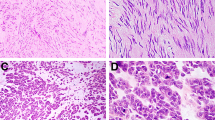
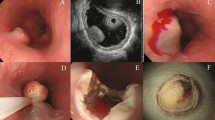
Abstract
Gastrointestinal stromal cell tumors (GISTs) are the most common mesenchymal neoplasm of the gastrointestinal tract and are frequently detected on routine endoscopy. Although only ∼10–30% of GISTs are clinically malignant, all may have some degree of malignant potential. Preoperative determination of malignancy risk can be estimated from tumor size and location, but reliable histopathologic criteria are not currently available. Given such biological uncertainty, accurate diagnosis is essential to differentiate these lesions from other truly benign, subepithelial tumors. Endoscopic ultrasound-guided fine-needle aspiration has emerged as an important procedure to secure a tissue diagnosis of a GIST. When encountering GISTs, gastroenterologists are faced with challenging management decisions, especially in the face of small, incidentally discovered lesions. The majority of localized GISTs are managed via surgical resection, although a select few may be observed using serial endoscopic ultrasound examinations. This Review provides a general overview of GISTs, with an emphasis on their endoscopic diagnosis, the management of localized disease, and the management of incidentally discovered GISTs.
Key Points
-
Gastrointestinal stromal cell tumors are the most common mesenchymal neoplasm of the gastrointestinal tract and are frequently discovered on routine endoscopy
-
Approximately 10–30% of gastrointestinal stromal cell tumors are clinically malignant, but all such tumors may have some malignant potential
-
Preoperative malignancy risk can be estimated from tumor size and location, but a set of reliable histologic criteria do not currently exist
-
The diagnosis of a gastrointestinal stromal cell tumor can be suggested by esophagogastroduodenoscopy or endoscopic ultrasonography, but definitive diagnosis requires tissue acquisition via endoscopic ultrasound-guided fine-needle aspiration
-
Complete surgical resection is the mainstay of treatment for localized disease, although, for a small subset of patients, surveillance via serial endoscopic ultrasound examinations can be undertaken
-
Imatinib therapy is the treatment of choice for locally advanced and metastatic disease, and can also be used as neoadjuvant therapy before surgical resection
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Miettinen, M. & Lasota, J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch. Pathol. Lab. Med. 130, 1466–1478 (2006).
Heinrich, M. C. et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299, 708–710 (2003).
Hirota, S. et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279, 577–580 (1998).
Miettinen, M., Sobin, L. H. & Lasota, J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am. J. Surg. Pathol. 29, 52–68 (2005) (2005).
Miettinen, M., Sarlomo-Rikala, M. & Lasota, J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum. Pathol. 30, 1213–1220 (1999).
Miettinen, M. & Lasota, J. Gatrointestinal stromal tumors: pathology and prognosis at different sites. Semin. Diagn. Pathol. 23, 70–83 (2006).
Besana-Ciani, I., Boni, L., Dionigi, G., Benevento, A. & Dionigi, R. Outcome and long term results of surgical resection for gastrointestinal stromal tumors (GIST). Scand. J. Surg. 92, 195–199 (2003).
Dematteo, R. P. et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 112, 608–615 (2008).
DeMatteo, R. P. et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann. Surg. 231, 51–58 (2000).
Nilsson, B. et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era-—a population-based study in western Sweden. Cancer 103, 821–829 (2005).
Tryggvason, G., Gíslason, H. G., Magnússon, M. K. & Jónasson, J. G. Gastrointestinal stromal tumors in Iceland, 1990–2003: the Icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int. J. Cancer 117, 289–293 (2005).
Miettinen, M. & Lasota, J. Gastrointestinal stromal tumors—definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 438, 1–12 (2001).
Dong, Q., McKee, G., Pitman, M., Geisinger, K. & Tambouret, R., Epithelioid variant of gastrointestinal stromal tumor: diagnosis by fine-needle aspiration. Diagn. Cytopathol. 29, 55–60 (2003).
Fletcher, C. D. et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum. Pathol. 33, 459–465 (2002).
Hirota, S. et al. Gain-of-function mutations of platelet-derived growth factor receptor α gene in gastrointestinal stromal tumors. Gastroenterology 125, 660–667 (2003).
Tryggvason, G. et al. Clinical study on gastrointestinal stromal tumors (GIST) in Iceland, 1990–2003. Dig. Dis. Sci. 52, 2249–2253 (2007).
Abraham, S. C., Krasinskas, A. M., Hofstetter, W. L., Swisher, S. G. & Wu, T. T. “Seedling” mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am. J. Surg. Pathol. 31, 1629–1635 (2007).
Agaimy, A. et al. Microscopic gastrointestinal stromal tumors in esophageal and intestinal surgical resection specimens: a clinicopathologic, immunohistochemical, and molecular study of 19 lesions. Am. J. Surg. Pathol. 32, 867–873 (2008).
Agaimy, A. et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am. J. Surg. Pathol. 31, 113–120 (2007).
Kawanowa, K. et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum. Pathol. 37, 1527–1535 (2006).
Hedenbro, J. L., Ekelund, M. & Wetterberg, P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg. Endosc. 5, 20–23 (1991).
Ghanem, N. et al. Computed tomography in gastrointestinal stromal tumors. Eur. Radiol. 13, 1669–1678 (2003).
Kamiyama, Y. et al. 18F-fluorodeoxyglucose positron emission tomography: useful technique for predicting malignant potential of gastrointestinal stromal tumors. World J. Surg. 29, 1429–1435 (2005).
Hwang, J. H. et al. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest. Endosc. 62, 202–208 (2005).
Motoo, Y. et al. Endoscopic ultrasonography in the diagnosis of extraluminal compressions mimicking gastric submucosal tumors. Endoscopy 26, 239–242 (1994).
Nickl, N. J. Endoscopic approach to gastrointestinal stromal tumors. Gastrointest Endosc. Clin. N. Am. 15, 455–466 (2005).
Nickl, N. J. et al. Clinical implications of endoscopic ultrasound: the American endosonography club study. Gastrointest. Endosc. 44, 371–377 (1996).
Rösch, T. et al. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand. J. Gastroenterol. 37, 856–862 (2002).
Hunt, G. C., Smith, P. P. & Faigel, D. O. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest. Endosc. 57, 68–72 (2003).
Cantor, M. J., Davila, R. E. & Faigel, D. O. Yield of tissue sampling for subepithelial lesions evaluated by EUS: a comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest. Endosc. 64, 29–34 (2006).
Hunt, G. C., Rader, A. E. & Faigel, D. O. A comparison of EUS features between CD-117 positive GI stromal tumors and CD-117 negative GI spindle cell tumors. Gastrointest. Endosc. 57, 469–474 (2003).
Polkowski, M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy 37, 635–645 (2005).
Brand, B. et al. Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract. Dig. Liver Dis. 34, 290–297 (2002).
Okai, T. et al. Endosonographic evaluation of c-kit-positive gastrointestinal stromal tumor. Abdom. Imaging 28, 301–303 (2003).
Gress, F. et al. Interobserver agreement for EUS in the evaluation and diagnosis of submucosal masses. Gastrointest. Endosc. 53, 71–76 (2001).
Chak, A. et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest. Endosc. 45, 468–473 (1997).
Palazzo, L. et al. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut 46, 88–92 (2000).
Jeon, S. W. et al. Gastrointestinal stromal tumors of the stomach: endosonographic differentiation in relation to histological risk. J. Gastroenterol. Hepatol. 22, 2069–2075 (2007).
Shah, P., Gao, F., Edmundowicz, S. A., Azar, R. R. & Early, D. S. Predicting malignant potential of gastrointestinal stromal tumors using endoscopic ultrasound. Dig. Dis. Sci. doi:10.1007/s10620-008-0484–0487 (2008).
Nickl, N. et al. Hypoechoic intramural tumor study: final report [Abstract]. Gastrointest. Endosc. 55, AB98 (2002).
Casali, P. G. et al. Gastrointestinal stromal tumors: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 19 (Suppl. 2), ii35–ii38 (2008).
Demetri, G. D. et al. NCCN clinical practice guidelines in oncology soft tissue sarcoma. http://www.nccn.org/professionals/physician_gls/PDF/sarcoma.pdf (2009).
Hwang, J. H., Rulyak, S. D. & Kimmey, M. B. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology 130, 2217–2228 (2006).
Chhieng, D. C. et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy: a study of 103 cases. Cancer 96, 232–239 (2002).
Eloubeidi, M. A. et al. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer 99, 285–292 (2003).
Giovannini, M., Seitz, J. F., Monges, G., Perrier, H. & Rabbia, I. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy 27, 171–177 (1995).
Shin, H. J., Lahoti, S. & Sneige, N. Endoscopic ultrasound-guided fine-needle aspiration in 179 cases: the M. D. Anderson Cancer Center experience. Cancer 96, 174–180 (2002).
Wiersema, M. J., Vilmann, P., Giovannini, M., Chang, K. J. & Wiersema, L. M. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 112, 1087–1095 (1997).
Wiersema, M. J., Wiersema, L. M., Khusro, Q., Cramer, H. M. & Tao, L. C. Combined endosonography and fine-needle aspiration cytology in the evaluation of gastrointestinal lesions. Gastrointest. Endosc. 40, 199–206 (1994).
Williams, D. B. et al. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut 44, 720–726 (1999).
Ando, N. et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest. Endosc. 55, 37–43 (2002).
Vander Noot, M. R. et al. Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer 102, 157–163 (2004).
Akahoshi, K. et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J. Gastroenterol. 13, 2077–2082 (2007).
Sepe, P. et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of gastrointestinal stromal cell tumors: sensitivity and cytologic yield. Gastrointest. Endosc. (in press).
Klapman, J. B., Logrono, R., Dye, C. E. & Waxman, I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am. J. Gastroenterol. 98, 1289–1294 (2003).
Pellise Urquiza, M. et al., Endoscopic ultrasound-guided fine needle aspiration: predictive factors of accurate diagnosis and cost-minimization analysis of on-site pathologist. Gastroenterol. Hepatol. 30, 319–324 (2007).
Rodriguez, S. A. & Faigel, D. O. Endoscopic diagnosis of gastrointestinal stromal cell tumors. Curr. Opin. Gastroenterol. 23, 539–543 (2007).
Levy, M. J. & Wiersema, M. J. EUS-guided Tru-cut biopsy. Gastrointest. Endosc. 62, 417–426 (2005).
Gines, A. et al. Prospective study of a Tru-cut needle for performing EUS-guided biopsy with EUS-guided FNA rescue. Gastrointest. Endosc. 62, 597–601 (2005).
Levy, M. J., Jondal, M. L., Clain, J. & Wiersema, M. J. Preliminary experience with an EUS-guided Tru-cut biopsy needle compared with EUS-guided FNA. Gastrointest. Endosc. 57, 101–106 (2003).
Varadarajulu, S. et al. Comparison of EUS-guided 19-gauge Tru-cut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy 36, 397–401 (2004).
Aithal, G. P. et al. EUS-guided tissue sampling: comparison of “dual sampling” (Tru-cut biopsy plus FNA) with “sequential sampling” (Tru-cut biopsy and then FNA as required). Endoscopy 39, 725–730 (2007).
Storch, I. et al. Advantage of EUS Tru-cut biopsy combined with fine-needle aspiration without immediate on-site cytopathologic examination. Gastrointest. Endosc. 64, 505–511 (2006).
Wittmann, J., Kocjan, G., Sgouros, S. N., Deheragoda, M. & Pereira, S. P. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and Tru-cut needle biopsy: a prospective study. Cytopathology 17, 27–33 (2006).
Gomes, A. L., Bardales, R. H., Milanezi, F., Reis, R. M. & Schmitt, F. Molecular analysis of c-Kit and PDGFRA in GISTs diagnosed by EUS. Am. J. Clin. Pathol. 127, 89–96 (2007).
Rader, A. E. et al. Fine-needle aspiration biopsy diagnosis of gastrointestinal stromal tumors using morphology, immunocytochemistry, and mutational analysis of c-kit. Cancer 93, 269–275 (2001).
Willmore-Payne, C., Layfield, L. J. & Holden, J. A. c-KIT mutation analysis for diagnosis of gastrointestinal stromal tumors in fine needle aspiration specimens. Cancer 105, 165–170 (2005).
Debiec-Rychter, M. et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur. J. Cancer 42, 1093–1103 (2006).
Heinrich, M. C. et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J. Clin. Oncol. 24, 4764–4774 (2006).
Heinrich, M. C. et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J. Clin. Oncol. 21, 4342–4349 (2003).
Singer, S. et al. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J. Clin. Oncol. 20, 3898–3905 (2002).
Lasota, J. & Miettinen, M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology 53, 245–266 (2008).
Demetri, G. D. et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)—update of the NCCN clinical practice guidelines. J. Natl Compr. Canc. Netw. 5 (Suppl. 2), 1–29 (2007).
Blay, J. Y. et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20–21 March 2004 under the auspices of ESMO. Ann. Oncol. 16, 566–578 (2005).
Bedard, E. L., Mamazza, J., Schlachta, C. M. & Poulin, E. C. Laparoscopic resection of gastrointestinal stromal tumors: not all tumors are created equal. Surg. Endosc. 20, 500–503 (2006).
Catena, F. et al. Laparoscopic treatment of gastric GIST: report of 21 cases and literature's review. J. Gastrointest. Surg. 12, 561–568 (2008).
Choi, S. M. et al. Laparoscopic wedge resection for gastric GIST: long-term follow-up results. Eur. J. Surg. Oncol. 33, 444–447 (2007).
Huguet, K. L. et al. Laparoscopic gastric gastrointestinal stromal tumor resection: the Mayo clinic experience. Arch. Surg. 143, 587–590 (2008).
Lai, I. R., Lee, W. J. & Yu, S. C. Minimally invasive surgery for gastric stromal cell tumors: intermediate follow-up results. J. Gastrointest. Surg. 10, 563–566 (2006).
Nguyen, S. Q., Divino, C. M., Wang, J. L. & Dikman, S. H. Laparoscopic management of gastrointestinal stromal tumors. Surg. Endosc. 20, 713–716 (2006).
Novitsky, Y. W., Kercher, K. W., Sing, R. F. & Heniford, B. T. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann. Surg. 243, 738–745 (2006).
Rivera, R. E., Eagon, J. C., Soper, N. J., Klingensmith, M. E. & Brunt, L. M. Experience with laparoscopic gastric resection: results and outcomes for 37 cases. Surg. Endosc. 19, 1622–1626 (2005).
Melzer, E. & Fidder, H. The natural course of upper gastrointestinal submucosal tumors: an endoscopic ultrasound survey. Isr. Med. Assoc. J. 2, 430–432 (2000).
Hwang, J. H. & Kimmey, M. B. The incidental upper gastrointestinal subepithelial mass. Gastroenterology 126, 301–307 (2004).
Lee, I. L. et al. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy 38, 1024–1028 (2006).
Park, Y. S. et al. Endoscopic enucleation of upper-GI submucosal tumors by using an insulated-tip electrosurgical knife. Gastrointest. Endosc. 59, 409–415 (2004).
Sun, S., Ge, N., Wang, C., Wang, M. & Lü, Q. Endoscopic band ligation of small gastric stromal tumors and follow-up by endoscopic ultrasonography. Surg. Endosc. 21, 574–578 (2007).
Zhou, P. H., Yao, L. Q. & Qin, X. Y. Endoscopic submucosal dissection for gastrointestinal stromal tumors: a report of 20 cases. Zhonghua Wei Chang Wai Ke Za Zhi 11, 219–222 (2008).
Demetri, G. D. et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 347, 472–480 (2002).
Buchdunger, E. et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J. Pharmacol. Exp. Ther. 295, 139–145 (2000).
Demetri, G. D. et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368, 1329–1338 (2006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
W. R. Brugge declares associations with the following companies: Pentax Corporation, as recipient of grant/research support, and Boston Scientific, as consultant. P. S. Sepe declares no competing interests.
Rights and permissions
About this article
Cite this article
Sepe, P., Brugge, W. A guide for the diagnosis and management of gastrointestinal stromal cell tumors. Nat Rev Gastroenterol Hepatol 6, 363–371 (2009). https://doi.org/10.1038/nrgastro.2009.43
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2009.43
This article is cited by
-
Chirurgische und interdisziplinäre Therapie gastrointestinaler Stromatumoren
Der Chirurg (2022)
-
Gastrointestinal stromal tumor: a review of current and emerging therapies
Cancer and Metastasis Reviews (2021)
-
The Impact of Imatinib on Survival and Treatment Trends for Small Bowel and Colorectal Gastrointestinal Stromal Tumors
Journal of Gastrointestinal Surgery (2020)
-
Endoscopic Enucleation Is Effective and Relatively Safe in Small Gastric Subepithelial Tumors Originating from Muscularis Propria
Digestive Diseases and Sciences (2019)
-
Feasibility of using computed tomography texture analysis parameters as imaging biomarkers for predicting risk grade of gastrointestinal stromal tumors: comparison with visual inspection
Abdominal Radiology (2019)