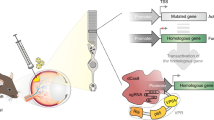
Key Points
-
Inherited diseases still represent a substantial burden to modern societies. The identification of causative gene defects for several of these diseases facilitated the development of gene-based therapeutic strategies. Over the past few years, clinical translation of bench research resulted in the first examples of therapeutic success for the field of gene transfer.
-
Adeno-associated virus (AAV) vectors are among the most suitable tools for in vivo gene delivery given their superior efficiency of transduction of several tissues, including liver, muscle and nervous tissue. Preclinical studies with these vectors have shown stable transgene expression and long-term correction of disease phenotype with little to no toxicity in small and large animal models of disease.
-
In humans, some of the most promising results were obtained when AAV was used to transfer therapeutic genes to the retina for the treatment of congenital blindness caused by retinal pigment epithelium-specific protein 65kDa (RPE65) deficiency, to the liver to correct the bleeding phenotype of haemophilia B, and to focal areas of the brain to ameliorate the course of Parkinson's disease, an acquired neurodegenerative disease.
-
The clinical experience with AAV vectors also highlighted a number of issues, some of which are not predicted by animal studies. Among them, vector or transgene immunogenicity remains the major obstacle to long-term therapeutic transgene expression. Target-tissue- and disease-specific hurdles will also need to be addressed to expand the scope of success with the AAV gene transfer platform.
-
The eye represents an ideal target tissue for gene transfer as widespread organ transduction can be achieved at low vector doses, and because presentation of a foreign antigen in this compartment is usually not associated with inflammatory responses. Experience with RPE65 deficiency is paving the way for the development of a whole new class of gene therapeutics targeting the retina.
-
The liver is also one of the most attractive targets for gene transfer owing to its unique biosynthetic capabilities and its ability to favour antigen-specific tolerance upon gene transfer. Some exciting results have recently been obtained in the clinic with haemophilia B; overcoming the limit of capsid-driven immune responses promises even greater success.
-
Gene transfer to the central nervous system has also given promising results. Efficient global gene transfer — achieved with novel delivery techniques and/or novel serotypes — will be the key to successfully treating some neurodegenerative diseases, such as lysosomal storage disorders.
-
Efficient delivery techniques are under development to target large areas of muscle using AAV vectors. Hopefully this will help us to reach the levels of transgene expression needed for therapeutic efficacy in some diseases.
-
The identification of the hurdles for clinical translation of AAV gene transfer, along with the development of solutions to overcome these problems, will enable us to provide improved therapeutic options for a range of inherited diseases by fully exploiting the potential of in vivo gene transfer.
Abstract
In vivo gene replacement for the treatment of inherited disease is one of the most compelling concepts in modern medicine. Adeno-associated virus (AAV) vectors have been extensively used for this purpose and have shown therapeutic efficacy in a range of animal models. Successful translation to the clinic was initially slow, but long-term expression of donated genes at therapeutic levels has now been achieved in patients with inherited retinal disorders and haemophilia B. Recent exciting results have raised hopes for the treatment of many other diseases. As we discuss here, the prospects and challenges for AAV gene therapy are to a large extent dependent on the target tissue and the specific disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
24 May 2011
In the above article, information on clinical trials using adeno-associated virus (AAV) vectors for the treatment of Leber's congenital amaurosis was omitted from table 1. This table has now been corrected online. The authors apologize for this omission.
References
McCandless, S. E., Brunger, J. W. & Cassidy, S. B. The burden of genetic disease on inpatient care in a children's hospital. Am. J. Hum. Genet. 74, 121–127 (2004).
Emery, A. E. H. & Rimoin, D. L. Principles and Practice of Medical Genetics 2nd edn (Churchill Livingstone, New York, 1990).
Aiuti, A. et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 360, 447–458 (2009).
Bainbridge, J. W. et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 358, 2231–2239 (2008). References 3 and 4 (together with references 7 and 17) are the first reports of efficacy in studies of AAV gene transfer in subjects with RPE65 deficiency.
Cartier, N. et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 326, 818–823 (2009).
Maguire, A. M. et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet 374, 1597–1605 (2009). This is the largest study of AAV gene transfer in RPE65-deficient subjects published to date. The results presented in this manuscript highlight the importance of early intervention in retinal degenerative diseases.
Maguire, A. M. et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 (2008).
Nathwani, A. C. et al. Early clinical trial results following administration of a low dose of a novel self complementary adeno-associated viral vector encoding human Factor IX in two subjects with severe hemophilia B. Blood (ASH Annual Meeting Abstracts) 116, abstract 248 (2010). This is an early report showing efficacy in a clinical trial of AAV factor IX gene transfer for haemophilia B in which an AAV vector optimized for liver expression was delivered intravenously in affected subjects. See also the next reference.
Ponder, K. P. Hemophilia gene therapy: a holy grail found. Mol. Ther. 19, 427–428 (2011).
Kay, M. A. State-of-the-art gene-based therapies: the road ahead. Nature Rev. Genet. 12, 316–328 (2011).
Amsterdam Molecular Therapeutics. AMT submits its lead product Glybera® application for marketing authorisation to EMA. Amsterdam Molecular Therapeutics [online] (2010).
Gaudet, D. et al. Review of the clinical development of alipogene tiparvovec gene therapy for lipoprotein lipase deficiency. Atheroscler. Suppl. 11, 55–60 (2010).
Naldini, L. Ex vivo gene transfer and correction for cell-based therapies. Nature Rev. Genet. 12, 301–315 (2011).
den Hollander, A. I., Roepman, R., Koenekoop, R. K. & Cremers, F. P. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 27, 391–419 (2008).
Jacobson, S. G. et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc. Natl Acad. Sci. USA 102, 6177–6182 (2005).
Acland, G. M. et al. Gene therapy restores vision in a canine model of childhood blindness. Nature Genet. 28, 92–95 (2001).
Hauswirth, W. W. et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 19, 979–990 (2008).
Cideciyan, A. V. et al. Vision 1 year after gene therapy for Leber's congenital amaurosis. N. Engl. J. Med. 361, 725–727 (2009).
Simonelli, F. et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol. Ther. 18, 643–650 (2010).
Acland, G. M. et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol. Ther. 12, 1072–1082 (2005).
Hiraki, S. & Green, N. S. Newborn screening for treatable genetic conditions: past, present and future. Obstet. Gynecol. Clin. North Am. 37, 11–21 (2010).
Lam, B. L. et al. Leber hereditary optic neuropathy gene therapy clinical trial recruitment: year 1. Arch. Ophthalmol. 128, 1129–1135 (2010).
Anand, V. et al. Gene therapy for choroideremia: in vitro rescue mediated by recombinant adenovirus. Vision Res. 43, 919–926 (2003).
Moscioni, D. et al. Long-term correction of ammonia metabolism and prolonged survival in ornithine transcarbamylase-deficient mice following liver-directed treatment with adeno-associated viral vectors. Mol. Ther. 14, 25–33 (2006).
Carrillo-Carrasco, N., Chandler, R. J., Chandrasekaran, S. & Venditti, C. P. Liver-directed recombinant adeno-associated viral gene delivery rescues a lethal mouse model of methylmalonic acidemia and provides long-term phenotypic correction. Hum. Gene Ther. 21, 1147–1154 (2010).
Lofqvist, T., Nilsson, I. M., Berntorp, E. & Pettersson, H. Haemophilia prophylaxis in young patients-a long-term follow-up. J. Intern. Med. 241, 395–400 (1997).
Manco-Johnson, M. J. et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 357, 535–544 (2007).
Evatt, B. L., Black, C., Batorova, A., Street, A. & Srivastava, A. Comprehensive care for haemophilia around the world. Haemophilia 10, 9–13 (2004).
Lin, H. F., Maeda, N., Smithies, O., Straight, D. L. & Stafford, D. W. A coagulation factor IX-deficient mouse model for human hemophilia B. Blood 90, 3962–3966 (1997).
Wang, L. et al. A factor IX-deficient mouse model for hemophilia B gene therapy. Proc. Natl Acad. Sci. USA 94, 11563–11566 (1997).
Yao, S. N. & Kurachi, K. Expression of human factor IX in mice after injection of genetically modified myoblasts. Proc. Natl Acad. Sci. USA 89, 3357–3361 (1992).
Bi, L. et al. Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nature Genet. 10, 119–121 (1995).
Evans, J. P., Brinkhous, K. M., Brayer, G. D., Reisner, H. M. & High, K. A. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc. Natl Acad. Sci. USA 86, 10095–10099 (1989).
Lozier, J. N. et al. Efficient transfection of primary cells in a canine hemophilia B model using adenovirus-polylysine-DNA complexes. Hum. Gene Ther. 5, 313–322 (1994).
Mauser, A. E., Whitlark, J., Whitney, K. M. & Lothrop, C. D. Jr. A deletion mutation causes hemophilia B in Lhasa Apso dogs. Blood 88, 3451–3455 (1996).
Manno, C. S. et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nature Med. 12, 342–347 (2006). This is the first study of AAV gene transfer to the liver of subjects with haemophilia B in which therapeutic transgene expression was achieved, albeit transiently. This study also brought to the attention of the AAV gene transfer field the issue of capsid immunogenicity.
Mount, J. D. et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood 99, 2670–2676 (2002).
Wang, L. et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood 105, 3079–3086 (2005).
Manno, C. S. et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 101, 2963–2972 (2003). This was the first clinical study of AAV intramuscular gene transfer in subjects with haemophilia B. Successful transduction of muscle tissue was documented in biopsy samples; however, circulating plasma factor IX levels were subtherapeutic. See also references 99 and 148.
Franco, L. M. et al. Evasion of immune responses to introduced human acid α-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol. Ther. 12, 876–884 (2005).
Mingozzi, F. et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J. Clin. Invest. 111, 1347–1356 (2003). This was the first study describing the induction of immunological tolerance to an antigen by hepatic gene transfer. This study also suggests that hepatic tolerance is mediated by CD4+ regulatory T cells. References 40, 42, 43, 44 and 48 are examples of studies confirming or extending these results.
Ziegler, R. J. et al. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of α-galactosidase A and the induction of immune tolerance in Fabry mice. Mol. Ther. 9, 231–240 (2004).
Follenzi, A. et al. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood 103, 3700–3709 (2004).
Xu, L. et al. Immune response after neonatal transfer of a human factor IX-expressing retroviral vector in dogs, cats, and mice. Thromb. Res. 120, 269–280 (2007).
Raper, S. E. et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 80, 148–158 (2003).
Mingozzi, F. et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nature Med. 13, 419–422 (2007). This manuscript describes the characterization of the T cell responses directed against the AAV capsid in humans undergoing gene transfer and healthy donors. See references 52–57 for additional work around the issue of AAV vector immunogenicity.
Jiang, H. et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 108, 3321–3328 (2006).
Mingozzi, F. et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood 110, 2334–2341 (2007).
Hauck, B. et al. Undetectable transcription of cap in a clinical AAV vector: implications for preformed capsid in immune responses. Mol. Ther. 17, 144–152 (2009).
National Institutes of Health Office of Biotechnology Activities. Immune responses to adeno-associated virus (AAV) vectors: NIH Recombinant DNA Advisory Committee Meeting. National Institutes of Health Office of Biotechnology Activities [online] (2007).
Li, C. et al. Cellular immune response to cryptic epitopes during therapeutic gene transfer. Proc. Natl Acad. Sci. USA 106, 10770–10774 (2009).
Vandenberghe, L. H. et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nature Med. 12, 967–971 (2006).
Basner-Tschakarjan, E. et al. Dose-dependent activation of capsid-specific T cells after AAV serotype 8 vector administration in a clinical study for hemophilia. Mol. Ther. (in the press).
Li, H. et al. Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol. Ther. 15, 792–800 (2007).
Li, C. et al. Adeno-associated virus type 2 (AAV2) capsid-specific cytotoxic T lymphocytes eliminate only vector-transduced cells coexpressing the AAV2 capsid in vivo. J. Virol. 81, 7540–7547 (2007).
Wang, L., Figueredo, J., Calcedo, R., Lin, J. & Wilson, J. M. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum. Gene Ther. 18, 185–194 (2007).
Pien, G. C. et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J. Clin. Invest. 119, 1688–1695 (2009). This manuscript describes the generation of an in vitro model to study AAV capsid antigen presentation on MHC class I.
Nathwani, A. C. et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood 109, 1414–1421 (2007). This manuscript is an example of optimization of an AAV vector for hepatic gene transfer (see also reference 60).
McCarty, D. M. et al. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 10, 2112–2118 (2003).
Nathwani, A. C. et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood 107, 2653–2661 (2006).
Calcedo, R., Vandenberghe, L. H., Gao, G., Lin, J. & Wilson, J. M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 199, 381–390 (2009). This is a comprehensive study on the prevalence of anti-AAV antibodies in humans.
Donsante, A. et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science 317, 477 (2007). This manuscript addresses the problem of vector integration within the host genome and its potential for genotoxicity (see also reference 63).
Li, H. et al. Assessing the potential for AAV vector genotoxicity in a murine model. Blood 117, 3311–3319 (2011).
Christine, C. W. et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology 73, 1662–1669 (2009).
Eberling, J. L. et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology 70, 1980–1983 (2008).
Kaplitt, M. G. et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet 369, 2097–2105 (2007).
Marks, W. J. Jr. et al. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 9, 1164–1172 (2010).
Marks, W. J. Jr. et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 7, 400–408 (2008).
LeWitt, P. A. et al. AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 10, 309–319 (2011).
Davidson, B. L. & McCray, P. B. Jr. Current prospects for RNA interference-based therapies. Nature Rev. Genet. 12, 329–340 (2011).
Davidson, B. L. et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc. Natl Acad. Sci. USA 97, 3428–3432 (2000). This is an early report of efficient transduction of brain tissue using AAV vectors.
Cabrera-Salazar, M. A. et al. Timing of therapeutic intervention determines functional and survival outcomes in a mouse model of late infantile Batten disease. Mol. Ther. 15, 1782–1788 (2007).
Cearley, C. N. & Wolfe, J. H. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol. Ther. 13, 528–537 (2006).
Chen, Y. H., Chang, M. & Davidson, B. L. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nature Med. 15, 1215–1218 (2009). This is an example of capsid engineering to redirect the AAV vector tropism to the endothelium of diseased brain and overcome the limitation of the BBB. See reference 75 for an example of a similar strategy.
Gray, S. J. et al. Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB). Mol. Ther. 18, 570–578 (2010).
Foust, K. D. et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nature Biotech. 27, 59–65 (2009). This is the first report describing the ability of AAV serotype 9 to cross the BBB when injected intravenously in neonatal mice. See also references 77 and 78 for examples in adult animals.
Fu, H., Dirosario, J., Killedar, S., Zaraspe, K. & McCarty, D. M. Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood-brain barrier gene delivery. Mol. Ther. 8 Mar 2011 (doi:10.1038/mt.2011.34).
Duque, S. et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 17, 1187–1196 (2009).
Inagaki, K. et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 14, 45–53 (2006).
Bobo, R. H. et al. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl Acad. Sci. USA 91, 2076–2080 (1994).
Kells, A. P. et al. Efficient gene therapy-based method for the delivery of therapeutics to primate cortex. Proc. Natl Acad. Sci. USA 106, 2407–2411 (2009).
Liu, G., Martins, I., Wemmie, J. A., Chiorini, J. A. & Davidson, B. L. Functional correction of CNS phenotypes in a lysosomal storage disease model using adeno-associated virus type 4 vectors. J. Neurosci. 25, 9321–9327 (2005).
Harper, S. Q. et al. RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc. Natl Acad. Sci. USA 102, 5820–5825 (2005).
Palfi, S. et al. ProSavin® a gene therapy approach for Parkinson disease: Phase I clinical trial update. Hum. Gene Ther. 21, 1364 (2010).
Mandel, R. J. CERE-110, an adeno-associated virus-based gene delivery vector expressing human nerve growth factor for the treatment of Alzheimer's disease. Curr. Opin. Mol. Ther. 12, 240–247 (2010).
Binder, D. K., Rau, G. M. & Starr, P. A. Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery 56, 722–732 (2005).
Matalon, R. et al. Aspartoacylase deficiency and N-acetylaspartic aciduria in patients with Canavan disease. Am. J. Med. Genet. 29, 463–471 (1988).
Janson, C. et al. Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum. Gene Ther. 13, 1391–1412 (2002).
McPhee, S. W. et al. Immune responses to AAV in a phase I study for Canavan disease. J. Gene Med. 8, 577–588 (2006).
Worgall, S. et al. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum. Gene Ther. 19, 463–474 (2008). This is the first report of a safety and efficacy study of AAV gene transfer in subjects with Batten's disease. In this study, an AAV2 vector was introduced into the brain's cortex with catheters inserted though burr holes.
Hofman, S. & Peltonen, L. The Neuronal Ceroid Lipofuscinoses (eds Scriver, C., Beaudet, A., Sly, W. & Valle, D.) (McGraw-Hill, New York, 2001).
Sondhi, D. et al. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol. Ther. 15, 481–491 (2007).
Brooks, A. I. et al. Functional correction of established central nervous system deficits in an animal model of lysosomal storage disease with feline immunodeficiency virus-based vectors. Proc. Natl Acad. Sci. USA 99, 6216–6221 (2002).
Mattsson, N. et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302, 385–393 (2009).
Mendell, J. R. et al. Sustained α-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann. Neurol. 68, 629–638 (2010).
Mendell, J. R. et al. Limb-girdle muscular dystrophy type 2D gene therapy restores α-sarcoglycan and associated proteins. Ann. Neurol. 66, 290–297 (2009).
Mendell, J. R. et al. Dystrophin immunity in Duchenne's muscular dystrophy. N. Engl. J. Med. 363, 1429–1437 (2010). This is the first human study showing that humans can mount a T cell response against epitopes that are present in the wild-type therapeutic transgene but absent in the endogenous, mutated gene. An intriguing finding of this study is that subjects with Duchenne muscular dystrophy present CD8+ T cell immunity against the wild-type dystrophin gene even before gene transfer.
Mas, A. et al. Reversal of type 1 diabetes by engineering a glucose sensor in skeletal muscle. Diabetes 55, 1546–1553 (2006).
Kay, M. A. et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nature Genet. 24, 257–261 (2000).
Hanley, J. P. et al. Investigation of chronic hepatitis C infection in individuals with haemophilia: assessment of invasive and non-invasive methods. Br. J. Haematol. 94, 159–165 (1996).
Brantly, M. L. et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 αl-antitrypsin (AAT) vector in AAT-deficient adults. Hum. Gene Ther. 17, 1177–1186 (2006).
Brantly, M. L. et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl Acad. Sci. USA 106, 16363–16368 (2009).
Stroes, E. S. et al. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler. Thromb. Vasc. Biol. 28, 2303–2304 (2008).
Arruda, V. R. et al. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol. Ther. 4, 586–592 (2001).
Herzog, R. W. et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum. Gene Ther. 13, 1281–1291 (2002).
Herzog, R. W. et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nature Med. 5, 56–63 (1999).
Chao, H. et al. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2, 619–623 (2000).
Arruda, V. R. et al. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood 103, 85–92 (2004).
Lu, Y. et al. Therapeutic level of functional human α 1 antitrypsin (hAAT) secreted from murine muscle transduced by adeno-associated virus (rAAV1) vector. J. Gene Med. 8, 730–735 (2006).
Su, L. T. et al. Uniform scale-independent gene transfer to striated muscle after transvenular extravasation of vector. Circulation 112, 1780–1788 (2005). The first description of the use of an intravascular delivery route to transduce large areas of skeletal muscle. See also references 111–115.
Arruda, V. R. et al. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood 115, 4678–4688 (2010).
Haurigot, V. et al. Safety of AAV factor IX peripheral transvenular gene delivery to muscle in hemophilia B dogs. Mol. Ther. 18, 1318–1329 (2010).
Rodino-Klapac, L. R. et al. Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol. Ther. 18, 109–117 (2010).
Powers, W. et al. Safety and feasibility of transvenous limb perfusion with saline in human muscular dystrophy. Mol. Ther. 18 (Suppl. 1), 126 (2010).
Toromanoff, A. et al. Lack of immunotoxicity after regional intravenous (RI) delivery of rAAV to nonhuman primate skeletal muscle. Mol. Ther. 18, 151–160 (2010).
Mingozzi, F. et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood 114, 2077–2086 (2009). This is the first report of dose-dependent activation of capsid-specific T cells in humans undergoing AAV gene transfer. See references 95, 96 and 102 for other human studies.
Mingozzi, F. et al. Modulation of T cell response to the AAV capsid in subjects undergoing intramuscular gene transfer for lipoprotein lipase deficiency. Hum. Gene Ther. 19, 1090 (2008).
Hudig, D., Haverty, T., Fulcher, C., Redelman, D. & Mendelsohn, J. Inhibition of human natural cytotoxicity by macromolecular antiproteases. J. Immunol. 126, 1569–1574 (1981). This work suggests that certain transgenes, such as one encoding α1 antitrypsin, may have immunomodulatory properties that can influence the outcome of gene transfer by blocking capsid-specific cytotoxic T lymphocytes (see in association with reference 102).
Matzinger, P. The danger model: a renewed sense of self. Science 296, 301–305 (2002).
Velazquez, V. M., Bowen, D. G. & Walker, C. M. Silencing of T lymphocytes by antigen-driven programmed death in recombinant adeno-associated virus vector-mediated gene therapy. Blood 113, 538–545 (2009).
Wright, J. F. Manufacturing and characterizing AAV-based vectors for use in clinical studies. Gene Ther. 15, 840–848 (2008).
Lock, M. et al. Characterization of a recombinant adeno-associated virus type 2 reference standard material. Hum. Gene Ther. 21, 1273–1285 (2010).
Urnov, F. D., Rebar, E. J., Holmes, M. C., Zhang, H. S. & Gregory, P. D. Genome editing with engineered zinc finger nucleases. Nature Rev. Genet. 11, 636–646 (2010).
Kang, E. M. et al. Retrovirus gene therapy for X-linked chronic granulomatous disease can achieve stable long-term correction of oxidase activity in peripheral blood neutrophils. Blood 115, 783–791 (2010).
European Society of Gene Therapy. One of three successfully treated CGD patients in a Swiss-German gene therapy trial died due to his underlying disease: a position statement from the European Society of Gene Therapy (ESGT). J. Gene Med. 8, 1435 (2006).
Hacein-Bey-Abina, S. et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 118, 3132–3142 (2008).
Hacein-Bey-Abina, S. et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302, 415–419 (2003).
Grimm, D. et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 441, 537–541 (2006).
Ross, C. J. et al. Correction of feline lipoprotein lipase deficiency with adeno-associated virus serotype 1-mediated gene transfer of the lipoprotein lipase S447X beneficial mutation. Hum. Gene Ther. 17, 487–499 (2006).
Walter, J., You, Q., Hagstrom, J. N., Sands, M. & High, K. A. Successful expression of human factor IX following repeat administration of adenoviral vector in mice. Proc. Natl Acad. Sci. USA 93, 3056–3061 (1996).
Favre, D. et al. Immediate and long-term safety of recombinant adeno-associated virus injection into the nonhuman primate muscle. Mol. Ther. 4, 559–566 (2001).
Gene therapy and the germline. Nature Med. 5, 245 (1999).
Favaro, P. et al. Host and vector-dependent effects on the risk of germline transmission of AAV vectors. Mol. Ther. 17, 1022–1030 (2009).
Schuettrumpf, J. et al. Inadvertent germline transmission of AAV2 vector: findings in a rabbit model correlate with those in a human clinical trial. Mol. Ther. 13, 1064–1073 (2006).
Srivastava, A., Lusby, E. W. & Berns, K. I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J. Virol. 45, 555–564 (1983).
Dong, J. Y., Fan, P. D. & Frizzell, R. A. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum. Gene Ther. 7, 2101–2112 (1996).
Matsushita, T. et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 5, 938–945 (1998).
Gao, G. P. et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl Acad. Sci. USA 99, 11854–11859 (2002). This article describes the isolation of novel AAV serotypes from monkeys that have high tropism for hepatocytes. One of the serotypes described in this manuscript is AAV8.
Nakai, H. et al. AAV serotype 2 vectors preferentially integrate into active genes in mice. Nature Genet. 34, 297–302 (2003).
Miller, D. G., Rutledge, E. A. & Russell, D. W. Chromosomal effects of adeno-associated virus vector integration. Nature Genet. 30, 147–148 (2002).
Wright, J. F., Wellman, J. & High, K. A. Manufacturing and regulatory strategies for clinical AAV2-hRPE65. Curr. Gene Ther. 10, 341–349 (2010).
Powell, J. S. et al. Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood 102, 2038–2045 (2003).
Xu, L. et al. Absence of a desmopressin response after therapeutic expression of factor VIII in hemophilia A dogs with liver-directed neonatal gene therapy. Proc. Natl Acad. Sci. USA 102, 6080–6085 (2005).
Cavazzana-Calvo, M. et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature 467, 318–322 (2010).
Driskell, R. R. & Engelhardt, J. F. Current status of gene therapy for inherited lung diseases. Ann. Rev. Physiol. 65, 585–512 (2003).
Chuah, M. K., Collen, D. & VandenDriessche, T. Clinical gene transfer studies for hemophilia A. Semin. Thromb. Hemost. 30, 249–256 (2004).
Brunetti-Pierri, N. et al. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol. Ther. 17, 327–333 (2009).
Jiang, H. et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol. Ther. 14, 452–455 (2006).
Le, M. et al. Therapeutic levels of functional human factor X in rats after retroviral-mediated hepatic gene therapy. Blood 89, 1254–1259 (1997).
Muzyczka, N. & Berns, K. I. in Parvoviridae: The Viruses and their Replication (eds Knipe, D. M. & Howley, P. M.) 2437–2477 (Lippincott, Williams and Wilkins, Philadelphia, 2001).
Yan, Z. et al. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J. Virol. 76, 2043–2053 (2002).
Finn, J. D. et al. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol. Ther. 18, 135–142 (2010).
Thomas, C. E., Storm, T. A., Huang, Z. & Kay, M. A. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 78, 3110–3122 (2004). This work highlights important differences between the AAV2 and AAV8 serotypes in terms of the rate of uncoating of viral genomes after the vectors enter a cell.
Aitken, M. L. et al. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 12, 1907–1916 (2001).
Moss, R. B. et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum. Gene Ther. 18, 726–732 (2007).
Wagner, J. A. et al. Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 109, 266–274 (1999).
Wagner, J. A. et al. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum. Gene Ther. 13, 1349–1359 (2002).
Wagner, J. A. et al. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet 351, 1702–1703 (1998).
Jaski, B. E. et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J. Card. Fail. 15, 171–181 (2009).
Frank, K. M. et al. Investigation of the cause of death in a gene-therapy trial. N. Engl. J. Med. 361, 161–169 (2009).
Mease, P. J. et al. Local delivery of a recombinant adenoassociated vector containing a tumour necrosis factor α antagonist gene in inflammatory arthritis: a phase 1 dose-escalation safety and tolerability study. Ann. Rheum. Dis. 68, 1247–1254 (2009).
Mease, P. J. et al. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase 1/2 study. J. Rheumatol. 37, 692–703 (2010).
Cardone, M. et al. Correction of Hunter syndrome in the MPSII mouse model by AAV2/8-mediated gene delivery. Hum. Mol. Genet. 15, 1225–1236 (2006).
McEachern, K. A. et al. AAV8-mediated expression of glucocerebrosidase ameliorates the storage pathology in the visceral organs of a mouse model of Gaucher disease. J. Gene Med. 8, 719–729 (2006).
Seppen, J. et al. Adeno-associated virus vector serotypes mediate sustained correction of bilirubin UDP glucuronosyltransferase deficiency in rats. Mol. Ther. 13, 1085–1092 (2006).
Koeberl, D. D. et al. Early, sustained efficacy of adeno-associated virus vector-mediated gene therapy in glycogen storage disease type Ia. Gene Ther. 13, 1281–1289 (2006).
Park, E. S., Oh, H. J., Kruger, W. D., Jung, S. C. & Lee, J. S. Recombinant adeno-associated virus mediated gene transfer in a mouse model for homocystinuria. Exp. Mol. Med. 38, 652–661 (2006).
Scallan, C. D. et al. Sustained phenotypic correction of canine hemophilia A using an adeno-associated viral vector. Blood 102, 2031–2037 (2003).
Daly, T. M., Ohlemiller, K. K., Roberts, M. S., Vogler, C. A. & Sands, M. S. Prevention of systemic clinical disease in MPS VII mice following AAV-mediated neonatal gene transfer. Gene Ther. 8, 1291–1298 (2001).
Watson, G. et al. Intrathecal administration of AAV vectors for the treatment of lysosomal storage in the brains of MPS I mice. Gene Ther. 13, 917–925 (2006).
Gao, J. et al. Hepatocyte-targeted HFE and TFR2 control hepcidin expression in mice. Blood 115, 3374–3381 (2011).
Conlon, T. J. et al. Efficient hepatic delivery and expression from a recombinant adeno-associated virus 8 pseudotyped α1-antitrypsin vector. Mol. Ther. 12, 867–875 (2005).
Ding, Z. et al. Correction of murine PKU following AAV-mediated intramuscular expression of a complete phenylalanine hydroxylating system. Mol. Ther. 16, 673–681 (2008).
Jung, S. C. et al. Adeno-associated viral vector-mediated gene transfer results in long-term enzymatic and functional correction in multiple organs of Fabry mice. Proc. Natl Acad. Sci. USA 98, 2676–2681 (2001).
Lebherz, C. et al. Gene therapy with novel adeno-associated virus vectors substantially diminishes atherosclerosis in a murine model of familial hypercholesterolemia. J. Gene Med. 6, 663–672 (2004).
Chandler, R. J. et al. Adeno-associated virus serotype 8 (AAV8) Gene transfer rescues a neonatal lethal murine model of propionic acidemia. Hum. Gene Ther. 16 Feb 2011 (doi:10.1089/hum.2010.164).
Acknowledgements
The authors would like to acknowledge the support of the Center for Cellular and Molecular Therapeutics at The Children's Hospital of Philadelphia, the Howard Hughes Medical Institute, and the US National Institutes of Health (HL64190 and HL078810). The authors are also grateful to collaborators and colleagues who provided access to unpublished data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Federico Mingozzi consulted for companies developing adeno-associated virus (AAV) vector therapeutics. He also consulted for companies that are not involved in AAV gene transfer. Federico Mingozzi is an inventor of a patent licensed to Amsterdam Molecular Therapeutics.
Katherine A. High is a member of the Scientific Advisory Board of Bluebird Bio, Cambridge, Massachusetts, USA. She is also a consultant for: Genzyme, Cambridge, Massachusetts, USA; Tacere Therapeutics, San Jose, California, USA; Forrest Research Laboratories, New York, USA; and Shire PLC.
Related links
Related links
DATABASES
Gene Therapy Clinical Trials Worldwide (provided by the Journal of Gene Medicine)
FURTHER INFORMATION
Glossary
- Tropism
-
Term used in virology to define the affinity of a virus for a particular host tissue. The tropism is mostly determined by the expression of specific molecules at the surface of a tissue to which the virus binds.
- Immunoprivileged
-
Term used to refer to tissues or organs (such as the eye or the brain) where immune responses are less pronounced than in other tissues or body compartments.
- Duchenne muscular dystrophy
-
An X-linked inherited disorder caused by mutations in the dystrophin gene associated with rapidly worsening muscle weakness, which leads to death in early adulthood.
- Batten's disease
-
A family of fatal neurodegenerative disorders caused by enzyme deficiencies associated with the pathological accumulation of substrates leading to neuronal cell toxicity.
- Haemarthrosis
-
The narrow definition of haemarthrosis is blood within the joint space. This occurs commonly in the bleeding disorder haemophilia and is associated with inflammation, increased chance of recurrent bleeding and gradual loss of joint function.
- Lysosomal storage disorders
-
A family of inherited disorders associated with loss of function of lysosomal enzymes with consequent pathological accumulation of substrates. The symptoms include neurodegeneration, liver disease and bone abnormalities.
- Serotype
-
In virology the term refers to the reactivity of a virus to certain antiviral antibodies. In vectorology, the term identifies different viral vector capsids, which might be isolated in nature or genetically engineered.
- Stereotactic injection
-
A delivery method used in neurosurgery to locate points within the brain using a three-dimensional coordinates system.
Rights and permissions
About this article
Cite this article
Mingozzi, F., High, K. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet 12, 341–355 (2011). https://doi.org/10.1038/nrg2988
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg2988
This article is cited by
-
Brain-targeted delivery of neuroprotective survival gene minimizing hematopoietic cell contamination: implications for Parkinson’s disease treatment
Journal of Translational Medicine (2024)
-
Acoustically targeted noninvasive gene therapy in large brain volumes
Gene Therapy (2024)
-
Sildenafil increases AAV9 transduction after a systemic administration and enhances AAV9-dystrophin therapeutic effect in mdx mice
Gene Therapy (2024)
-
Biophysical characterization of adeno-associated virus capsid through the viral transduction life cycle
Journal of Genetic Engineering and Biotechnology (2023)
-
Rational immunosilencing of a promiscuous T-cell epitope in the capsid of an adeno-associated virus
Nature Biomedical Engineering (2023)