Key Points
-
Genomic instability, including whole-chromosome aneuploidy, is a hallmark of cancer.
-
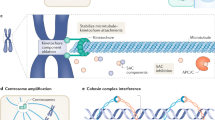
The disruption of multiple pathways, including defects in kinetochore–microtubule attachments and dynamics, centrosome number, spindle assembly checkpoint (SAC) and chromosome cohesion, can lead to aneuploidy.
-
Aneuploidy is generally detrimental in non-transformed cells and can result in imbalances at the level of the transcriptome and proteome.
-
A key question and area of research is how cells can adapt to tolerate aneuploidy.
-
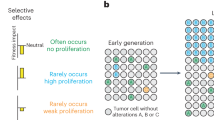
Aneuploidy can be an effective mechanism to generate phenotypic variation and adaptation under a selective pressure.
-
Aneuploidy can both promote and inhibit tumorigenesis.
-
Aneuploidy is a highly attractive target in cancer therapy.
-
Therapeutics that target aneuploidy could either target aneuploidy generally or target specific recurrent aneuploidies that are associated with certain cancers.
Abstract
Genetic instability, which includes both numerical and structural chromosomal abnormalities, is a hallmark of cancer. Whereas the structural chromosome rearrangements have received substantial attention, the role of whole-chromosome aneuploidy in cancer is much less well-understood. Here we review recent progress in understanding the roles of whole-chromosome aneuploidy in cancer, including the mechanistic causes of aneuploidy, the cellular responses to chromosome gains or losses and how cells might adapt to tolerate these usually detrimental alterations. We also explore the role of aneuploidy in cellular transformation and discuss the possibility of developing aneuploidy-specific therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mitelman, F., Johansson, B. & Mertens, F. The impact of translocations and gene fusions on cancer causation. Nature Rev. Cancer 7, 233–245 (2007).
Ricke, R. M., van Ree, J. H. & van Deursen, J. M. Whole chromosome instability and cancer: a complex relationship. Trends Genet. 24, 457–466 (2008).
Teixeira, M. R. & Heim, S. Multiple numerical chromosome aberrations in cancer: what are their causes and what are their consequences? Semin. Cancer Biol. 15, 3–12 (2005).
Schvartzman, J. M., Sotillo, R. & Benezra, R. Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nature Rev. Cancer 10, 102–115 (2010).
Hanks, S. et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nature Genet. 36, 1159–1161 (2004).
Thompson, S. L. & Compton, D. A. Chromosomes and cancer cells. Chromosome Res. 19, 433–444 (2011).
Thompson, S. L., Bakhoum, S. F. & Compton, D. A. Mechanisms of chromosomal instability. Curr. Biol. 20, R285–R295 (2010).
Chandhok, N. S. & Pellman, D. A little CIN may cost a lot: revisiting aneuploidy and cancer. Curr. Opin. Genet. Dev. 19, 74–81 (2009).
Beroukhim, R. et al. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010). This study shows that one-quarter of the genome of a typical cancer cell is affected by either whole-arm or whole-chromosome somatic copy number alterations.
Mitelman, F., Johannson, B. & Mertens, F. Mitelman Database of Chromosome Aberrations in Cancer [online], (2012).
Ozery-Flato, M., Linhart, C., Trakhtenbrot, L., Izraeli, S. & Shamir, R. Large-scale analysis of chromosomal aberrations in cancer karyotypes reveals two distinct paths to aneuploidy. Genome Biol. 12, R61 (2011).
Barnard, D. R. et al. Morphologic, immunologic, and cytogenetic classification of acute myeloid leukemia and myelodysplastic syndrome in childhood: a report from the Childrens Cancer Group. Leukemia 10, 5–12 (1996).
Maurici, D. et al. Frequency and implications of chromosome 8 and 12 gains in Ewing sarcoma. Cancer Genet. Cytogenet. 100, 106–110 (1998).
Qi, H. et al. Trisomies 8 and 20 in desmoid tumors. Cancer Genet. Cytogenet. 92, 147–149 (1996).
Barnard, D. R. et al. Acute myeloid leukemia and myelodysplastic syndrome in children treated for cancer: comparison with primary presentation. Blood 100, 427–434 (2002).
Paulsson, K. & Johansson, B. Trisomy 8 as the sole chromosomal aberration in acute myeloid leukemia and myelodysplastic syndromes. Pathol. Biol. 55, 37–48 (2007).
Thompson, S. L. & Compton, D. A. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 180, 665–672 (2008).
Cimini, D., Tanzarella, C. & Degrassi, F. Differences in malsegregation rates obtained by scoring ana-telophases or binucleate cells. Mutagenesis 14, 563–568 (1999).
Compton, D. A. Mechanisms of aneuploidy. Curr. Opin. Cell Biol. 23, 109–113 (2011).
Cimini, D. Merotelic kinetochore orientation, aneuploidy, and cancer. Biochim. Biophys. Acta 1786, 32–40 (2008).
Cimini, D. et al. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 153, 517–527 (2001).
Gregan, J., Polakova, S., Zhang, L., Tolic´-Nørrelykke, I. M. & Cimini, D. Merotelic kinetochore attachment: causes and effects. Trends Cell Biol. 21, 374–381 (2011).
Ganem, N. J., Godinho, S. A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 (2009). This study provides a mechanistic link between extra centrosomes and CIN by demonstrating that supernumerary centrosomes increase the frequency of merotelic attachments and chromosome segregation errors.
Silkworth, W. T., Nardi, I. K., Scholl, L. M. & Cimini, D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS ONE 4, e6564 (2009).
Bakhoum, S. F., Genovese, G. & Compton, D. A. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr. Biol. 19, 1937–1942 (2009).
Tada, K., Susumu, H., Sakuno, T. & Watanabe, Y. Condensin association with histone H2A shapes mitotic chromosomes. Nature 474, 477–483 (2011).
Corbett, K. D. et al. The monopolin complex crosslinks kinetochore components to regulate chromosome-microtubule attachments. Cell 142, 556–567 (2010).
Bakhoum, S. F., Thompson, S. L., Manning, A. L. & Compton, D. A. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nature Cell Biol. 11, 27–35 (2009).
Nigg, E. A. Origins and consequences of centrosome aberrations in human cancers. Int. J. Cancer 119, 2717–2723 (2006).
Pihan, G. A. et al. Centrosome defects and genetic instability in malignant tumors. Cancer Res. 58, 3974–3985 (1998).
Pihan, G. A., Wallace, J., Zhou, Y. & Doxsey, S. J. Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res. 63, 1398–1404 (2003).
Nigg, E. A. Centrosome aberrations: cause or consequence of cancer progression? Nature Rev. Cancer 2, 815–825 (2002).
Boveri, T. The Origin of Malignant Tumors (Waverly Press, Baltimore, Maryland, 1929).
Holland, A. J. & Cleveland, D. W. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nature Rev. Mol. Cell Biol. 10, 478–487 (2009).
Brinkley, B. R. Managing the centrosome numbers game: from chaos to stability in cancer cell division. Trends Cell Biol. 11, 18–21 (2001).
Ring, D., Hubble, R. & Kirschner, M. Mitosis in a cell with multiple centrioles. J. Cell Biol. 94, 549–556 (1982).
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instability in colorectal cancers. Nature 386, 623–627 (1997).
Rajagopalan, H., Nowak, M. A., Vogelstein, B. & Lengauer, C. The significance of unstable chromosomes in colorectal cancer. Nature Rev. Cancer 3, 695–701 (2003).
Cahill, D. P. et al. Characterization of MAD2B and other mitotic spindle checkpoint genes. Genomics 58, 181–187 (1999).
Haruki, N. et al. Molecular analysis of the mitotic checkpoint genes BUB1, BUBR1 and BUB3 in human lung cancers. Cancer Lett. 162, 201–205 (2001).
Myrie, K. A., Percy, M. J., Azim, J. N., Neeley, C. K. & Petty, E. M. Mutation and expression analysis of human BUB1 and BUB1B in aneuploid breast cancer cell lines. Cancer Lett. 152, 193–199 (2000).
Kops, G. J., Weaver, B. A. & Cleveland, D. W. On the road to cancer: aneuploidy and the mitotic checkpoint. Nature Rev. Cancer 5, 773–785 (2005).
Wang, Z. et al. Three classes of genes mutated in colorectal cancers with chromosomal instability. Cancer Res. 64, 2998–3001 (2004).
Gascoigne, K. E. & Taylor, S. S. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell 14, 111–122 (2008).
Tighe, A., Johnson, V. L., Albertella, M. & Taylor, S. S. Aneuploid colon cancer cells have a robust spindle checkpoint. EMBO Rep. 2, 609–614 (2001).
Haruta, M. et al. Combined BubR1 protein down-regulation and RASSF1A hypermethylation in Wilms tumors with diverse cytogenetic changes. Mol. Carcinog. 47, 660–666 (2008).
Park, H. Y. et al. Differential promoter methylation may be a key molecular mechanism in regulating BubR1 expression in cancer cells. Exp. Mol. Med. 39, 195–204 (2007).
Morgan, D. O. The Cell Cycle: Principles of Control (Sinauer Associates, Sunderland, Maryland, 2007).
Musacchio, A. & Salmon, E. D. The spindle-assembly checkpoint in space and time. Nature Rev. Mol. Cell Biol. 8, 379–393 (2007).
Barber, T. D. et al. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc. Natl Acad. Sci. USA 105, 3443–3448 (2008).
Solomon, D. A. et al. Mutational inactivation of STAG2 causes aneuploidy in human cancer. Science 333, 1039–1043 (2011).
Dorsett, D. Cohesin: genomic insights into controlling gene transcription and development. Curr. Opin. Genet. Dev. 21, 199–206 (2011).
Torres, E. M., Williams, B. R. & Amon, A. Aneuploidy: cells losing their balance. Genetics 179, 737–746 (2008).
Torres, E. M. et al. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science 317, 916–924 (2007). This study uses a chromosome transfer strategy and selectable markers to generate isogenic aneuploid yeast strains with a single extra chromosome.
Sheltzer, J. M. et al. Aneuploidy drives genomic instability in yeast. Science 333, 1026–1030 (2011). This study shows that aneuploidy in yeast can generate genomic instability, including increased chromosome loss, mutation rate and defective DNA damage repair.
Niwa, O., Tange, Y. & Kurabayashi, A. Growth arrest and chromosome instability in aneuploid yeast. Yeast 23, 937–950 (2006).
Williams, B. R. et al. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science 322, 703–709 (2008). This study establishes and characterizes isogenic MEF cell lines that are trisomic for chromosomes 1, 13, 16 or 19 using balanced Robertsonian translocations.
Rasmussen, S. A., Wong, L. Y., Yang, Q., May, K. M. & Friedman, J. M. Population-based analyses of mortality in trisomy 13 and trisomy 18. Pediatrics 111, 777–784 (2003).
Segal, D. J. & McCoy, E. E. Studies on Down's syndrome in tissue culture. I. Growth rates and protein contents of fibroblast cultures. J. Cell Physiol. 83, 85–90 (1974).
Taylor, A. I. Cell selection in vivo in normal-G trisomic mosaics. Nature 219, 1028–1030 (1968).
Yurov, Y. B. et al. Aneuploidy and confined chromosomal mosaicism in the developing human brain. PLoS ONE 2, e558 (2007).
Yurov, Y. B. et al. The variation of aneuploidy frequency in the developing and adult human brain revealed by an interphase FISH study. J. Histochem. Cytochem. 53, 385–390 (2005).
Gasch, A. P. et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11, 4241–4257 (2000).
Pavelka, N. et al. Aneuploidy confers quantitative proteome changes and phenotypic variation in budding yeast. Nature 468, 321–325 (2010). This study describes the induction of meiosis in yeast strains with an odd ploidy (3N or 5N) to produce isogenic aneuploid strains.
ElBaradi, T. T., van der Sande, C. A., Mager, W. H., Raue, H. A. & Planta, R. J. The cellular level of yeast ribosomal protein L25 is controlled principally by rapid degradation of excess protein. Curr. Genet. 10, 733–739 (1986).
Maicas, E., Pluthero, F. G. & Friesen, J. D. The accumulation of three yeast ribosomal proteins under conditions of excess mRNA is determined primarily by fast protein decay. Mol. Cell. Biol. 8, 169–175 (1988).
Torres, E. M. et al. Identification of aneuploidy-tolerating mutations. Cell 143, 71–83 (2010).
Collier, T. S. et al. Comparison of stable-isotope labeling with amino acids in cell culture and spectral counting for relative quantification of protein expression. Rapid Commun. Mass Spectrom. 25, 2524–2532 (2011).
Collier, T. S. et al. Direct comparison of stable isotope labeling by amino acids in cell culture and spectral counting for quantitative proteomics. Anal. Chem. 82, 8696–8702 (2010).
St. Charles, J., Hamilton, M. L. & Petes, T. D. Meiotic chromosome segregation in triploid strains of Saccharomyces cerevisiae. Genetics 186, 537–550 (2010).
Storchova, Z. et al. Genome-wide genetic analysis of polyploidy in yeast. Nature 443, 541–547 (2006).
Ganem, N. J., Storchova, Z. & Pellman, D. Tetraploidy, aneuploidy and cancer. Curr. Opin. Genet. Dev. 17, 157–162 (2007).
Thompson, S. L. & Compton, D. A. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J. Cell Biol. 188, 369–381 (2010).
Tomasini, R., Mak, T. W. & Melino, G. The impact of p53 and p73 on aneuploidy and cancer. Trends Cell Biol. 18, 244–252 (2008).
Bunz, F. et al. Targeted inactivation of p53 in human cells does not result in aneuploidy. Cancer Res. 62, 1129–1133 (2002).
Kingsbury, M. A. et al. Aneuploid neurons are functionally active and integrated into brain circuitry. Proc. Natl Acad. Sci. USA 102, 6143–6147 (2005).
Rehen, S. K. et al. Chromosomal variation in neurons of the developing and adult mammalian nervous system. Proc. Natl Acad. Sci. USA 98, 13361–13366 (2001).
Li, M. et al. The ATM–p53 pathway suppresses aneuploidy-induced tumorigenesis. Proc. Natl Acad. Sci. USA 107, 14188–14193 (2010).
Guo, Z., Kozlov, S., Lavin, M. F., Person, M. D. & Paull, T. T. ATM activation by oxidative stress. Science 330, 517–521 (2010).
Weaver, B. A., Silk, A. D., Montagna, C., Verdier-Pinard, P. & Cleveland, D. W. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell 11, 25–36 (2007).
Weaver, B. A. & Cleveland, D. W. The role of aneuploidy in promoting and suppressing tumors. J. Cell Biol. 185, 935–937 (2009).
Weaver, B. A. & Cleveland, D. W. The aneuploidy paradox in cell growth and tumorigenesis. Cancer Cell 14, 431–433 (2008).
Manning, A. L., Longworth, M. S. & Dyson, N. J. Loss of pRB causes centromere dysfunction and chromosomal instability. Genes Dev. 24, 1364–1376 (2010).
Zheng, L. & Lee, W. H. Retinoblastoma tumor suppressor and genome stability. Adv. Cancer Res. 85, 13–50 (2002).
Knudsen, E. S., Sexton, C. R. & Mayhew, C. N. Role of the retinoblastoma tumor suppressor in the maintenance of genome integrity. Curr. Mol. Med. 6, 749–757 (2006).
Manning, A. L. & Dyson, N. J. pRB, a tumor suppressor with a stabilizing presence. Trends Cell Biol. 21, 433–441 (2011).
Coschi, C. H. et al. Mitotic chromosome condensation mediated by the retinoblastoma protein is tumor-suppressive. Genes Dev. 24, 1351–1363 (2010).
van Harn, T. et al. Loss of Rb proteins causes genomic instability in the absence of mitogenic signaling. Genes Dev. 24, 1377–1388 (2010).
Schvartzman, J. M., Duijf, P. H., Sotillo, R., Coker, C. & Benezra, R. Mad2 is a critical mediator of the chromosome instability observed upon Rb and p53 pathway inhibition. Cancer Cell 19, 701–714 (2011). This study shows that the overexpression of the mitotic checkpoint protein MAD2 is required for the CIN that results from the inhibition of the RB and p53 pathways, two pathways that are frequently inactivated in human cancer.
Sotillo, R. et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell 11, 9–23 (2007).
Pavelka, N., Rancati, G. & Li, R. Dr Jekyll and Mr Hyde: role of aneuploidy in cellular adaptation and cancer. Curr. Opin. Cell Biol. 22, 809–815 (2010).
Rancati, G. et al. Aneuploidy underlies rapid adaptive evolution of yeast cells deprived of a conserved cytokinesis motor. Cell 135, 879–893 (2008). This work demonstrates the beneficial and synergistic effects of small changes in gene expression owing to aneuploidy in yeast with defects in cytokinesis.
Bianchi, A. B., Aldaz, C. M. & Conti, C. J. Nonrandom duplication of the chromosome bearing a mutated Ha-ras-1 allele in mouse skin tumors. Proc. Natl Acad. Sci. USA 87, 6902–6906 (1990).
Zhuang, Z. et al. Trisomy 7-harbouring non-random duplication of the mutant MET allele in hereditary papillary renal carcinomas. Nature Genet. 20, 66–69 (1998).
Fischer, J. et al. Duplication and overexpression of the mutant allele of the MET proto-oncogene in multiple hereditary papillary renal cell tumours. Oncogene 17, 733–739 (1998).
Beghini, A. et al. Trisomy 4 leading to duplication of a mutated KIT allele in acute myeloid leukemia with mast cell involvement. Cancer Genet. Cytogenet. 119, 26–31 (2000).
Langabeer, S. E., Beghini, A. & Larizza, L. AML with t(8;21) and trisomy 4: possible involvement of c-kit? Leukemia 17, 1915; author reply 1915–1916 (2003).
Sotillo, R., Schvartzman, J. M., Socci, N. D. & Benezra, R. Mad2-induced chromosome instability leads to lung tumour relapse after oncogene withdrawal. Nature 464, 436–440 (2010). This study shows that KRAS-driven tumours that develop CIN and aneuploidy owing to transient MAD2 overexpression recur at a markedly elevated rate after the withdrawal of the KRAS oncogene.
Malinge, S., Izraeli, S. & Crispino, J. D. Insights into the manifestations, outcomes and mechanisms of leukemogenesis in Down syndrome. Blood 113, 2619–2628 (2009).
Baek, K. H. et al. Down's syndrome suppression of tumour growth and the role of the calcineurin inhibitor DSCR1. Nature 459, 1126–1130 (2009).
Ng, A. P. et al. Trisomy of Erg is required for myeloproliferation in a mouse model of Down syndrome. Blood 115, 3966–3969 (2010).
Baetz, K., Measday, V. & Andrews, B. Revealing hidden relationships among yeast genes involved in chromosome segregation using systematic synthetic lethal and synthetic dosage lethal screens. Cell Cycle 5, 592–595 (2006).
Measday, V. et al. Systematic yeast synthetic lethal and synthetic dosage lethal screens identify genes required for chromosome segregation. Proc. Natl Acad. Sci. USA 102, 13956–13961 (2005).
Lin, H. et al. Polyploids require Bik1 for kinetochore–microtubule attachment. J. Cell Biol. 155, 1173–1184 (2001).
Kramer, A., Maier, B. & Bartek, J. Centrosome clustering and chromosomal (in) stability: A matter of life and death. Mol. Oncol. 5, 324–335 (2011).
Kwon, M. et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 22, 2189–2203 (2008). In this study, a genome-wide siRNA screen was used to identify new mechanisms by which cells suppress multipolar divisions.
Tang, Y. C., Williams, B. R., Siegel, J. J. & Amon, A. Identification of aneuploidy-selective antiproliferation compounds. Cell 144, 499–512 (2011). This study identifies drugs with aneuploidy-specific lethality.
Chng, W. J. et al. Molecular dissection of hyperdiploid multiple myeloma by gene expression profiling. Cancer Res. 67, 2982–2989 (2007).
Mateos, M. V. et al. Outcome according to cytogenetic abnormalities and DNA ploidy in myeloma patients receiving short induction with weekly bortezomib followed by maintenace. Blood 118, 4547–4553 (2011).
Usmani, S. Z., Bona, R. & Li, Z. 17 AAG for HSP90 inhibition in cancer—from bench to bedside. Curr. Mol. Med. 9, 654–664 (2009).
Taub, J. W. & Ge, Y. Down syndrome, drug metabolism and chromosome 21. Pediatr. Blood Cancer 44, 33–39 (2005).
Zhang, L. et al. Reduced folate carrier gene expression in childhood acute lymphoblastic leukemia: relationship to immunophenotype and ploidy. Clin. Cancer Res. 4, 2169–2177 (1998).
Belkov, V. M. et al. Reduced folate carrier expression in acute lymphoblastic leukemia: a mechanism for ploidy but not lineage differences in methotrexate accumulation. Blood 93, 1643–1650 (1999).
Meaburn, K. J., Parris, C. N. & Bridger, J. M. The manipulation of chromosomes by mankind: the uses of microcell-mediated chromosome transfer. Chromosoma 114, 263–274 (2005).
Doherty, A. M. & Fisher, E. M. Microcell-mediated chromosome transfer (MMCT): small cells with huge potential. Mamm. Genome 14, 583–592 (2003).
Upender, M. B. et al. Chromosome transfer induced aneuploidy results in complex dysregulation of the cellular transcriptome in immortalized and cancer cells. Cancer Res. 64, 6941–6949 (2004).
Hughes, T. R. et al. Widespread aneuploidy revealed by DNA microarray expression profiling. Nature Genet. 25, 333–337 (2000).
Selmecki, A., Forche, A. & Berman, J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 313, 367–370 (2006). This study demonstrates that that the in vivo acquisition of extra copies of an isochromosome by Candida albicans confers resistance to fluconazole through the action of two specific genes in a copy-number-dependent manner.
Selmecki, A., Gerami-Nejad, M., Paulson, C., Forche, A. & Berman, J. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol. Microbiol. 68, 624–641 (2008).
Duesberg, P. et al. How aneuploidy may cause cancer and genetic instability. Anticancer Res. 19, 4887–4906 (1999).
Duesberg, P., Li, R., Fabarius, A. & Hehlmann, R. Aneuploidy and cancer: from correlation to causation. Contrib. Microbiol. 13, 16–44 (2006).
Su, X. et al. Mechanism underlying the dual-mode regulation of microtubule dynamics by Kip/3kinesin-8. Mol. Cell 43, 751–763 (2011).
Fujiwara, T. et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437, 1043–1047 (2005).
Acknowledgements
We apologize to the authors whose work was not discussed or cited owing to space limitations. The authors are grateful to members of the Pellman laboratory, in particular Hubo Li, for helpful discussions. The authors thank Z. Storchova, A. Amon and R. Li for sharing results before publication. The authors thank E. Ivanova for providing the SKY karyotype for Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Aneuploidy
-
The presence of an abnormal number of chromosomes, either more or less than the diploid number. Aneuploidy is associated with cell and organismal inviability, cancer and birth defects.
- Chromosomal instability
-
(CIN). A persistently high rate of gain and loss of chromosomes.
- Kinetochore
-
A large protein complex that assembles at centromeres. It is composed of inner and outer regions that contain >80 proteins, which are required for spindle attachment, chromosome movement and regulation of the mitotic checkpoint.
- Centrosome
-
An organelle that serves as the main microtubule-organizing centre of the cell, as well as a regulator of cell cycle progression.
- Spindle assembly checkpoint
-
(SAC). A highly conserved surveillance mechanism in mitosis and meiosis that minimizes chromosome loss by preventing chromosomes from initiating anaphase until all kinetochores have successfully captured spindle microtubules.
- Merotelic attachments
-
Abnormal kinetochore–microtubule attachments that occur when a single kinetochore attaches to microtubules that arise from both poles of the spindle.
- Mosaic variegated aneuploidy
-
A rare, recessive condition that is characterized by growth restriction, microcephaly, childhood cancer and constitutional mosaicism for chromosomal gains and losses.
- Co-culture
-
A cell culture containing a mixture of two different cell types.
- Proteasome inhibitors
-
A class of drugs, including MG132 and bortezomib, that block the action of proteasomes, which are cellular complexes involved in protein degradation.
- Dosage compensation
-
The counterbalancing of gene and protein imbalances that arise from unequal numbers of chromosomes, such as sex chromosomes in normal cells or potentially any chromosome in aneuploid cells.
- Environmental stress response
-
(ESR). A gene set signature defined in yeast that has been grown under stressful conditions and at slow growth rates.
- Proteotoxic stress
-
Results from the accumulation of unfolded, misfolded and aggregated proteins in a cell.
- Robertsonian translocation
-
A chromosomal abnormality in which two acrocentric chromosomes become joined by a common centromere.
- Reactive oxygen species
-
(ROS). Ions or small molecules — including oxygen ions, free radicals, inorganic peroxides and organic peroxides — that are highly reactive owing to the presence of unpaired valence shell electrons. They are a by-product of the normal metabolism of oxygen and have important roles in cell signalling. Increased levels owing to environmental stress can result in damage to cells.
- Mutator phenotype
-
The loss-of-function of one gene, such as one for the repair of damaged DNA, that greatly increases the mutation rates at other loci.
- Chromosome congression
-
The process of aligning chromosomes on the spindle during mitosis.
- Oncogene addiction
-
The dependence of a cancer cell on one overactive gene or pathway for survival, growth and proliferation.
- Synthetic lethality
-
Two genes are synthetic lethal if mutation of either alone is compatible with viability, but mutation of both leads to cell death.
- AICAR
-
Short for 5-aminoimidazole-4-carboxamide ribonucleotide, this is an activator of AMP-activated protein kinase (AMPK), which is a metabolic master regulator that is activated in times of reduced energy availability.
- HSP90 inhibitor
-
A class of drugs, including 17-allylaminogeldanamycin (17-AAG), that inhibit the function of the molecular chaperone heat shock protein 90 (HSP90), which is involved in protein folding.
Rights and permissions
About this article
Cite this article
Gordon, D., Resio, B. & Pellman, D. Causes and consequences of aneuploidy in cancer. Nat Rev Genet 13, 189–203 (2012). https://doi.org/10.1038/nrg3123
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3123
This article is cited by
-
RIT1 regulates mitosis and promotes proliferation by interacting with SMC3 and PDS5 in hepatocellular carcinoma
Journal of Experimental & Clinical Cancer Research (2023)
-
Cancer genomes tolerate deleterious coding mutations through somatic copy number amplifications of wild-type regions
Nature Communications (2023)
-
Tracking circulating PD-L1-positive cells to monitor the outcome of patients with gastric cancer receiving anti-HER2 plus anti-PD-1 therapy
Human Cell (2023)
-
Disentangling the roles of aneuploidy, chromosomal instability and tumour heterogeneity in developing resistance to cancer therapies
Chromosome Research (2023)
-
Heterogeneous circulating tumor cells correlate with responses to neoadjuvant chemotherapy and prognosis in patients with locally advanced breast cancer
Breast Cancer Research and Treatment (2023)