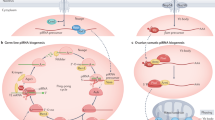
Key Points
-
PIWI-interacting RNAs (piRNAs) are made from dedicated precursor transcripts that are transcribed from specialized loci.
-
piRNA biogenesis from their precursors involves at least three different steps: first, the 5′ end is created by an endonuclease; second, the piRNA intermediate is loaded into a PIWI protein; and third, the 3′ end is trimmed and modified.
-
Target selection by PIWI–piRNA complexes may depend on perfect or near perfect base-pairing interactions — for example, as occurs in Drosophila melanogaster and mice — or on interactions involving substantial mismatches, as occurs in Caenorhabditis elegans.
-
PIWI proteins can trigger changes to the chromatin of their target loci. The exact mechanisms are thus far still not clear but bear similarities to the RNAi-heterochromatin pathway of fission yeast.
-
The heterochromatin changes imposed by PIWI proteins can be inherited stably across generations. In some cases, maintenance of this silencing can occur in absence of further PIWI pathway activity.
-
Changes to the chromatin structure of PIWI targets may convert such targets into piRNA-producing loci. In such cases, transcripts from these targets are efficiently routed into piRNA biogenesis pathways.
Abstract
Small-RNA-guided gene regulation is a recurring theme in biology. Animal germ cells are characterized by an intriguing small-RNA-mediated gene-silencing mechanism known as the PIWI pathway. For a long time, both the biogenesis of PIWI-interacting RNAs (piRNAs) as well as their mode of gene silencing has remained elusive. A recent body of work is shedding more light on both aspects and implicates PIWI in the establishment of transgenerational epigenetic states. In fact, the epigenetic states imposed by PIWI on targets may actually drive piRNA production itself. These findings start to couple small RNA biogenesis with small-RNA-mediated epigenetics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cox, D. N. et al. A novel class of evolutionarily conserved genes defined by PIWI are essential for stem cell self-renewal. Genes Dev. 12, 3715–3727 (1998).
Deng, W. & Lin, H. Miwi, a murine homolog of Piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev. Cell 2, 819–830 (2002).
Ghildiyal, M. & Zamore, P. D. Small silencing RNAs: an expanding universe. Nature Rev. Genet. 10, 94–108 (2009).
Song, J. J. Crystal structure of argonaute and its implications for RISC slicer activity. Science 305, 1434–1437 (2004).
Tolia, N. H. & Joshua-Tor, L. Slicer and the Argonautes. Nature Chem. Biol. 3, 36–43 (2007).
Wang, Y. et al. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature 461, 754–761 (2009).
Schirle, N. T. & MacRae, I. J. The crystal structure of human Argonaute2. Science 336, 1037–1040 (2012).
Elkayam, E. et al. The structure of human argonaute-2 in complex with miR-20a. Cell 150, 100–110 (2012).
Wang, Y. et al. Structure of an Argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature 456, 921–926 (2008).
van Wolfswinkel, J. C. & Ketting, R. F. The role of small non-coding RNAs in genome stability and chromatin organization. J. Cell Sci. 123, 1825–1839 (2010).
Czech, B. & Hannon, G. J. Small RNA sorting: matchmaking for Argonautes. Nature Rev. Genet. 12, 19–31 (2010).
Siomi, H. & Siomi, M. C. On the road to reading the RNA-interference code. Nature 457, 396–404 (2009).
Jinek, M. & Doudna, J. A. A three-dimensional view of the molecular machinery of RNA interference. Nature 457, 405–412 (2009).
Rana, T. M. Illuminating the silence: understanding the structure and function of small RNAs. Nature Rev. Mol. Cell Biol. 8, 23–36 (2007).
Malone, C. D. & Hannon, G. J. Small RNAs as guardians of the genome. Cell 136, 656–668 (2009).
Kazazian, H. H. Mobile elements: drivers of genome evolution. Science 303, 1626–1632 (2004).
Rouget, C. et al. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature 467, 1128–1132 (2010).
Castel, S. E. & Martienssen, R. A. RNA interference in the nucleus: roles for small RNAs in transcription, epigenetics and beyond. Nature Rev. Genet. 14, 100–112 (2013).
Robine, N. et al. A broadly conserved pathway generates 3′UTR-directed primary piRNAs. Curr. Biol. 19, 2066–2076 (2009).
Seto, A. G., Kingston, R. E. & Lau, N. C. The coming of age for PIWI proteins. Mol. Cell 26, 603–609 (2007).
Rajasethupathy, P. et al. A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell 149, 693–707 (2012).
Houwing, S. et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in zebrafish. Cell 129, 69–82 (2007).
Vagin, V. V. A. Distinct small RNA pathway silences selfish genetic elements in the germline. Science 313, 320–324 (2006).
Brennecke, J. et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128, 1089–1103 (2007).
Li, X. Z. et al. An ancient transcription factor initiates the burst of piRNA production during early meiosis in mouse testes. Mol. Cell 50, 67–81 (2013). This paper describes the first transcription factor that initiates the transcription of pachytene piRNA precursors and genes involved in piRNA biogenesis, thereby creating a feed-forward loop to boost piRNA abundance.
Al-Muktar, K. A. K. & Webb, A. C. An ultrastructural study of primordial germ cells, oogonia and early oocytes in Xenopus laevis. J. Embryol. Exp. Morphol. 26, 195–217 (1971).
Ketting, R. F. The many faces of RNAi. Dev. Cell 20, 148–161 (2011).
Aravin, A. A., Sachidanandam, R., Girard, A., Fejes-Toth, K. & Hannon, G. J. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 316, 744–747 (2007).
Gunawardane, L. S. et al. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science 315, 1587–1590 (2007).
Li, C. et al. Collapse of germline piRNAs in the absence of Argonaute3 reveals somatic piRNAs in flies. Cell 137, 509–521 (2009).
Grentzinger, T. et al. piRNA-mediated transgenerational inheritance of an acquired trait. Genome Res. 22, 1877–1888 (2012).
Brennecke, J. et al. An epigenetic role for maternally inherited piRNAs in transposon silencing. Science 322, 1387–1392 (2008).
Klattenhoff, C. & Theurkauf, W. Biogenesis and germline functions of piRNAs. Development 135, 3–9 (2007).
Siomi, M. C., Sato, K., Pezic, D. & Aravin, A. A. PIWI-interacting small RNAs: the vanguard of genome defence. Nature Rev. Mol. Cell Biol. 12, 246–258 (2011).
Grivna, S. T., Pyhtila, B. & Lin, H. MIWI associates with translational machinery and PIWI-interacting RNAs (piRNAs) in regulating spermatogenesis. Proc. Natl Acad. Sci. USA 103, 13415–13420 (2006).
Molnar, A., Melnyk, C. & Baulcombe, D. C. Silencing signals in plants: a long journey for small RNAs. Genome Biol. 12, 215 (2011).
hler, M. B. U. & Gasser, S. M. Silent chromatin at the middle and ends: lessons from yeasts. EMBO J. 28, 2149–2161 (2009).
Grewal, S. I. RNAi-dependent formation of heterochromatin and its diverse functions. Curr. Opin. Genet. Dev. 20, 134–141 (2010).
Das, P. P. et al. Piwi and piRNAs act upstream of an endogenous siRNA pathway to suppress Tc3 transposon mobility in the Caenorhabditis elegans germline. Mol. Cell 31, 79–90 (2008).
Batista, P. J. et al. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol. Cell 31, 67–78 (2008).
Billi, A. C. et al. A conserved upstream motif orchestrates autonomous, germline-enriched expression of Caenorhabditis elegans piRNAs. PLoS Genet. 9, e1003392 (2013).
Ruby, J. G. et al. Large-scale sequencing reveals 21U-RNAs and additional microRNAs and endogenous siRNAs in C. elegans. Cell 127, 1193–1207 (2006).
Cecere, G., Zheng, G. X. Y., Mansisidor, A. R., Klymko, K. E. & Grishok, A. Promoters recognized by forkhead proteins exist for individual 21U-RNAs. Mol. Cell 47, 734–745 (2012).
Gu, W. et al. CapSeq and CIP-TAP identify Pol II start sites and reveal capped small RNAs as C. elegans piRNA precursors. Cell 151, 1488–1500 (2012).
Brannan, K. et al. mRNA decapping factors and the exonuclease Xrn2 function in widespread premature termination of RNA polymerase II transcription. Mol. Cell 46, 311–324 (2012).
Aravin, A. et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442, 203–207 (2006).
Aravin, A. A., Hannon, G. J. & Brennecke, J. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science 318, 761–764 (2007).
Lau, N. C. et al. Characterization of the piRNA complex from rat testes. Science 313, 363–367 (2006).
Girard, A., Sachidanandam, R., Hannon, G. J. & Carmell, M. A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442, 199–202 (2006).
Klattenhoff, C. et al. The Drosophila HP1 homolog rhino is required for transposon silencing and piRNA production by dual-strand clusters. Cell 138, 1137–1149 (2009).
Grewal, S. I. S. & Jia, S. Heterochromatin revisited. Nature Rev. Genet. 8, 35–46 (2007).
Keller, C. et al. HP1(Swi6) mediates the recognition and destruction of heterochromatic RNA transcripts. Mol. Cell 47, 215–227 (2012). This work shows the RNA-binding activity of SWI6 is required for silencing but leaves heterochromatin formation intact.
Zhang, F. et al. UAP56 couples piRNA clusters to the perinuclear transposon silencing machinery. Cell 151, 871–884 (2012). This paper describes the roles of nuclear UAP56 and perinuclear Vasa proteins in forming a piRNA-processing compartment that spans the nuclear envelope and is essential for piRNA production and transposon silencing.
Pane, A. Wehr, K. & Schüpbach, T. Zucchini and squash encode two putative nucleases required for rasiRNA production in the Drosophila germline. Dev. Cell 12, 851–862 (2007).
Huang, H. et al. piRNA-associated germline nuage formation and spermatogenesis require MitoPLD profusogenic mitochondrial-surface lipid signaling. Dev. Cell 20, 376–387 (2011).
Watanabe, T. et al. MITOPLD is a mitochondrial protein essential for nuage formation and piRNA biogenesis in the mouse germline. Dev. Cell 20, 364–375 (2011).
Saito, K. et al. Roles for the Yb body components Armitage and Yb in primary piRNA biogenesis in Drosophila. Genes Dev. 24, 2493–2498 (2010).
Choi, S.-Y. et al. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nature Cell Biol. 8, 1255–1262 (2006).
Voigt, F. et al. Crystal structure of the primary piRNA biogenesis factor Zucchini reveals similarity to the bacterial PLD endonuclease Nuc. RNA 18, 2128–2134 (2012).
Nishimasu, H. et al. Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature 491, 284–287 (2012).
Ipsaro, J. J., Haase, A. D., Knott, S. R., Joshua-Tor, L. & Hannon, G. J. The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature 491, 279–283 (2012). References 59, 60 and 61 show crystal structures of Zuc, showing that it has a nuclease domain and possesses single-stranded nuclease activity. These studies provide proof that Zuc is a nucleasethat probably acts to specify piRNA 5′ ends.
Mi, S. et al. Sorting of small RNAs into Arabidopsis Argonaute complexes is directed by the 5′ terminal nucleotide. Cell 133, 116–127 (2008).
Kawaoka, S., Izumi, N., Katsuma, S. & Tomari, Y. 3′ end formation of PIWI-interacting RNAs in vitro. Mol. Cell 43, 1015–1022 (2011).
Iwasaki, S. et al. Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. Mol. Cell 39, 292–299 (2010).
Johnston, M., Geoffroy, M.-C., Sobala, A., Hay, R. & Hutvagner, G. HSP90 protein stabilizes unloaded Argonaute complexes and microscopic P-bodies in human cells. Mol. Biol. Cell 21, 1462–1469 (2010).
Miyoshi, T., Takeuchi, A., Siomi, H. & Siomi, M. C. A direct role for Hsp90 in pre-RISC formation in Drosophila. Nature Struct. Mol. Biol. 17, 1024–1026 (2010).
Iki, T., Yoshikawa, M., Meshi, T. & Ishikawa, M. Cyclophilin 40 facilitates HSP90-mediated RISC assembly in plants. EMBO J. 31, 267–278 (2012).
Gangaraju, V. K. et al. Drosophila Piwi functions in Hsp90-mediated suppression of phenotypic variation. Nature Genet. 43, 153–158 (2010).
Olivieri, D., Senti, K.-A., Subramanian, S., Sachidanandam, R. & Brennecke, J. The cochaperone shutdown defines a group of biogenesis factors essential for all piRNA populations in Drosophila. Mol. Cell 47, 954–969 (2012).
Specchia, V. et al. Hsp90 prevents phenotypic variation by suppressing the mutagenic activity of transposons. Nature 463, 662–665 (2010).
Vagin, V. V. et al. Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev. 23, 1749–1762 (2009).
Xiol, J. et al. A role for Fkbp6 and the chaperone machinery in piRNA amplification and transposon silencing. Mol. Cell 47, 970–979 (2012).
Kamminga, L. M. et al. Hen1 is required for oocyte development and piRNA stability in zebrafish. EMBO J. 29, 3688–3700 (2010).
Saito, K. et al. Pimet, the Drosophila homolog of HEN1, mediates 2′-O-methylation of Piwi-interacting RNAs at their 3′ ends. Genes Dev. 21, 1603–1608 (2007).
Horwich, M. D. et al. The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Curr. Biol. 17, 1265–1272 (2007).
Yu, B. Methylation as a crucial step in plant microRNA biogenesis. Science 307, 932–935 (2005).
Kurth, H. M. & Mochizuki, K. 2′-O-methylation stabilizes Piwi-associated small RNAs and ensures DNA elimination in Tetrahymena. RNA 15, 675–685 (2009).
Ameres, S. L. et al. Target RNA-directed trimming and tailing of small silencing RNAs. Science 328, 1534–1539 (2010).
Ameres, S. L., Hung, J.-H., Xu, J., Weng, Z. & Zamore, P. D. Target RNA-directed tailing and trimming purifies the sorting of endo-siRNAs between the two Drosophila Argonaute proteins. RNA 17, 54–63 (2011).
Vourekas, A. et al. Mili and Miwi target RNA repertoire reveals piRNA biogenesis and function of Miwi in spermiogenesis. Nature Struct. Mol. Biol. 19, 773–781 (2012).
Bagijn, M. P. et al. Function, targets, and evolution of Caenorhabditis elegans piRNAs. Science 337, 574–578 (2012).
Lee, H.-C. et al. C. elegans piRNAs mediate the genome-wide surveillance of germline Transcripts. Cell 150, 78–87 (2012). References 81 and 82 show that the C. elegans -specific PIWI protein PRG-1 binds its targets by imperfectly base pairing and silences independently of its endonuclease activity. Instead, it recruits a downstream small RNA pathway that generates endogenous siRNAs (endo-siRNAs) to induce silencing.
Reuter, M. et al. Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing. Nature 480, 264–267 (2011).
Huang, X. A. et al. A major epigenetic programming mechanism guided by piRNAs. Dev. Cell 24, 502–516 (2013).
Cox, D. N., Chao, A. & Lin, H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 127, 503–514 (2000).
Aravin, A. A. et al. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol. Cell 31, 785–799 (2008).
Houwing, S., Berezikov, E. & Ketting, R. F. Zili is required for germ cell differentiation and meiosis in zebrafish. EMBO J. 27, 2702–2711 (2008).
Qi, H. et al. The Yb body a major site for Piwi-associated RNA biogenesis and a gateway for Piwi expression and transport to the nucleus in somatic cells. J. Biol. Chem. 286, 3789–3797 (2011).
Olivieri, D., Sykora, M. M., Sachidanandam, R., Mechtler, K. & Brennecke, J. An in vivo RNAi assay identifies major genetic and cellular requirements for primary piRNA biogenesis in Drosophila. EMBO J. 29, 3301–3317 (2010).
Kuramochi-Miyagawa, S. et al. MVH in piRNA processing and gene silencing of retrotransposons. Genes Dev. 24, 887–892 (2010).
Luteijn, M. J. et al. Extremely stable Piwi-induced gene silencing in Caenorhabditis elegans. EMBO J. 31, 3422–3430 (2012).
Ashe, A. et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell 150, 88–99 (2012).
Shirayama, M. et al. piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline. Cell 150, 65–77 (2012).
Buckley, B. A. et al. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature 489, 447–451 (2012). References 91, 92, 93 and 94 show a role for PRG-1 in inducing stably inherited silenced state by recruiting a nuclear RNAi machinery that triggers heterochromatin formation. Reference 94 demonstrates the nuclear activity of WAGO-9 in RNAi-induced transgenerational silencing and reveals a role in germline mortality.
Burton, N. O., Burkhart, K. B. & Kennedy, S. Nuclear RNAi maintains heritable gene silencing in Caenorhabditis elegans. Proc. Natl Acad. Sci. 108, 19683–19688 (2011).
Burkhart, K. B. et al. A pre-mRNA-associating factor links endogenous siRNAs to chromatin regulation. PLoS Genet. 7, e1002249 (2011).
Kuramochi-Miyagawa, S. et al. Mili, a mammalian member of Piwi family gene, is essential for spermatogenesis. Development 131, 839–849 (2004).
Morgan, H. D., Santos, F., Green, K., Dean, W. & Reik, W. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 14 (Suppl. 1), R47–R58 (2005).
Claycomb, J. M. et al. The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell 139, 123–134 (2009).
Wolfswinkel, J. C. V. et al. CDE-1 affects chromosome segregation through uridylation of CSR-1-bound siRNAs. Cell 139, 135–148 (2009).
Fang, W., Wang, X., Bracht, J. R., Nowacki, M. & Landweber, L. F. Piwi-interacting RNAs protect DNA against loss during oxytricha genome rearrangement. Cell 151, 1243–1255 (2012).
Yin, H. & Lin, H. An epigenetic activation role of Piwi and a Piwi-associated piRNA in Drosophila melanogaster. Nature 450, 304–308 (2007).
Brower-Toland, B. et al. Drosophila PIWI associates with chromatin and interacts directly with HP1a. Genes Dev. 21, 2300–2311 (2007).
Rozhkov, N. V., Hammell, M. & Hannon, G. J. Multiple roles for Piwi in silencing Drosophila transposons. Genes Dev. 27, 400–412 (2013).
Sienski, G., Donertas, D. & Brennecke, J. Transcriptional silencing of transposons by Piwi and maelstrom and its impact on chromatin state and gene expression. Cell 151, 964–980 (2012).
Le Thomas, A. et al. Piwi induces piRNA-guided transcriptional silencing and establishment of a repressive chromatin state. Genes Dev. 27, 390–399 (2013). References 104, 105 and 106, along with reference 82, show that D. melanogaster Piwi is required to establish heterochromatic H3K9me3 marks on transposons and their genomic surroundings. Reference 105 also shows that the high-mobility group (HMG) protein Mael is essential for transposon silencing but not for heterochromatin formation.
Darricarrère, N., Liu, N., Watanabe, T. & Lin, H. Function of Piwi, a nuclear Piwi/Argonaute protein, is independent of its slicer activity. Proc. Natl Acad. Sci. 110, 1297–1302 (2013).
Aravin, A. A. et al. Cytoplasmic compartmentalization of the fetal piRNA pathway in mice. PLoS Genet. 5, e1000764 (2009).
de Vanssay, A. et al. Paramutation in Drosophila linked to emergence of a piRNA-producing locus. Nature 490, 112–115 (2012). This work shows that piRNA targets can be converted into piRNA-producing loci, resulting in genetic behavior that closely mirrors that of paramutation.
Erhard, K. F. Jr & Hollick, J. B. Paramutation: a process for acquiring trans-generational regulatory states. Curr. Opin. Plant Biol. 14, 210–216 (2011).
Pilu, R. Paramutation: just a curiosity or fine tuning of gene expression in the next generation? Curr. Genom. 12, 298–306 (2011).
Muerdter, F. et al. A genome-wide RNAi screen draws a genetic framework for transposon control and primary piRNA biogenesis in Drosophila. Mol. Cell. http://dx.doi.org/10.1016/j.molcel.2013.04.006 (2013).
Czech, B., Preall, J. B., McGinn, J. & Hannon, G. J. A transcriptome-wide RNAi screen in the Drosophila ovary reveals factors of the germline piRNA pathway. Mol. Cell http://dx.doi.org/10.1016/j.molcel.2013.04.007 (2013).
Handler, D. et al. The genetic makeup of the Drosophila piRNA pathway. Mol. Cell http://dx.doi.org/10.1016/j.molcel.2013.04.031 (2013).
Acknowledgements
The authors thank members of their laboratory for helpful discussions. This work is supported by grants from the European Research Council (ERC; StG 202819), the Netherlands Organization for Scientific Research (NWO; ECHO 700.57.006 and Vici 724.011.001) and the Boehringer Ingelheim Stiftung. The authors apologize to those colleagues whose work could not be cited owing to space restrictions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- 21U RNAs
-
Short RNA molecules found in Caenorhabditis elegans that are characterized by a length of mostly 21 nt and a uracil at their 5′ end. 21U RNAs are bound by the Piwi proteins PRG-1 and PRG-2.
- Pachytene
-
The third stage of meiotic prophase. Homologous chromosomes are tightly held together by the synaptonemal complex, and homologous recombination ('crossing over') begins.
- RNA-induced transcriptional silencing complex
-
(RITS complex). An RNA interference (RNAi) effector complex required for heterochromatin assembly in fission yeast. It targets centromeric transcripts to induce both histone H3 at lysine 9 (H3K9) methylation and small interfering RNA amplification.
- 22G RNAs
-
Short RNA molecules in Caenorhabditis elegans made by RNA-dependent RNA polymerase (RdRP) activity on an RNA template. 22G RNAs are characterized by a triphosphate group at their 5′ end and a strong preference for a G at their 5′ end. They are bound by Argonaute proteins.
- RNA-dependent RNA polymerases
-
(RdRP). RNA polymerases that use single-stranded RNA as a template to synthesize double-stranded RNA.
- RNA-induced epigenetic silencing
-
(RNAe). A form of epigenetic silencing that can be induced by various forms of RNA interference (RNAi)-related pathways, resulting in trimethylation of histone H3 at lysine 9 (H3K9) residues at the silenced locus. The silencing is maintained through both mitosis and meiosis and can last for tens of generations.
- Paramutation
-
The interaction between two alleles through which the epigenetic state of one allele can be imposed on the other allele.
Rights and permissions
About this article
Cite this article
Luteijn, M., Ketting, R. PIWI-interacting RNAs: from generation to transgenerational epigenetics. Nat Rev Genet 14, 523–534 (2013). https://doi.org/10.1038/nrg3495
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3495
This article is cited by
-
Epigenetic modifications in the development of bronchopulmonary dysplasia: a review
Pediatric Research (2024)
-
Small RNAs, spermatogenesis, and male infertility: a decade of retrospect
Reproductive Biology and Endocrinology (2023)
-
PIWI-interacting RNA expression regulates pathogenesis in a Caenorhabditis elegans model of Lewy body disease
Nature Communications (2023)
-
Insights into the microRNA landscape of Rhodnius prolixus, a vector of Chagas disease
Scientific Reports (2023)
-
Molecular mechanisms of transgenerational epigenetic inheritance
Nature Reviews Genetics (2022)