Key Points
-
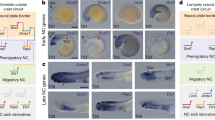
Neural crest cells are a migratory population of cells that originate from the border between the neural plate and the non-neural ectoderm.
-
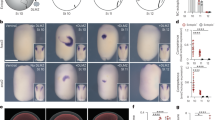
Induction at the neural plate border is difficult to define; it probably involves many steps and the cells show remarkable plasticity in their cell-fate determination (here, induction of Slug expression is considered to be coincident with the induction of the neural crest).
-
Two models have been proposed for induction of the neural crest: the neural induction model and the two-signal model, both of which involve an interplay between BMP signalling and inhibition, as well as WNT (Wingless-related) and FGF (fibroblast growth factor) signalling.
-
The source of signalling that is required for neural crest induction is still controversial — some experiments indicate that it is the paraxial mesoderm, whereas others indicate that the signals come from the epithelial–neural plate border.
-
Notch signalling, a secreted protein Noelin and zebrafish Narrowminded have also been implicated in neural crest induction.
-
Signals that induce the neural crest turn on the expression of transcription factors such as Slug, Pax, Fox, Zic, Sox and Meis, all of which direct neural crest differentiation.
Abstract
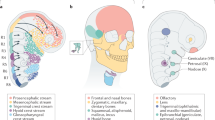
In the embryo, the neural crest is an important population of cells that gives rise to diverse derivatives, including the peripheral nervous system and the craniofacial skeleton. Evolutionarily, the neural crest is of interest as an important innovation in vertebrates. Experimentally, it represents an excellent system for studying fundamental developmental processes, such as tissue induction. Classical embryologists have identified interactions between tissues that lead to neural crest formation. More recently, geneticists and molecular biologists have identified the genes that are involved in these interactions; this recent work has revealed that induction of the neural crest is a complex multistep process that involves many genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Le Douarin, N. M. & Kalcheim, C. The Neural Crest 2nd edn (Cambridge Univ. Press, Cambridge, UK, 1999).
Selleck, M. A. J. & Bronner-Fraser, M. Origins of the avian neural crest: the role of neural plate–epidermal interactions. Development 121, 525–538 (1995).
Collazo, A., Bronner-Fraser, M. & Fraser, S. E. Vital dye labelling of Xenopus laevis trunk neural crest reveals multipotency and novel pathways of migration. Development 118, 363–376 (1993).
Ruffins, S., Artinger, K. B. & Bronner-Fraser, M. Early migrating neural crest cells can form ventral neural tube derivatives when challenged by transplantation. Dev. Biol. 203, 295–304 (1998).
Nieto, M. A., Sargent, M. G., Wilkinson, D. G. & Cooke, J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264, 835–839 (1994).
Mayor, R., Morgan, R. & Sargent, M. G. Induction of the prospective neural crest in Xenopus. Development 121, 767–777 (1995).
Wilson, P. A. & Hemmati-Brivanlou, A. Vertebrate neural induction: inducers, inhibitors, and a new synthesis. Neuron 18, 699–710 (1997).
Hogan, B. L. Bone morphogenetic proteins in development. Curr. Opin. Genet. Dev. 6, 432–438 (1996).
Streit, A. et al. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development 125, 507–519 (1998).
Faure, S., Santa Barbara, P., Roberts, D. J. & Whitman, M. Endogenous patterns of BMP signaling during early chick development. Dev. Biol. 244, 44–65 (2002).
Wilson, S. I., Graziano, E., Harland, R., Jessell, T. M. & Edlund, T. An early requirement for FGF signaling in the acquisition of neural cell fate in the chick embryo. Curr. Biol. 10, 421–429 (2000).
Streit, A., Berliner, A. J., Papanayotou, C., Sirulnik, A. & Stern, C. D. Initiation of neural induction by FGF signalling before gastrulation. Nature 406, 74–78 (2000).
Storey, K. G. et al. Early posterior neural tissue is induced by FGF in the chick embryo. Development 125, 473–484 (1998).
Wilson, S. et al. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature 411, 325–330 (2001).
Mayor, R. & Aybar, M. J. Induction and development of neural crest in Xenopus laevis. Cell Tissue Res. 305, 203–209 (2001).
Streit, A. & Stern, C. D. Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech. Dev. 82, 51–66 (1999).
Nguyen, V. H. et al. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev. Biol. 199, 93–110 (1998).
LaBonne, C. & Bronner-Fraser, M. Neural crest induction in Xenopus: evidence for a two-signal model. Development 125, 2403–2414 (1998).
Wilson, P. A. & Hemmati-Brivanlou, A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature 376, 331–333 (1995).
Knecht, A. K. & Harland, R. M. Mechanisms of dorsal–ventral patterning in noggin-induced neural tissue. Development 124, 2477–2488 (1997).
Essex, L. J., Mayor, R. & Sargent, M. A. Expression of Xenopus snail in mesoderm and prospective neural fold ectoderm. Dev. Dyn. 198, 108–122 (1993).
Bang, A. G., Papalopulu, N., Kintner, C. & Goulding, M. D. Expression of Pax-3 is initiated in the early neural plate by posteriorizing signals produced by the organizer and by posterior non-axial mesoderm. Development 124, 2075–2085 (1997).
Linker, C., Bronner-Fraser, M. & Mayor, R. Relationship between gene expression domains of Xsnail, Xslug, and Xtwist and cell movement in the prospective neural crest of Xenopus. Dev. Biol. 224, 215–225 (2000).
Marchant, L., Linker, C., Ruiz, P., Guerrero, N. & Mayor, R. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev. Biol. 198, 319–329 (1998).
Villanueva, S., Glavic, A., Ruiz, P. & Mayor, R. Posteriorization by FGF, Wnt, and retinoic acid is required for neural crest induction. Dev. Biol. 241, 289–301 (2002).
Saint-Jeannet, J.-P., He, X., Varmus, H. E. & Dawid, I. B. Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc. Natl Acad. Sci. USA 94, 13713–13718 (1997).
Chang, C. & Hemmati-Brivanlou, A. Neural crest induction by Xwnt7B in Xenopus. Dev. Biol. 194, 129–134 (1998).
Isaacs, H. V., Tannahill, D. & Slack, J. M. W. Expression of a novel FGF in the Xenopus embryo. A new candidate inducing factor for mesoderm formation and anteroposterior specification. Development 114, 711–720 (1992).
Christian, J. L., McMahon, J. A., McMahon, A. P. & Moon, R. T. XWnt-8, a Xenopus Wnt-1/int-1 related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development 111, 1045–1055 (1991).
Deardorff, M. A., Tan, C., Saint-Jeannet, J.-P. & Klein, P. A role for frizzled 3 in neural crest development. Development 128, 3655–3663 (2001).
Papalopulu, N. & Kintner, C. A posteriorising factor, retinoic acid, reveals that anteroposterior patterning controls the timing of neuronal differentiation in Xenopus neuroectoderm. Development 122, 3409–3418 (1996).
Lamb, T. M. & Harland, R. M. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior–posterior neural pattern. Development 121, 3627–3636 (1995).
McGrew, L. L., Lai, C. J. & Moon, R. T. Specification of the antero-posterior neural axis through synergistic interaction of the Wnt signaling cascade with noggin and follistatin. Dev. Biol. 172, 337–342 (1995).
Ikeya, M., Lee, S. M., Johnson, J. E., McMahon, A. P. & Takada, S. Wnt signaling required for expansion of neural crest and CNS progenitors. Nature 389, 966–970 (1997).
Mayor, R., Guerrero, N. & Martinez, C. Role of FGF and noggin in neural crest induction. Dev. Biol. 189, 1–12 (1997).
Tan, C. et al. Kermit, a frizzled interacting protein, regulates frizzled 3 signaling in neural crest development. Development 128, 3665–3674 (2001).
Raven, C. R. & Kloos, J. Induction by medial and lateral pieces of the archenteron roof, with special reference to the determination of neural crest. Acta Neerl. Morphol. 5, 384–362 (1945).
Bonstein, L., Elias, S. & Frank, D. Paraxial-fated mesoderm is required for neural crest induction in Xenopus embryos. Dev. Biol. 193, 156–168 (1998).
Rollhäuser-ter Horst, J. Artificial neural crest formation in amphibia. Anat. Embryol. 157, 113–120 (1979).
Moury, J. D. & Jacobson, A. G. Neural fold formation at newly created boundaries between neural plate and epidermis in the axolotl. Dev. Biol. 133, 44–57 (1989).
Woo, K. & Fraser, S. E. Specification of the hindbrain fate in the zebrafish. Dev. Biol. 197, 283–296 (1998).
Moury, J. D. & Jacobson, A. G. The origins of neural crest cells in the axolotl. Dev. Biol. 141, 243–253 (1990).
Mancilla, A. & Mayor, R. Neural crest formation in Xenopus laevis: mechanisms of Xslug induction. Dev. Biol. 177, 580–589 (1996).
Liem, K. F., Tremml, G., Roelink, H. & Jessell, T. M. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 82, 969–979 (1995).
Liem, K. F., Tremml, G. & Jessell, T. M. A role for the roof plate and its resident TGFβ-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91, 127–138 (1997).
Selleck, M. A. J., García-Castro, M. I., Artinger, K. B. & Bronner-Fraser, M. Effects of Shh and Noggin on neural crest formation demonstrate that BMP is required in the neural tube but not ectoderm. Development 125, 4919–4930 (1998).
García-Castro, M. I., Marcelle, C. & Bronner-Fraser, M. Wnt in the ectoderm functions as a neural crest inducer. Science (in the press).
Sela-Donenfeld, D. & Kalcheim, C. Regulation of the onset of neural crest migration by coordinated activity of BMP4 and Noggin in the dorsal neural tube. Development 126, 4749–4762 (1999).
Coffman, C. R., Skoglund, P., Harris, W. A. & Kintner, C. R. Expression of an extracellular deletion of Xotch diverts cell fate in Xenopus embryos. Cell 73, 659–671 (1993).
Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 (1999).
Cornell, R. A. & Eisen, J. S. Delta signaling mediates segregation of neural crest and spinal sensory neurons from zebrafish lateral neural plate. Development 127, 2873–2882 (2000).
Endo, Y., Osumi, N. & Wakamatsu, Y. Bimodal functions of Notch-mediated signaling are involved in neural crest formation during avian ectoderm development. Development 129, 863–873 (2002).
Barembaum, M., Moreno, T. A., LaBonne, C., Sechrist, J. & Bronner-Fraser, M. Noelin-1 is a secreted glycoprotein involved in generation of the neural crest. Nature Cell Biol. 2, 219–225 (2000).
Artinger, K. B., Chitnis, A. B., Mercola, M. & Driever, W. Zebrafish narrowminded suggests a genetic link between formation of neural crest and primary sensory neurons. Development 126, 3969–3979 (1999).
LaBonne, C. & Bronner-Fraser, M. Snail-related transcriptional repressors are required in Xenopus for both the induction of the neural crest and its subsequent migration. Dev. Biol. 221, 195–205 (2000).
Epstein, D. J., Vekemans, M. & Gros, P. splotch (Sp2H), a mutation affecting development of the mouse neural tube, shows a deletion within the Paired homeodomain of Pax-3. Cell 67, 767–774 (1991).
Mansouri, A., Stoykova, A., Torres, M. & Gruss, P. Dysgenesis of cephalic neural crest derivatives in Pax7−/− mutant mice. Development 122, 831–838 (1996).
Dottori, M., Gross, M. K., Labosky, P. & Goulding, M. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development 128, 4127–4138 (2001).
Kos, R., Reedy, M. V., Johnson, R. L. & Erickson, C. A. The winged-helix transcription factor Foxd3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development 128, 1467–1479 (2001).
Sasai, N., Mizuseki, K. & Sasai, Y. Requirement of FoxD3 -class signaling for neural crest determination in Xenopus. Development 128, 2525–2536 (2001).
Nakata, K., Nagai, T., Aruga, J. & Mikoshiba, K. Xenopus Zic3, a primary regulator both in neural and neural crest development. Proc. Natl Acad. Sci. USA 94, 11980–11985 (1997).
Nakata, K., Nagai, T., Aruga, J. & Mikoshiba, K. Xenopus Zic family and its role in neural and neural crest development. Mech. Dev. 75, 43–51 (1998).
Kuo, J. S. et al. Opl: a zinc finger protein that regulates neural determination and patterning in Xenopus. Development 125, 2867–2882 (1998).
Brewster, R., Lee, J. & Ruiz í Altaba, A. Gli/Zic factors pattern the neural plate by defining domains of cell differentiation. Nature 393, 579–583 (1998).
Nakata, K., Koyabu, Y., Aruga, J. & Mikoshiba, K. A novel member of the Xenopus Zic family, Zic5, mediates neural crest development. Mech. Dev. 99, 83–91 (2000).
Dutton, K. A. et al. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development 128, 4113–4125 (2001).
Spokony, R. F., Aoki, Y., Saint-Germain, N., Magner-Fink, E. & Saint-Jeannet, J.-P. The transcription factor Sox9 is required for cranial neural crest development in Xenopus. Development 129, 421–432 (2002).
Maeda, R. et al. Xmeis1, a protooncogene involved in specifying neural crest cell fate in Xenopus embryos. Oncogene 20, 1329–1342 (2001).
Reeves, F. C., Burdge, G. C., Fredericks, W. J., Rauscher, F. J. & Lillycrop, K. A. Induction of antisense Pax-3 expression leads to the rapid morphological differentiation of neuronal cells and an altered response to the mitogenic growth factor bFGF. J. Cell Sci. 112, 253–261 (1999).
Vallin, J. et al. Cloning and characterization of three Xenopus Slug promoters reveal direct regulation by Lef/β-catenin signaling. J. Biol. Chem. 276, 30353–30358 (2001).
del Barrio, M. G. & Nieto, M. A. Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development 129, 1583–1593 (2002).
Jiang, R., Lan, Y., Norton, C., Sundberg, J. P. & Gridley, T. The Slug gene is not essential for mesoderm or neural crest development in mice. Dev. Biol. 198, 277–285 (1998).
Sefton, M., Sanchez, S. & Nieto, M. A. Conserved and divergent roles for members of the Snail family of transcription factors in the chick and mouse embryo. Development 125, 3111–3121 (1998).
Baker, C. V. H. & Bronner-Fraser, M. The origins of the neural crest. II. An evolutionary perspective. Mech. Dev. 69, 13–29 (1997).
Maisey, J. G. Heads and tails: a chordate phylogeny. Cladistics 2, 201–256 (1986).
Peterson, K. J. A phylogenetic test of the calcichordate scenario. Lethaia 28, 25–38 (1995).
Kuratani, S., Nobusada, Y., Horigome, N. & Shigetani, Y. Embryology of the lamprey and evolution of the vertebrate jaw: insights from molecular and developmental perspectives. Phil. Trans. R. Soc. Lond. B356, 1615–1632 (2001).
Holland, L. Z. & Holland, N. D. Evolution of neural crest and placodes: amphioxus as a model for the ancestral vertebrate? J. Anat. 199, 85–98 (2001).
Neidert, A. H., Panopoulou, G. & Langeland, J. A. Amphioxus goosecoid and the evolution of the head organizer and prechordal plate. Evol. Dev. 2, 303–310 (2000).
Acknowledgements
The authors thank T. Moreno for help with the figures, and M. García-Castro and M. Albrecht for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- NEURAL TUBE
-
A cylindrical structure that runs through the midline of the embryo; it expands in the head to form the brain and in the trunk to form the spinal cord.
- NEURAL FOLDS
-
Tips of invaginating ectoderm that will close to form the dorsal portion of the neural tube.
- PLACODES
-
Thickenings in the vertebrate cranial ectoderm that invaginate and form parts of cranial sensory ganglia and paired sensory organs.
- AUTONOMIC NEURONS
-
Nerve cells of the peripheral nervous system that innervate the viscera, smooth muscles and exocrine glands.
- PHARYNGEAL DENTICLES
-
Dense structures on the surface of pharyngeal arches of early vertebrates that are thought to be pressure sensitive.
- GASTRULATION
-
Morphogenetic movement that transforms a single-layered embryo into an embryo with three germ layers.
- HAMBURGER–HAMILTON STAGES
-
Stages that describe the age of chick embryos; stage 2 refers to the time before gastrulation.
- EPIBLAST
-
A term for the embryonic layer in chicks, mice and humans from which the embryo proper arises during gastrulation.
- MORPHOLINO ANTISENSE OLIGONUCLEOTIDES
-
Modified antisense oligonucleotides that are designed to block translation by pairing with the translation start site in the 5′ untranslated region.
- PARAXIAL MESODERM
-
Mesoderm that is adjacent to the neural tube and that is destined to form somites.
- SOMITES
-
Mesodermal balls of cells adjacent to the neural tube that will differentiate into the muscle, vertebrae and dermis.
- ROHON–BEARD SENSORY NEURONS
-
Early-differentiating neurons in the dorsal neural tube of fish and amphibians.
Rights and permissions
About this article
Cite this article
Knecht, A., Bronner-Fraser, M. Induction of the neural crest: a multigene process. Nat Rev Genet 3, 453–461 (2002). https://doi.org/10.1038/nrg819
Issue Date:
DOI: https://doi.org/10.1038/nrg819
This article is cited by
-
Mesenchymal properties of iPSC-derived neural progenitors that generate undesired grafts after transplantation
Communications Biology (2023)
-
From neural crest cells to melanocytes: cellular plasticity during development and beyond
Cellular and Molecular Life Sciences (2019)
-
Tissue biology perspective on macrophages
Nature Immunology (2016)
-
Transferrin receptor facilitates TGF-β and BMP signaling activation to control craniofacial morphogenesis
Cell Death & Disease (2016)
-
Live Image Profiling of Neural Crest Lineages in Zebrafish Transgenic Lines
Molecules and Cells (2013)