Key Points
-
The budding yeast Saccharomyces cerevisiae, because of its compact genome and tractable genetics, has served as a prime model organism for developing and applying large-scale genomic technologies.
-
During the past few years, several comparable genome-wide functional studies have been published. In many cases, the majority of results can be confirmed by completely independent methods, but it has also become apparent that there are limitations and uncertainties associated with these technologies.
-
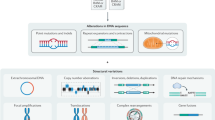
These problems and possible solutions are discussed by highlighting yeast work in which gene expression, protein abundance, protein–DNA interactions, DNA replication, protein interactions and genome-wide mutagenesis have been investigated.
-
Each method has its own drawbacks and might not be comprehensive.
-
Differences in the genetic background of strains, in experimental set-ups and in analytical methods contribute to inconsistencies between databases. Technical difficulties and experimental variation are also the cause of false-positive and false-negative results. Because of the work involved, some large-scale studies have not been replicated, and appropriate controls might not be feasible. In some cases, it might be difficult to judge the rate of erroneous results.
-
The integration of genome-wide data sets increases fidelity and generates the most relevant information. Computational approaches towards this aim are discussed.
Abstract
Since the publication of the Saccharomyces cerevisiae genome sequence, much effort has been dedicated to developing high-throughput techniques to generate comprehensive information about the function and dynamics of all genes in this yeast's genome. These techniques have generated data sets that typically contain large amounts of reliable and valuable biological information. Nevertheless, there are also uncertainties that are associated with such large-scale studies, which we discuss in this review. These uncertainties increase with the complexity of the organism under study. On the basis of the results from yeast, we should learn much from human and mouse genomic data sets. However, as with yeast data sets, they might also contain misleading results.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Goffeau, A. et al. Life with 6000 genes. Science 274, 546, 563–567 (1996).
Kumar, A. & Snyder, M. Emerging technologies in yeast genomics. Nature Rev. Genet. 2, 302–312 (2001).
Pandey, A. & Mann, M. Proteomics to study genes and genomes. Nature 405, 837–846 (2000).
DeRisi, J. L., Iyer, V. R. & Brown, P. O. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278, 680–686 (1997).
Wodicka, L., Dong, H., Mittmann, M., Ho, M.-H. & Lockhart, D. J. Genome-wide expression monitoring in Saccharomyces cerevisiae. Nature Biotechnol. 15, 1359–1367 (1997).
Velculescu, V. E., Zhang, L., Vogelstein, B. & Kinzler, K. W. Serial analysis of gene expression. Science 270, 484–487 (1995).
Schena, M., Shalon, D., Davis, R. W. & Brown, P. O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270, 467–470 (1995).
Primig, M. et al. The core meiotic transcriptome in budding yeasts. Nature Genet. 26, 415–423 (2000).
Jelinsky, S. A., Estep, P., Church, G. M. & Samson, L. D. Regulatory networks revealed by transcriptional profiling of damaged Saccharomyces cerevisiae cells: Rpn4 links base excision repair with proteasomes. Mol. Cell. Biol. 20, 8157–8167 (2000).
Cho, R. J. et al. A genome-wide transcriptional analysis of the mitotic cell cycle. Mol. Cell 2, 65–73 (1998).
Spellman, P. T. et al. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell 9, 3273–3297 (1998).
Gasch, A. P. et al. Genomic expression responses to DNA-damaging agents and the regulatory role of the yeast ATR homolog Mec1p. Mol. Biol. Cell 12, 2987–3003 (2001).
Jelinsky, S. A. & Samson, L. D. Global response of Saccharomyces cerevisiae to an alkylating agent. Proc. Natl Acad. Sci. USA 96, 1486–1491 (1999).
Velculescu, V. E. et al. Characterization of the yeast transcriptome. Cell 88, 243–251 (1997).
Chu, S. et al. The transcriptional program of sporulation in budding yeast. Science 282, 699–705 (1998).
Hughes, T. R. et al. Functional discovery via a compendium of expression profiles. Cell 102, 109–126 (2000).State-of-the-art DNA microarray study showing that cellular pathways affected by drug or genetic perturbations can be determined by comparing the gene expression profile with a compendium of 300 expression profiles that were measured under different conditions.
Hughes, J. D., Estep, P. W., Tavazoie, S. & Church, G. M. Computational identification of cis-regulatory elements associated with groups of functionally related genes in Saccharomyces cerevisiae. J. Mol. Biol. 296, 1205–1214 (2000).
Dirick, L., Bohm, T. & Nasmyth, K. Roles and regulation of Cln-Cdc28 kinases at the start of the cell cycle of Saccharomyces cerevisiae. EMBO J. 14, 4803–4813 (1995).
Gygi, S. P., Rochon, Y., Franza, B. R. & Aebersold, R. Correlation between protein and mRNA abundance in yeast. Mol. Cell. Biol. 19, 1720–1730 (1999).
Futcher, B., Latter, G. I., Monardo, P., McLaughlin, C. S. & Garrels, J. I. A sampling of the yeast proteome. Mol. Cell. Biol. 19, 7357–7368 (1999).
Washburn, M. P., Wolters, D. & Yates, J. R. III. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nature Biotechnol. 19, 242–247 (2001).
Ren, B. et al. Genome-wide location and function of DNA binding proteins. Science 290, 2306–2309 (2000).
Iyer, V. R. et al. Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature 409, 533–538 (2001).References 22 and 23 describe an approach to mapping the interaction sites of DNA-binding proteins in vivo by combining DNA microarrays with chromatin immunoprecipitation.
Newlon, C. S. & Theis, J. F. DNA replication joins the revolution: whole-genome views of DNA replication in budding yeast. Bioessays 24, 300–304 (2002).
Raghuraman, M. K. et al. Replication dynamics of the yeast genome. Science 294, 115–121 (2001).
Wyrick, J. J. et al. Genome-wide distribution of ORC and MCM proteins in S. cerevisiae: high-resolution mapping of replication origins. Science 294, 2357–2360 (2001).
Fields, S. & Song, O. A novel genetic system to detect protein–protein interactions. Nature 340, 245–246 (1989).
Uetz, P. et al. A comprehensive analysis of protein–protein interactions in Saccharomyces cerevisiae. Nature 403, 623–627 (2000).
Ito, T. et al. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl Acad. Sci. USA 98, 4569–4574 (2001).References 28 and 29 are independent studies in which hundreds of binary protein–protein interactions were determined in a high-throughput yeast two-hybrid assay.
Hodges, P. E., McKee, A. H., Davis, B. P., Payne, W. E. & Garrels, J. I. The Yeast Proteome Database (YPD): a model for the organization and presentation of genome-wide functional data. Nucleic Acids Res. 27, 69–73 (1999).
Fromont-Racine, M., Rain, J. C. & Legrain, P. Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nature Genet. 16, 277–282 (1997).
Gavin, A. C. et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415, 141–147 (2002).
Ho, Y. et al. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415, 180–183 (2002).References 32 and 33 are independent approaches by which hundreds of protein complexes were purified and identified using mass spectrometry.
Kumar, A. & Snyder, M. Protein complexes take the bait. Nature 415, 123–124 (2002).
Winzeler, E. et al. Functional characterization of the Saccharomyces cerevisiae genome by precise deletion and parallel analysis. Science 285, 901–906 (1999).
Giaever, G. et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418, 387–391 (2002).References 35 and 36 describe the creation of a complete set of barcoded yeast deletion strains.
Ball, C. A. et al. Integrating functional genomic information into the Saccharomyces genome database. Nucleic Acids Res. 28, 77–80 (2000).
Tong, A. H. et al. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294, 2364–2368 (2001).
Giaever, G. et al. Genomic profiling of drug sensitivities via induced haploinsufficiency. Nature Genet. 21, 278–283 (1999).
Raamsdonk, L. M. et al. A functional genomics strategy that uses metabolome data to reveal the phenotype of silent mutations. Nature Biotechnol. 19, 45–50 (2001).
Hughes, T. R. et al. Widespread aneuploidy revealed by DNA microarray expression profiling. Nature Genet. 25, 333–337 (2000).
Elledge, S. J. & Davis, R. W. Two genes differentially regulated in the cell cycle and by DNA-damaging agents encode alternative regulatory subunits of ribonucleotide reductase. Genes Dev. 4, 740–751 (1990).
Winzeler, E. A., Lee, B., McCusker, J. H. & Davis, R. W. Whole genome genetic-typing in yeast using high-density oligonucleotide arrays. Parasitology 118, S73–S80 (1999).
Ross-Macdonald, P. et al. Large-scale analysis of the yeast genome by transposon tagging and gene disruption. Nature 402, 413–418 (1999).
Valencia, M. et al. NEJ1 controls non-homologous end joining in Saccharomyces cerevisiae. Nature 414, 666–669 (2001).
Ooi, S. L., Shoemaker, D. D. & Boeke, J. D. A DNA microarray-based genetic screen for nonhomologous end-joining mutants in Saccharomyces cerevisiae. Science 294, 2552–2556 (2001).
Marcotte, E. M., Pellegrini, M., Thompson, M. J., Yeates, T. O. & Eisenberg, D. A combined algorithm for genome-wide prediction of protein function. Nature 402, 83–86 (1999).
Ge, H., Liu, Z., Church, G. M. & Vidal, M. Correlation between transcriptome and interactome mapping data from Saccharomyces cerevisiae. Nature Genet. 29, 482–486 (2001).
Jansen, R., Greenbaum, D. & Gerstein, M. Relating whole-genome expression data with protein–protein interactions. Genome Res. 12, 37–46 (2002).
von Mering, C. et al. Comparative assessment of large-scale data sets of protein–protein interactions. Nature 417, 399–403 (2002).Computational approach to assess the accuracy, potential and bias of different genomic approaches to determine protein–protein interactions.
Steinmetz, L. M. et al. Dissecting the architecture of a quantitative trait locus in yeast. Nature 416, 326–330 (2002).
Acknowledgements
E.A.W. is supported by a New Scholars Award from The Ellison Medical Foundation. B.G. is supported by a fellowship from the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
<i>Saccharomyces</i> Genome Database
FURTHER INFORMATION
Munich Information Centre for Protein Sciences — Comprehensive Yeast Genome Database
Saccharomyces Genome Deletion Project
Transposon-Insertion Phenotypes, Localization and Expression in Saccharomyces (TRIPLES)
Glossary
- TRANSCRIPTOME
-
The entire mRNA complement that is expressed by an organism. The expression of mRNA transcripts is dynamic and changes under different conditions.
- TWO-DIMENSIONAL GEL
-
A gel-electrophoresis method by which proteins are separated by charge in the first dimension and by size in the second.
- MASS SPECTROMETRY
-
A technique that provides accurate information about the molecular mass of complex molecules. It can identify extremely small amounts of proteins by their mass-fragment spectra.
- CODON-USAGE BIAS
-
A measurement that takes into account the frequency of less commonly used alternative codons in a protein-coding sequence.
- LIQUID CHROMATOGRAPHY
-
A method that separates molecules from mixtures by selective adsorption.
- PHEROMONE
-
A molecule that is produced for signalling between individuals of the same species.
- AUTONOMOUS REPLICATION SEQUENCE
-
(ARS). A consensus sequence that is necessary, but not sufficient, to allow the replication and maintenance of an otherwise non-replicating plasmid in yeast.
- MESELSON–STAHL EXPERIMENT
-
The experiment that was originally used to prove semiconservative DNA replication. Cells were first grown in a medium that contains heavy isotopes and then moved to a medium that contains light isotopes. The newly replicated DNA was analysed by density-gradient centrifugation. Because only heavy–light hybrids were observed versus heavy- or light-labelled DNA, it was concluded that DNA replicates semi-conservatively.
- BAIT
-
In a yeast two-hybrid approach, this is the protein that is fused to the Gal4 DNA-binding domain.
- PREY
-
In a yeast two-hybrid approach, this is the protein that is fused to the Gal4 activating domain.
- ORTHOLOGUE
-
A homologous gene that originated through speciation.
- STOICHIOMETRIC
-
The molar ratio of interacting molecules.
- TETRAD DISSECTION
-
Phenotypic analysis of the four haploid daughter cells that come from the meiotic cell division of a single diploid cell in yeast. In Saccharomyces cerevisiae, the four meiotic daughter cells are in a single ascus, or spore. Physically associated with one another in this 'tetrad', they can be dissected away from each other, allowing phenotypic segregation ratios to be scored precisely.
- SYNTHETIC INTERACTIONS
-
These occur when a double mutant has a phenotype that is more severe or milder than the phenotype of either single-mutant parent. For suppressors (synthetic viable), the double mutant is viable when at least one of the single mutants is not. For synthetic-lethal mutants, the double mutant is inviable under conditions in which both parental mutations are viable.
- COLLATERAL MUTATION
-
A random second-site mutation that might have been introduced inadvertently during site-directed mutagenesis.
- ANEUPLOIDY
-
The presence of extra copies, or no copies, of some chromosomes.
Rights and permissions
About this article
Cite this article
Grünenfelder, B., Winzeler, E. Treasures and traps in genome-wide data sets: case examples from yeast. Nat Rev Genet 3, 653–661 (2002). https://doi.org/10.1038/nrg886
Issue Date:
DOI: https://doi.org/10.1038/nrg886
This article is cited by
-
Single-cell and multivariate approaches in genetic perturbation screens
Nature Reviews Genetics (2015)
-
Architecture of transcriptional regulatory circuits is knitted over the topology of bio-molecular interaction networks
BMC Systems Biology (2008)
-
Autocorrelation analysis reveals widespread spatial biases in microarray experiments
BMC Genomics (2007)
-
Information Theory in Living Systems, Methods, Applications, and Challenges
Bulletin of Mathematical Biology (2007)
-
MIMAS: an innovative tool for network-based high density oligonucleotide microarray data management and annotation
BMC Bioinformatics (2006)