Key Points
-
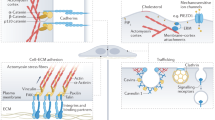
Spatial, temporal and mechanical cues control gene expression and protein activity that regulate force production and mechanical tissue responses in embryos. It is not well understood how mechanical cues determine cell fate or alter gene expression.
-
Various experiments have aimed to understand the role of mechanically activated cell signalling during morphogenesis. Molecular and genetic manipulations have been used, along with analyses of the spatial and temporal mechanisms that transmit and coordinate forces in the tissue. In vivo and in vitro studies have identified the roles of biomechanical cues in guiding cell fate and behaviour.
-
Recent experiments have shown that cellular mechanosensing is broken down into mechanical signals from the microenvironment of the cell and from the plasma membrane and cell cortex. These signals might alter the activity of transcription factors through cell signalling.
-
Mechanical signals have been shown to have a role in complex and poorly understood feedback loops that underlie the robust programmes of development.
Abstract
Force production and the propagation of stress and strain within embryos and organisms are crucial physical processes that direct morphogenesis. In addition, there is mounting evidence that biomechanical cues created by these processes guide cell behaviours and cell fates. In this Review we discuss key roles for biomechanics during development to directly shape tissues, to provide positional information for cell fate decisions and to enable robust programmes of development. Several recently identified molecular mechanisms suggest how cells and tissues might coordinate their responses to biomechanical cues. Finally, we outline long-term challenges in integrating biomechanics with genetic analysis of developing embryos.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
His, W. On the principles of animal morphology. Proc. R. Soc. Edinburgh 15, 287–298 (1888).
Rhumbler, L. Zur mechanik des gastrulationsvorganges insbesondere der invagination. Archiv für Entwicklungsmechanik der Organismen 14, 401–476 (1902) (in German).
Morgan, T. H. Experimental Embryology (Columbia Univ. Press, 1927).
Lewis, W. H. Mechanics of Invagination. Anatom. Record 97, 139–156 (1947).
Howard, J. Mechanics of Motor Proteins and the Cytoskeleton (Sinauer Associates, 2001).
Selman, G. G. Studies on the forces producing neural closure in amphibia. Proc. R. Phys. Soc. Edinburgh 24, 24–27 (1955).
Selman, G. G. The forces producing neural closure in amphibia. J. Embryol. Exp. Morphol. 6, 448–465 (1958).
Smith, J. L. & Schoenwolf, G. C. Further evidence of extrinsic forces in bending of the neural plate. J. Comp. Neurol. 307, 225–236 (1991).
Alvarez, I. S. & Schoenwolf, G. C. Expansion of surface epithelium provides the major extrinsic force for bending of the neural plate. J. Exp. Zool. 261, 340–348 (1992).
Smith, J. L., Schoenwolf, G. C. & Quan, J. Quantitative analyses of neuroepithelial cell shapes during bending of the mouse neural plate. J. Comp. Neurol. 342, 144–151 (1994).
Smith, J. L. & Schoenwolf, G. C. Neurulation: coming to closure. Trends Neurosci. 20, 510–517 (1997).
Ettensohn, C. A. Mechanisms of epithelial invagination. Quarterly Rev. Biol. 60, 289–307 (1985).
Gustafson, T. & Wolpert, L. The cellular basis of morphogenesis and sea urchin development. Int. Rev. Cytol. 15, 139–214 (1963).
Moore, A. R. & Burt, A. S. On the locus and nature of the forces causing gastrulation in the embryos of Dendraster excentricus. J. Exp. Zool. 82, 159–171 (1939).
Hutson, M. S. et al. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science 300, 145–149 (2003). This article described the mechanical steps required for D. melanogaster dorsal closure. The authors used laser ablation to describe the direction and relative magnitude of stress and test contribution of forces from different tissues.
Peralta, X. G. et al. Upregulation of forces and morphogenic asymmetries in dorsal closure during Drosophila development. Biophys. J. 92, 2583–2596 (2007).
Toyama, Y., Peralta, X. G., Wells, A. R., Kiehart, D. P. & Edwards, G. S. Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science 321, 1683–1686 (2008).
Kiehart, D. P., Galbraith, C. G., Edwards, K. A., Rickoll, W. L. & Montague, R. A. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell Biol. 149, 471–490 (2000). These authors examined the forces responsible for dorsal closure in D. melanogaster using three techniques: time-lapse confocal microscopy of actomyosin dynamics, laser cutting and repeated ablation of cells. These techniques were used to test the relative contribution of distinct morphogenetic 'motors' to dorsal closure.
Solon, J., Kaya-Copur, A., Colombelli, J. & Brunner, D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 137, 1331–1342 (2009).
Gorfinkiel, N., Blanchard, G. B., Adams, R. J. & Martinez Arias, A. Mechanical control of global cell behaviour during dorsal closure in Drosophila. Development 136, 1889–1898 (2009).
David, D. J., Tishkina, A. & Harris, T. J. The PAR complex regulates pulsed actomyosin contractions during amnioserosa apical constriction in Drosophila. Development 137, 1645–1655 (2010).
Young, P. E., Richman, A. M., Ketchum, A. S. & Kiehart, D. P. Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev. 7, 29–41 (1993).
Edwards, K. A., Demsky, M., Montague, R. A., Weymouth, N. & Kiehart, D. P. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev. Biol. 191, 103–117 (1997).
Jacinto, A. et al. Dynamic analysis of actin cable function during Drosophila dorsal closure. Curr. Biol. 12, 1245–1250 (2002).
Millard, T. H. & Martin, P. Dynamic analysis of filopodial interactions during the zippering phase of Drosophila dorsal closure. Development 135, 621–626 (2008).
Davidson, L. A., Koehl, M. A., Keller, R. & Oster, G. F. How do sea urchins invaginate? Using biomechanics to distinguish between mechanisms of primary invagination. Development 121, 2005–2018 (1995). This study used finite element modelling to test hypothesized mechanisms for sea urchin primary invagination and applied the model results to develop experiments that would ascertain the in vivo mechanism.
Davidson, L. A., Oster, G. F., Keller, R. E. & Koehl, M. A. Measurements of mechanical properties of the blastula wall reveal which hypothesized mechanisms of primary invagination are physically plausible in the sea urchin Strongylocentrotus purpuratus. Dev. Biol. 209, 221–238 (1999).
Sherrard, K., Robin, F., Lemaire, P. & Munro, E. Sequential activation of apical and basolateral contractility drives ascidian endoderm invagination. Curr. Biol. 20, 1499–1510 (2010).
Koehl, M. A. R. Biomechanical approaches to morphogenesis. Seminars Dev. Biol. 1, 367–378 (1990).
Trinkaus, J. P. Cells Into Organs: The Forces That Shape The Embryo (Prentice-Hall, 1984).
Harris, A. K., Wild, P. & Stopak, D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science 208, 177–179 (1980).
Dembo, M. & Wang, Y. L. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 76, 2307–2316 (1999).
Wang, H. B., Dembo, M., Hanks, S. K. & Wang, Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl Acad. Sci. USA 98, 11295–11300 (2001). This study examined the role of focal adhesion kinase in mechanosensing. The researchers applied forces by pulling or pushing the substrate near the cell with a microneedle.
Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. M. & Ingber, D. E. Geometric control of cell life and death. Science 276, 1425–1428 (1997).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). Here, the authors tuned the elasticity of in vitro gels to physiological elasticity and observed the corresponding differentiation of human mesenchymal stem cells.
Trappmann, B. et al. Extracellular-matrix tethering regulates stem-cell fate. Nature Mater. 11, 642–649 (2012).
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K. & Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 (2004).
Brodland, G. W. et al. Furrowing surface contraction wave coincident with primary neural induction in amphibian embryos. J. Morphol. 219, 131–142 (1994).
Beloussov, L. V., Lakirev, A. V., Naumidi, I. I. & Novoselov, V. V. Effects of relaxation of mechanical tensions upon the early morphogenesis of Xenopus laevis embryos. Int. J. Dev. Biol. 34, 409–419 (1990).
Beloussov, L. V., Luchinskaya, N. N., Ermakov, A. S. & Glagoleva, N. S. Gastrulation in amphibian embryos, regarded as a succession of biomechanical feedback events. Int. J. Dev. Biol. 50, 113–122 (2006).
Desprat, N., Supatto, W., Pouille, P. A., Beaurepaire, E. & Farge, E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell 15, 470–477 (2008). These authors used laser ablation and magnetic tweezers to deform D. melanogaster embryos and draw conclusions about mechanically induced factors.
Farge, E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr. Biol. 13, 1365–1377 (2003). In this study, compression was applied to deform D. melanogaster embryos, and the authors observed the transcription patterns of multiple genes after compression.
Kumar, A. & Shivashankar, G. Mechanical force alters morphogenetic movements and segmental gene expression patterns during Drosophila embryogenesis. PLoS ONE 7, e33089 (2012).
Lecuit, T., Lenne, P. F. & Munro, E. Force generation, transmission, and integration during cell and tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 27, 157–184 (2011).
Keller, R., Davidson, L. A. & Shook, D. R. How we are shaped: the biomechanics of gastrulation. Differentiation 71, 171–205 (2003).
Sampath, K. et al. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature 395, 185–189 (1998).
Pouille, P. A., Ahmadi, P., Brunet, A. C. & Farge, E. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci. Signal 2, ra16 (2009).
Martin, A. C., Kaschube, M. & Wieschaus, E. F. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495–499 (2009). This study elucidated that apical constriction of ventral furrow cells in Drosophila melanogaster is caused by pulsed actomyosin contractions.
Mason, F. M., Tworoger, M. & Martin, A. C. Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nature Cell Biol. 15, 926–936 (2013).
Somogyi, K. & Rorth, P. Evidence for tension-based regulation of Drosophila MAL and SRF during invasive cell migration. Dev. Cell 7, 85–93 (2004).
Garcia, A. J., Vega, M. D. & Boettiger, D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol. Biol. Cell 10, 785–798 (1999).
Krammer, A., Lu, H., Isralewitz, B., Schulten, K. & Vogel, V. Forced unfolding of the fibronectin type III module reveals a tensile molecular recognition switch. Proc. Natl Acad. Sci. USA 96, 1351–1356 (1999).
Klotzsch, E. et al. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc. Natl Acad. Sci. USA 106, 18267–18272 (2009).
Baneyx, G., Baugh, L. & Vogel, V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc. Natl Acad. Sci. USA 99, 5139–5143 (2002).
Marsden, M. & DeSimone, D. W. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development 128, 3635–3647 (2001).
Davidson, L. A., Marsden, M., Keller, R. & Desimone, D. W. Integrin α5β1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr. Biol. 16, 833–844 (2006).
Trinh, L. A. & Stainier, D. Y. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev. Cell 6, 371–382 (2004).
Chiu, C. H., Chou, C. W., Takada, S. & Liu, Y. W. Development and fibronectin signaling requirements of the zebrafish interrenal vessel. PLoS ONE 7, e43040 (2012).
Pulina, M. V. et al. Essential roles of fibronectin in the development of the left-right embryonic body plan. Dev. Biol. 354, 208–220 (2011).
Weber, G. F., Bjerke, M. A. & Desimone, D. W. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev. Cell 22, 104–115 (2012).
Raich, W. B., Agbunag, C. & Hardin, J. Rapid epithelial-sheet sealing in the Caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr. Biol. 9, 1139–1146 (1999).
Yonemura, S. Wada, Y., Watanabe, T., Nagafuchi, A. & Shibata, M. α-Catenin as a tension transducer that induces adherens junction development. Nature Cell Biol. 12, 533–542 (2010).
Kong, F. et al. Cyclic mechanical reinforcement of integrin-ligand interactions. Mol. Cell 49, 1060–1068 (2013).
Xu, W., Baribault, H. & Adamson, E. D. Vinculin knockout results in heart and brain defects during embryonic development. Development 125, 327–337 (1998).
le Duc, Q. et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J. Cell Biol. 189, 1107–1115 (2010).
Mierke, C. T. et al. Mechano-coupling and regulation of contractility by the vinculin tail domain. Biophys. J. 94, 661–670 (2008).
del Rio, A. et al. Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641 (2009).
Ho, T. C., Horn, N. A., Huynh, T., Kelava, L. & Lansman, J. B. Evidence TRPV4 contributes to mechanosensitive ion channels in mouse skeletal muscle fibers. Channels (Austin) 6, 246–254 (2012).
Thodeti, C. K. et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res. 104, 1123–1130 (2009).
Kottgen, M. et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J. Cell Biol. 182, 437–447 (2008).
Cosens, D. J. & Manning, A. Abnormal electroretinogram from a Drosophila mutant. Nature 224, 285–287 (1969).
Liedtke, W., Tobin, D. M., Bargmann, C. I. & Friedman, J. M. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 100, 14531–14536 (2003).
Coste, B. et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 (2012).
Kim, S. E., Coste, B., Chadha, A., Cook, B. & Patapoutian, A. The role of Drosophila Piezo in mechanical nociception. Nature 483, 209–212 (2012).
Eisenhoffer, G. T. et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484, 546–549 (2012).
Coste, B. et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010).
Houk, A. R. et al. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell 148, 175–188 (2012).
Itoh, T. et al. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev. Cell 9, 791–804 (2005).
Suetsugu, S. The proposed functions of membrane curvatures mediated by the BAR domain superfamily proteins. J. Biochem. 148, 1–12 (2010).
Saarikangas, J. et al. Molecular mechanisms of membrane deformation by I-BAR domain proteins. Curr. Biol. 19, 95–107 (2009).
Schuler, S. et al. Ciliated sensory hair cell formation and function require the F-BAR protein syndapin I and the WH2 domain-based actin nucleator Cobl. J. Cell Sci. 126(Pt 1),196–208 (2013).
Parmar, K. M. et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Invest. 116, 49–58 (2006).
Lee, J. S. et al. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev. Cell 11, 845–857 (2006).
Groenendijk, B. C., Hierck, B. P., Gittenberger-De Groot, A. C. & Poelmann, R. E. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev. Dyn. 230, 57–68 (2004).
Wang, N. et al. Shear stress regulation of Kruppel-like factor 2 expression is flow pattern-specific. Biochem. Biophys. Res. Commun. 341, 1244–1251 (2006).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Gee, S. T., Milgram, S. L., Kramer, K. L., Conlon, F. L. & Moody, S. A. Yes-associated protein 65 (YAP) expands neural progenitors and regulates Pax3 expression in the neural plate border zone. PLoS ONE 6, e20309 (2011).
Morin-Kensicki, E. M. et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol. Cell. Biol. 26, 77–87 (2006).
Sandmann, T. et al. A core transcriptional network for early mesoderm development in Drosophila melanogaster. Genes Dev. 21, 436–449 (2007).
Pearson, R., Fleetwood, J., Eaton, S., Crossley, M. & Bao, S. Krüppel-like transcription factors: a functional family. Int. J. Biochem. Cell Biol. 40, 1996–2001 (2008).
Fu, J. et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nature Methods 7, 733–736 (2010).
Manu et al. Canalization of gene expression in the Drosophila blastoderm by gap gene cross regulation. PLoS Biol. 7, e1000049 (2009).
Tyszka, J. M., Ewald, A. J., Wallingford, J. B. & Fraser, S. E. New tools for visualization and analysis of morphogenesis in spherical embryos. Dev. Dyn. 234, 974–983 (2005).
Jiang, X., Bruzewicz, D. A., Wong, A. P., Piel, M. & Whitesides, G. M. Directing cell migration with asymmetric micropatterns. Proc. Natl Acad. Sci. USA 102, 975–978 (2005).
von Dassow, M. & Davidson, L. A. Physics and the canalization of morphogenesis: a grand challenge in organismal biology. Phys. Biol. 8, 045002 (2011). These authors used simple in vivo mechanical experiments and theoretical models to describe the viscoelastic properties of a vertebrate embryo and to rule out the presence of mechanical feedback in X. laevis embryonic tissues.
Farge, E. Mechanotransduction in development. Curr. Top. Dev. Biol. 95, 243–265 (2011).
Shook, D. R. & Keller, R. Morphogenic machines evolve more rapidly than the signals that pattern them: lessons from amphibians. J. Exp. Zool. B Mol. Dev. Evol. 310, 111–135 (2008).
Fernandez-Gonzalez, R., Simoes Sde, M., Roper, J. C., Eaton, S. & Zallen, J. A. Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 17, 736–743 (2009).
Sweeney, H. L. & Houdusse, A. Structural and functional insights into the Myosin motor mechanism. Annu. Rev. Biophys. 39, 539–557 (2010).
von Dassow, M., Strother, J. A. & Davidson, L. A. Surprisingly simple mechanical behavior from a complex embryonic tissue. PLoS ONE 5, e15359 (2010).
Ma, X., Lynch, H. E., Scully, P. C. & Hutson, M. S. Probing embryonic tissue mechanics with laser hole drilling. Phys. Biol. 6, 036004 (2009).
Rodriguez-Diaz, A. et al. Actomyosin purse strings: renewable resources that make morphogenesis robust and resilient. HFSP J. 2, 220–237 (2008).
Benazeraf, B. et al. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 466, 248–252 (2010).
Varner, V. D., Voronov, D. A. & Taber, L. A. Mechanics of head fold formation: investigating tissue-level forces during early development. Development 137, 3801–3811 (2010).
Beloussov, L. V., Lakirev, A. V. & Naumidi, I. I. The role of external tensions in differentiation of Xenopus laevis embryonic tissues. Cell Differ. Dev. 25, 165–176 (1988).
Beloussov, L. V., Dorfman, J. G. & Cherdantzev, V. G. Mechanical stresses and morphological patterns in amphibian embryos. J. Embryol. Exp. Morphol. 34, 559–574 (1975).
Sokolow, A., Toyama, Y., Kiehart, D. P. & Edwards, G. S. Cell ingression and apical shape oscillations during dorsal closure in Drosophila. Biophys. J. 102, 969–979 (2012).
Peralta, X. G., Toyama, Y., Kiehart, D. P. & Edwards, G. S. Emergent properties during dorsal closure in Drosophila morphogenesis. Phys. Biol. 5, 15004 (2008).
Blanchard, G. B. et al. Tissue tectonics: morphogenetic strain rates, cell shape change and intercalation. Nature Methods 6, 458–464 (2009).
Gibson, M. C., Patel, A. B., Nagpal, R. & Perrimon, N. The emergence of geometric order in proliferating metazoan epithelia. Nature 442, 1038–1041 (2006).
Zhou, J., Kim, H. Y. & Davidson, L. A. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development 136, 677–688 (2009).
Zhou, J., Kim, H. Y., Wang, J. H. & Davidson, L. A. Macroscopic stiffening of embryonic tissues via microtubules, RhoGEF and the assembly of contractile bundles of actomyosin. Development 137, 2785–2794 (2010).
Wiebe, C. & Brodland, G. W. Tensile properties of embryonic epithelia measured using a novel instrument. J. Biomech. 38, 2087–2094 (2005).
Zamir, E. A. & Taber, L. A. Material properties and residual stress in the stage 12 chick heart during cardiac looping. J. Biomech. Eng. 126, 823–830 (2004).
Foty, R. A., Pfleger, C. M., Forgacs, G. & Steinberg, M. S. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development 122, 1611–1620 (1996).
Forgacs, G., Foty, R. A., Shafrir, Y. & Steinberg, M. S. Viscoelastic properties of living embryonic tissues: a quantitative study. Biophys. J. 74, 2227–2234 (1998).
Joshi, S. D., von Dassow, M. & Davidson, L. A. Experimental control of excitable embryonic tissues: three stimuli induce rapid epithelial contraction. Exp. Cell Res. 316, 103–114 (2010).
Voronov, D. A. & Taber, L. A. Cardiac looping in experimental conditions: effects of extraembryonic forces. Dev. Dyn. 224, 413–421 (2002).
Zamir, E. A., Srinivasan, V., Perucchio, R. & Taber, L. A. Mechanical asymmetry in the embryonic chick heart during looping. Ann. Biomed. Eng. 31, 1327–1336 (2003).
Odell, G. M., Oster, G., Alberch, P. & Burnside, B. The mechanical basis of morphogenesis. I. Epithelial folding and invagination. Dev. Biol. 85, 446–462 (1981).
Brodland, G. W. et al. Video force microscopy reveals the mechanics of ventral furrow invagination in Drosophila. Proc. Natl Acad. Sci. USA 107, 22111–22116 (2010).
Savin, T. et al. On the growth and form of the gut. Nature 476, 57–62 (2011).
Brodland, G. W. & Veldhuis, J. H. Assessing the mechanical energy costs of various tissue reshaping mechanisms. Biomech. Model. Mechanobiol. 11, 1137–1147 (2012).
Acknowledgements
The authors would like to thank members of the Davidson laboratory for their support and ongoing discussions on the role of mechanics in development. This work was supported by the National Science Foundation (IOS-0845775, LAD and CJM) and the US National Institutes of Health (HD044750 and ES019259, L.A.D.; 2T32EB003392, C.J.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Gastrulation
-
Stage of embryonic development when large cell rearrangements occur and the three germ layers — endoderm, mesoderm and ectoderm — of the embryo are established.
- Vegetal plate
-
The columnar epithelium at the vegetal pole of an echinoderm embryo. The thickened vegetal plate forms a pocket and tube by invagination during gastrulation to form the archenteron, or primitive gut, of the embryo.
- Blastula
-
The early stage of a developing embryo after rapid cell divisions have created a sphere, sometimes hollow, of many cells.
- Ectoderm
-
The outermost germ layer of the embryo. Cells from this layer differentiate into skin and neural tissues.
- Dorsal closure
-
A step in Drosophila melanogaster development in which the epidermis closes over the exposed amnioserosa.
- Amnioserosa
-
A layer of epithelial cells that covers dorsal regions of the early Drosophila melanogaster embryo.
- Epidermis
-
The outermost epithelial layer of an embryo.
- Anterior
-
The axis of the embryo defined by the tissues fated to form the head.
- Posterior
-
The axis of the embryo defined by the tissues fated to form the tail.
- Invagination
-
The 'in-folding' of an epithelium.
- Apical
-
Surfaces that face the 'outside' or lumen.
- Basal
-
Surfaces that face in the opposite direction to apical surfaces; that is, away from the 'outside'.
- Traction force microscopy
-
A method used to determine the force that a cell or tissue exerts on a substrate to which it is adhered.
- Focal adhesion complex
-
A dynamic protein complex that connects the cytoskeleton of the cell to the extracellular matrix.
- Desmosomes
-
A spot-like junctional complex for cell–cell adhesion that is distinct from adherens junctions (which connect epithelial cells to neighbours at their apical ends).
- Cell cortex
-
A layer of cytoplasm just inside the plasma membrane. Cytoskeletal proteins in the cell cortex maintain the shape of the cell.
- Stomodeal primordium
-
A tissue fated to give rise to the Drosophila melanogaster foregut.
Rights and permissions
About this article
Cite this article
Miller, C., Davidson, L. The interplay between cell signalling and mechanics in developmental processes. Nat Rev Genet 14, 733–744 (2013). https://doi.org/10.1038/nrg3513
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3513
This article is cited by
-
Rapid biomechanical imaging at low irradiation level via dual line-scanning Brillouin microscopy
Nature Methods (2023)
-
Biophysics in tumor growth and progression: from single mechano-sensitive molecules to mechanomedicine
Oncogene (2023)
-
Time-lapse mechanical imaging of neural tube closure in live embryo using Brillouin microscopy
Scientific Reports (2023)
-
Mechanics of the cellular microenvironment as probed by cells in vivo during zebrafish presomitic mesoderm differentiation
Nature Materials (2023)
-
Extracting multiple surfaces from 3D microscopy images in complex biological tissues with the Zellige software tool
BMC Biology (2022)