan, T. G. & Cryan, J. F. Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disease. Int. J. Neuropsycholopharmacol. 22, 1–18 (2010).
Thoua, N. M. et al. Amitriptyline modifies the visceral hypersensitivity repsonse to acute stress in the irritable bowel syndrome. Aliment. Pharmacol. Ther. 29, 552–560 (2009).
Bjarnason, I., MacPherson, A. & Hollander, D. Intestinal permeabiity: an overview. Gastroenterology 108, 1566–1581 (1995).
Macdonald, T. T. & Montelenone, G. Immunity, inflammation, and allergy in the gut. Science 307, 1920–1925 (2005).
Spiller, R. C. et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47, 804–811 (2000).
Dunlop, S. P. et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am. J. Gastroenterol. 101, 1288–1294 (2006).
Ait-Belgnaoui, A., Bradesi, S., Fioramonit, J., Theodorou, V. & Bueno, L. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain 113, 141–147 (2005).
Zhou, Q., Zhang, B. & Verne, G. N. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 146, 41–46 (2009).
Souba, W. W. et al. The role of glutamine in maintaning a healthy gut and supporting the metabolic response to injury and infection. J. Surg. Res. 48, 383–391 (1990).
Yoshida, S. et al. Effects of glutamine supplements and radiochemotherapy on systemic immune and gut barrier function in patients with advanced esophageal cancer. Ann. Surg. 227, 485–491 (1998).
Sido, B., Seel, C., Hochlehnert, A., Breitkreutz, R. & Dröge, W. Low intestinal glutamine level and low glutaminase activity in Crohn's disease: a rational for glutamine supplementation? Dig. Dis. Sci. 51, 2170–2179 (2006).
Häberle, J. et al. Congenital glutamine deficiency with glutamine synthetase mutations. N. Engl. J. Med. 353, 1926–1933 (2005).
DeMarco, V., Dyess, K., Strauss, D., West, C. M. & Neu, J. Inhibition of glutamine synthetase decreases proliferation of cultured rat intestinal epithelial cells. J. Nutr. 129, 57–62 (1999).
Kim, J. et al. Identification of many microRNAs that copurify with polyribodomes in mammalian neurons. Proc. Natl Acad. Sci. USA 101, 360–365 (2004).
Farh, K. K. et al. The widespread impact of mammalian microRNAs on mRNA represssion and evolution. Science 310, 1817–1821 (2005).
Garson, R., Marcucci, G. & Croce, C. M. Targeting microRNAs in cancer: rationale, strategies, and challenges. Nat. Rev. Drug Discov. 9, 775–789 (2010).
Kapeller, J. et al. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum. Mol. Genet. 17, 2967–2977 (2008).
Wu, F. et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2α. Gastroenterology 135, 1624–1635 (2008).
miRBase The microRNA database [online], (2011).
Valadi, H. et al. Exosome-mediated transfer of mRNA and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Février, B. & Raposo, G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 16, 415–421 (2004).
Acknowledgements
The authors would like to acknowledge the support of NIH grants (NS053090, AT005291) and a VA Merit Review Award from the Medical Research Service of the Department of Veterans Affairs.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Zhou, Q., Verne, G. New insights into visceral hypersensitivity—clinical implications in IBS. Nat Rev Gastroenterol Hepatol 8, 349–355 (2011). https://doi.org/10.1038/nrgastro.2011.83
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2011.83
This article is cited by
-
Distinctions Between Fecal and Intestinal Mucosal Microbiota in Subgroups of Irritable Bowel Syndrome
Digestive Diseases and Sciences (2022)
-
Functional urological disorders: a sensitized defence response in the bladder–gut–brain axis
Nature Reviews Urology (2017)
-
Does central sensitization help explain idiopathic overactive bladder?
Nature Reviews Urology (2016)
-
The Relationship Between Colonic Macrophages and MicroRNA-128 in the Pathogenesis of Slow Transit Constipation
Digestive Diseases and Sciences (2015)
-
Computational functional genomics based analysis of pain-relevant micro-RNAs
Human Genetics (2015)