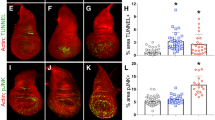
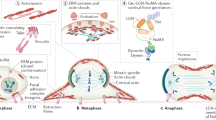
Key Points
-
Adult tissues are maintained by the proliferation of tissue stem cells that constitute only a small fraction of the total cell mass. The same applies to some tumours: a small number of cancer stem cells (CSCs) are able to sustain long-term tumour growth.
-
Being immortal, mitotically competent and located in tissues in which tumours appear, stem cells are likely candidates to initiate tumor growth. Consistently, CSCs that are isolated from different tumours have been shown to bear surface markers that are unique to the normal stem cells from the tissues in which the tumours arose.
-
Stem cells can undergo self-renewing asymmetric cell division to produce two unequal daughters; one enters a programme of differentiation, whereas the other retains stem-cell identity.
-
Using Drosophila melanogaster neuroblasts and male germline stem cells (mGSCs) as model stem cells, recent studies have revealed that mutation in any of several genes that are involved in this asymmetric division leads to overgrowth.
-
Proper spindle alignment is key for the correct implementation of the unequal fate of the stem-cell daughters. In both neuroblasts and mGSCs, the two centrosomes are unequal. One is a constitutively active microtubule-organizing centre (MTOC), which remains apical and stays in the stem cell after division; the other is active only during mitosis, when it is localized basally and inherited by the differentiating cell.
-
Consistent with the key role of spindle orientation during stem-cell asymmetric cell division, some mutations that impair spindle orientation in neuroblasts and mGSCs result in overgrowth.
-
Implantation of fly tumours in adult hosts provides a rigorous test to distinguish between hyperplastic, benign and malignant neoplastic growth. It also provides a means to age D. melanogaster tumours over extended periods of time, allowing for complex traits like metastasis and genome instability to appear, thus affording a more realistic model of tumour progression.
-
On the basis of recent data, a new hypothesis is put forward whereby centrosome abnormalities could have a role in tumour development — not by causing aneuploidy, as proposed by the classical Boveri's hypothesis, but through failed cell-fate determination during stem-cell asymmetric division.
-
Some of the issues that remain open for further investigation are: the actual mechanism by which misdetermined cell fate results in overgrowth; how complex traits like metastasis and genome instability appear in these tumours, and the contribution of genome instability to tumour progression; the role of CSCs in fly tumours; the molecular mechanisms that control spindle alignment in stem cells; other possible aspects of functional asymmetry in stem cells; and the extent to which these conclusions apply to tumours in vertebrates.
Abstract
Recent genetic studies in flies have added further support to an increasing body of evidence that suggests that stem cells might be the cell-of-origin of certain tumours. Malfunction of the mechanisms that control the division of stem cells and the developmental fate of the two resulting daughters could be one of the initial events that steers cells into malignant transformation. These studies suggest a role for controlled spindle orientation in suppressing stem-cell overgrowth. In parallel, the machinery that drives asymmetry in stem cells has been further characterized, identifying new components and uncovering the unique, highly sophisticated behaviour of centrosomes in these cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kay, H. E. How many cell-generations? Lancet 15, 418–419 (1965).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer and cancer stem cells. Nature 414, 105–111 (2001).
Al-Hajj, M. & Clarke, M. F. Self-renewal and solid tumor stem cells. Oncogene 23, 7274–7282 (2004).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003).
Dontu, G., Al-Hajj, M., Abdallah, W. M., Clarke, M. F. & Wicha, M. S. Stem cells in normal breast development and breast cancer. Cell Prolif. 36, S59–S72 (2003).
Kondo, T., Setoguchi, T. & Taga, T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc. Natl Acad. Sci. USA 101, 781–786 (2004).
Lapidot, T. et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367, 645–648 (1994).
Singh, S. K. et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 63, 5821–5828 (2003).
Rubin, G. M. et al. Comparative genomics of the eukaryotes. Science 287, 2204–2215 (2000).
Jaekel, R. & Klein, T. The Drosophila Notch inhibitor and tumor suppressor gene lethal (2) giant discs encodes a conserved regulator of endosomal trafficking. Dev. Cell 11, 655–669 (2006).
Yu, F. et al. A mouse homologue of Drosophila pins can asymmetrically localize and substitute for pins function in Drosophila neuroblasts. J. Cell Sci. 116, 887–896 (2003).
Sancho, E., Batlle, E. & Clevers, H. Signaling pathways in intestinal development and cancer. Annu. Rev. Cell Dev. Biol. 20, 695–723 (2004).
Krivtsov, A. V. et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL–AF9. Nature 442, 818–822 (2006).
Sell, S. & Pierce, G. B. Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers. Lab. Invest. 70, 6–22 (1994).
Potter, V. R. Phenotypic diversity in experimental hepatomas: the concept of partially blocked ontogeny. The 10th Walter Hubert Lecture. Br. J. Cancer 38, 1–23 (1978).
Zajicek, G. Streaming organism. Med. Hypotheses 45, 403–407 (1995).
Alison, M. R. & Lovell, M. J. Liver cancer: the role of stem cells. Cell Prolif. 38, 407–421 (2005).
Bonnet, D. & Dick, J. E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Med. 3, 730–737 (1997).
Al-Hajj, M., Becker, M. W., Wicha, M., Weissman, I. & Clarke, M. F. Therapeutic implications of cancer stem cells. Curr. Opin. Genet. Dev. 14, 43–47 (2004).
Dirks, P. B. Cancer: stem cells and brain tumours. Nature 444, 687–688 (2006).
Huntly, B. J. P. & Gilliland, D. G. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nature Rev. Cancer 5, 311–321 (2005).
Pilkington, G. J. Cancer stem cells in the mammalian central nervous system. Cell Prolif. 38, 423–433 (2005).
Wang, J. C. Y. & Dick, J. E. Cancer stem cells: Lessons from leukemia. Trends Cell Biol. 15, 494–501 (2005).
Wicha, M. S., Liu, S. & Dontu, G. Cancer stem cells: an old idea – a paradigm shift. Cancer Res. 66, 1883–1890 (2006).
Piccirillo, S. G. et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444, 761–765 (2006).
Bao, S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006).
Micchelli, C. A. & Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 (2006).
Ohlstein, B. & Spradling, A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470–474 (2006).
Ohlstein, B. & Spradling, A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science 315, 988–992 (2007).
Betschinger, J. & Knoblich, J. A. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 14, R674–R685 (2004).
Doe, C. Q. & Bowerman, B. Asymmetric cell division: Fly neuroblast meets worm zygote. Curr. Opin. Cell Biol. 13, 68–75 (2001).
Jan, Y. N. & Jan, L. Y. Asymmetric cell division. Nature 392, 775–778 (1998).
Matsuzaki, F. Asymmetric cell division in neurogenesis. Tanpakushitsu kakusan koso 50, 595–600 (2005). (in Japanese).
Roegiers, F. & Jan, Y. N. Asymmetric cell division. Curr. Opin. Cell Biol. 16, 195–205 (2004).
Wodarz, A. Molecular control of cell polarity and asymmetric cell division in Drosophila neuroblasts. Curr. Opin. Cell Biol. 17, 475–481 (2005).
Wodarz, A. & Huttner, W. B. Asymmetric cell division during neurogenesis in Drosophila and vertebrates. Mech. Dev. 120, 1297–1309 (2003).
Yu, F., Kuo, C. T. & Jan, Y. N. Drosophila neuroblast asymmetric cell division: recent advances and implications for stem cell biology. Neuron 51, 13–20 (2006).
Li, L. & Xie, T. Stem cell niche: structure and function. Annu. Rev. Cell Dev. Biol. 21, 605–631 (2005).
Yamashita, Y. M., Fuller, M. T. & Jones, D. L. Signaling in stem cell niches: lessons from the Drosophila germline. J. Cell Sci. 118, 665–672 (2005).
Wong, M. D., Jin, Z. & Xie, T. Molecular mechanisms of germline stem cell regulation. Annu. Rev. Genet. 39, 173–195 (2005).
Brumby, A. M. & Richardson, H. E. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 22, 5769–5779 (2003).
Gateff, E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science 200, 1448–1459 (1978).
Gateff, E., Loffler, T. & Wismar, J. A temperature-sensitive brain tumor suppressor mutation of Drosophila melanogaster: developmental studies and molecular localization of the gene. Mech. Dev. 41, 15–31 (1993).
Bilder, D. Epithelial polarity and proliferation control: links from the Drosophila neoplastictumor suppressors. Genes Dev. 18, 1909–1925 (2004).
Wodarz, A. Tumor suppressors: linking cell polarity and growth control. Curr. Biol. 10, R624–R626 (2000).
Bilder, D., Li, M. & Perrimon, N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113–116 (2000).
Humbert, P., Russell, S. & Richardson, H. Dlg, scribble and Lgl in cell polarity, cell proliferation and cancer. BioEssays 25, 542–553 (2003).
Pagliarini, R. A. & Xu, T. A Genetic screen in Drosophila for metastatic behavior. Science 302, 1227–1231 (2003).
Uhlirova, M. & Bohmann, D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 25, 5294–5304 (2006).
Albertson, R. & Doe, C. Q. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nature Cell Biol. 5, 166–170 (2003).
Ohshiro, T., Yagami, T., Zhang, C. & Matsuzaki, F. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature 408, 593–596 (2000).
Faubert, A., Lessard, J. & Sauvageau, G. Are genetic determinants of asymmetric stem cell division active in hematopoietic stem cells? Oncogene 23, 7247–7255 (2004).
Caussinus, E. & Gonzalez, C. Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nature Genet. 37, 1125–1129 (2005). This report showed that failed asymmetric division of D. melanogaster neuroblasts can trigger malignant growth.
Betschinger, J., Mechtler, K. & Knoblich, J. A. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 124, 1241–1253 (2006).
Lee, C. Y., Wilkinson, B. D., Siegrist, S. E., Wharton, R. P. & Doe, C. Q. Brat is a Miranda cargo protein that promotes neuronal differentiation and inhibits neuroblast self-renewal. Dev. Cell 10, 441–449 (2006). References 54 and 55 demonstrated that BRAT, initially known as a neoplastic brain tumour suppressor, is one of the cell-fate determinants that is asymmetrically segregated during neuroblast division.
Choksi, S. P. et al. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Dev. Cell 11, 775–789 (2006).
Bello, B., Reichert, H. & Hirth, F. The brain tumor gene negatively regulates neural progenitor cell proliferation in the larval central brain of Drosophila. Development 133, 2639–2648 (2006).
Lee, C. Y., Robinson, K. J. & Doe, C. Q. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature 439, 594–598 (2006).
Gateff, E. & Schneiderman, H. A. Neoplasms in mutant and cultured wild-type tissues of Drosophila. Natl Cancer Inst. Monogr. 31, 365–397 (1969).
Beaucher, M. et al. Drosophila brain tumor metastases express both neuronal and glial cell type markers. Dev. Biol. 301, 287–297 (2007).
Beaucher, M., Hersperger, E., Page-McCaw, A. & Shearn, A. Metastatic ability of Drosophila tumors depends on MMP activity. Dev. Biol. 303, 625–634 (2007).
Lengauer, C., Kinzler, K. W. & Vogelstein, B. Genetic instabilities in human cancers. Nature 396, 643–649 (1998).
Remensberger, P. Cytologic and histologic studies in cell stems of Drosophila melanogaster cultured in vivo. Chromosoma 23, 386–417 (1968).
Heisenberg, M. Mutants of brain structure and function: what is the significance of the mushroom bodies for behavior? Basic Life Sci. 16, 373–390 (1980).
Bowman, S. K., Neumüller, R. A., Novatchkova, M., Du, Q. & Knoblich, J. A. The Drosophila NuMA homolog Mud regulates spindle orientation in asymmetric cell division. Dev. Cell 10, 731–742 (2006).
Siller, K. H., Cabernard, C. & Doe, C. Q. The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nature Cell Biol. 8, 594–600 (2006).
Izumi, Y., Ohta, N., Hisata, K., Raabe, T. & Matsuzaki, F. Drosophila Pins-binding protein Mud regulates spindle-polarity coupling and centrosome organization. Nature Cell Biol. 8, 586–593 (2006). References 65–67 identified MUD as a key mediator of spindle alignment during neuroblast asymmetric division and showed a link between faulty spindle alingment and overgrowth.
Prokop, A. & Technau, G. M. Normal function of the mushroom body defect gene of Drosophila is required for the regulation of the number and proliferation of neuroblasts. Dev. Biol. 161, 321–337 (1994).
Sukhai, M. A. et al. Myeloid leukemia with promyelocytic features in transgenic mice expressing hCG–NuMA–RARα Oncogene 23, 665–678 (2004).
Wells, R. A., Catzavelos, C. & Kamel-Reid, S. Fusion of retinoic acid receptor α to NuMA, the nuclear mitotic apparatus protein, by a variant translocation in acute promyelocytic leukaemia. Nature Genet. 17, 109–113 (1997).
Kammerer, S. et al. Association of the NuMA region on chromosome 11q13 with breast cancer susceptibility. Proc. Natl Acad. Sci. USA 102, 2004–2009 (2005).
Lee, C. Y. et al. Drosophila Aurora-A-kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation. Genes Dev. 20, 3464–3474 (2006).
Wang, H. et al. Aurora-A-acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev. 20, 3453–3463 (2006). References 72 and 73 found a direct role of the protein kinase AUR in cell-fate determination during neuroblast division. They also provided evidence linking abnormal fate specification with overgrowth
McCartney, B. M. et al. Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J. Cell Biol. 146, 1303–1318 (1999).
Yu, X., Waltzer, L. & Bienz, M. A new Drosophila APC homologue associated with adhesive zones of epithelial cells. Nature Cell Biol. 1, 144–151 (1999).
Yamashita, Y. M., Jones, D. L. & Fuller, M. T. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301, 1547–1550 (2003). This paper showed that spindle orientation in mGSCs requires APC2 and functional centrosomes, and that misoriented spindles result in increased numbers of stem cells.
Megraw, T. L., Li, K., Kao, L. R. & Kaufman, T. C. The centrosomin protein is required for centrosome assembly and function during cleavage in Drosophila. Development 126, 2829–2839 (1999).
Vaizel-Ohayon, D. & Schejter, E. D. Mutations in centrosomin reveal requirements for centrosomal function during early Drosophila embryogenesis. Curr. Biol. 9, 889–898 (1999).
Kiger, A. A., Jones, D. L., Schulz, C., Rogers, M. B. & Fuller, M. T. Stem cell self-renewal specified by JAK–STAT activation in response to a support cell cue. Science 294, 2542–2545 (2001).
Tulina, N. & Matunis, E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK–STAT signaling. Science 294, 2546–2549 (2001).
Kaltschmidt, J. A., Davidson, C. M., Brown, N. H. & Brand, A. H. Rotation and asymmetry of the mitotic spindle direct asymmetric cell division in the developing central nervous system. Nature Cell Biol. 2, 7–12 (2000).
Savoian, M. S. & Rieder, C. L. Mitosis in primary cultures of Drosophila melanogaster larval neuroblasts. J. Cell Sci. 115, 3061–3072 (2002).
Kawamura, K. Studies on cytokinesis in neuroblasts of the grasshopper, Chortophaga viridifasciata (De Geer). I. Formation and behavior of the mitotic apparatus. Exp. Cell Res. 21, 1–18 (1960).
Kaltschmidt, J. A. & Brand, A. H. Asymmetric cell division: microtubule dynamics and spindle asymmetry. J. Cell Sci. 115, 2257–2264 (2002).
Yamashita, Y. M., Mahowald, A. P., Perlin, J. R. & Fuller, M. T. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science 315, 518–521 (2007).
Rebollo, E. et al. Functionally unequal centrosomes drive spindle orientation in asymmetrically dividing Drosophila neural stem cells. Dev. Cell 12, 467–474 (2007). References 85 and 86 showed that centrosome replication in mGSCs and neuroblasts produces two centrosomes that are functionally unequal, thus contributing to anchoring the spindle in the correct orientation during stem-cell division.
Izumi, Y., Ohta, N., Itoh-Furuya, A., Fuse, N. & Matsuzaki, F. Differential functions of G protein and Baz–aPKC signaling pathways in Drosophila neuroblast asymmetric division. J. Cell Biol. 164, 729–738 (2004).
Rujano, M. A. et al. Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes. PLoS Biol. 4, e417 (2006). This article documented that, during stem-cell division, damaged proteins are asymmetrically seggregated into the shorter-lived daughter cell.
Hardy, P. A. & Zacharias, H. Reappraisal of the Hansemann–Boveri hypothesis on the origin of tumors. Cell Biol. Int. 29, 983–992 (2005).
Duensing, S. & Munger, K. Centrosome abnormalities, genomic instability and carcinogenic progression. Biochim. Biophys. Acta 1471, M81–M88 (2001).
Lingle, W. L. & Salisbury, J. L. The role of the centrosome in the development of malignant tumors. Curr. Top. Dev. Biol. 49, 313–329 (2000).
Naruganahalli, K. S., Lakshmanan, M., Dastidar, S. G. & Ray, A. Therapeutic potential of Aurora kinase inhibitors in cancer. Curr. Opin. Investig. Drugs 7, 1044–1051 (2006).
Takai, N., Hamanaka, R., Yoshimatsu, J. & Miyakawa, I. Polo-like kinases (Plks) and cancer. Oncogene 24, 287–291 (2005).
Nigg, E. A. Centrosome aberrations: cause or consequence of cancer progression? Nature Rev. Cancer 2, 815–825 (2002).
Ring, D., Hubble, R. & Kirschner, M. Mitosis in a cell with multiple centrioles. J. Cell Biol. 94, 549–556 (1982).
Giansanti, M. G., Gatti, M. & Bonaccorsi, S. The role of centrosomes and astral microtubules during asymmetric division of Drosophila neuroblasts. Development 128, 1137–1145 (2001).
Woodhouse, E., Hersperger, E. & Shearn, A. Growth, metastasis, and invasiveness of Drosophila tumors caused by mutations in specific tumor suppressor genes. Dev. Genes Evol. 207, 542–550 (1998).
Cairns, J. Mutation selection and the natural history of cancer. Nature 255, 197–200 (1975).
Shinin, V., Gayraud-Morel, B., Gomès, D. & Tajbakhsh, S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nature Cell Biol. 8, 677–682 (2006).
Gateff, E. A. & Schneiderman, H. A. Developmental studies of a new mutation of Drosophila melanogaster: lethal(2)giant larvae4. Am. Zool. 7, 760 (1967).
Gateff, E. & Schneiderman, H. A. Developmental capacities of benign and malignant neoplasms of Drosophila. Rouxs Arch. Dev. Biol. 176, 23–65 (1974).
Beadle, G. W. & Ephrussi, B. Development of eye colors in Drosophila: transplantation experiments with suppressor of vermilion. Proc. Natl Acad. Sci. USA 22, 536–540 (1936).
Hadorn, E. The Genetics and Biology of Drosophila 2C, 557–558 (1978).
Ikeshima-Kataoka, H., Skeath, J. B., Nabeshima, Y. I., Doe, C. Q. & Matsuzaki, F. Miranda directs Prospero to a daughter cell during Drosophila asymmetric divisions. Nature 390, 625–629 (1997).
Knoblich, J. A., Jan, L. Y. & Jan, Y. N. Asymmetric segregation of Numb and Prospero during cell division. Nature 377, 624–627 (1995).
Lu, B., Rothenberg, M., Jan, L. Y. & Jan, Y. N. Partner of numb colocalizes with Numb during mitosis and directs Numb asymmetric localization in Drosophila neural and muscle progenitors. Cell 95, 225–235 (1998).
Rhyu, M. S., Jan, L. Y. & Jan, Y. N. Asymmetric distribution of Numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 76, 477–491 (1994).
Shen, C. P., Jan, L. Y. & Jan, Y. N. Miranda is required for the asymmetric localization of Prospero during mitosis in Drosophila. Cell 90, 449–458 (1997).
Kuchinke, U., Grawe, F. & Knust, E. Control of spindle orientation in Drosophila by the Par-3-related PDZ-domain protein Bazooka. Curr. Biol. 8, 1357–1365 (1998).
Petronczki, M. & Knoblich, J. A. DmPAR-6 directs epithelial polarity and asymetric cell division of neuroblasts in Drosophila. Nature Cell Biol. 3, 43–49 (2001).
Wodarz, A., Ramrath, A., Grimm, A. & Knust, E. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J. Cell Biol. 150, 1361–1374 (2000).
Kraut, R. & Campos-Ortega, J. A. inscuteable, a neural precursor gene of Drosophila, encodes a candidate for a cytoskeleton adaptor protein. Dev. Biol. 174, 65–81 (1996).
Schaefer, M., Petronczki, M., Dorner, D., Forte, M. & Knoblich, J. A. Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell 107, 183–194 (2001).
Yu, F., Cai, Y., Kaushik, R., Yang, X. & Chia, W. Distinct roles of GαI and Gβ13F subunits of the heterotrimeric G protein complex in the mediation of Drosophila neuroblast asymmetric divisions. J. Cell Biol. 162, 623–633 (2003).
Yu, F., Morin, X., Cai, Y., Yang, X. & Chia, W. Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in Inscuteable apical localization. Cell 100, 399–409 (2000).
Yu, F. et al. Locomotion defects, together with Pins, regulates heterotrimeric G-protein signaling during Drosophila neuroblast asymmetric divisions. Genes Dev. 19, 1341–1353 (2005).
Fuse, N., Hisata, K., Katzen, A. L. & Matsuzaki, F. Heterotrimeric G proteins regulate daughter cell size asymmetry in Drosophila neuroblast divisions. Curr. Biol. 13, 947–954 (2003).
Wang, H. & Chia, W. Drosophila neural progenitor polarity and asymmetric division. Biol. Cell 97, 63–74 (2005).
Hampoelz, B., Hoeller, O., Bowman, S. K., Dunican, D. & Knoblich, J. A. Drosophila RIC-8 is essential for plasma-membrane localization of heterotrimeric G proteins. Nature Cell Biol. 7, 1099–1105 (2005).
Peng, C. Y., Manning, L., Albertson, R. & Doe, C. Q. The tumour-suppresor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature 408, 596–600 (2000).
Betschinger, J., Mechtler, K. & Knoblich, J. A. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 422, 326–330 (2003).
Rusan, N. M. & Peifer, M. A role for a novel centrosome cycle in asymmetric cell divison. J. Cell Biol. 177, 13–20 (2007).
Acknowledgements
The help received from M. Calleja, E. Castellanos, E. Aguilar, L. Mendizabal and A. Janic, the comments on a first draft from P. Dominguez and J. Janushke, and the input provided by all members of my laboratory are very much appreciated. I am also indebted to A. Martinez-Arias and M. González-Gaitán who provided extensive criticism that contributed significantly to shaping this Review. Research in my laboratory is supported by grants from the European Union, the Spanish Government and the Generalitat de Catalunya.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Spindle alignment
-
The fixed orientation of the cell-division spindle with respect to certain cell-polarity cues.
- Centrosomes
-
The main microtubule-organizing centre of most animal cells, typically composed of two orthogonally arranged centrioles that are surrounded by a meshwork of pericentriolar material.
- Boveri's hypothesis
-
States that numerical and/or structural centrosome abnormalities that cause chromosome missegregation might be the origin of cancer.
- Progenitor cells
-
Partially committed, undifferentiated cells that retain certain plasticity (or multipotency) and limited self-renewal capability.
- Neoplastic transformation
-
A process by which normal cells start to grow out of control.
- Micrometastases
-
Small colonies of cancerous cells originally derived from cells that migrated away from the primary, much larger tumour.
- Transdetermination
-
An occasional process in which cells of cultured fly imaginal discs switch to a different developmental fate.
- Imaginal discs
-
Disc-shaped structures in holometabolous insects such as Drosophila melanogaster that are set aside during embryogenesis, grow during the larval stages and differentiate to form the adult epidermal structures during metamorphosis.
- Mushroom body
-
A structure of the adult Drosophila melanogaster brain that is required for olfactory learning and memory.
- Spindle asters
-
Star-shaped clusters of microtubules that radiate from the centrosomes during cell division.
- Pericentriolar material
-
The meshwork that surrounds the centrioles and contains the microtubule-nucleating activity of the centrosome.
Rights and permissions
About this article
Cite this article
Gonzalez, C. Spindle orientation, asymmetric division and tumour suppression in Drosophila stem cells. Nat Rev Genet 8, 462–472 (2007). https://doi.org/10.1038/nrg2103
Issue Date:
DOI: https://doi.org/10.1038/nrg2103
This article is cited by
-
Depletion of kinesin motor KIF20A to target cell fate control suppresses medulloblastoma tumour growth
Communications Biology (2021)
-
The last-born daughter cell contributes to division orientation of Drosophila larval neuroblasts
Nature Communications (2018)
-
Dynamics of Bone Marrow VSELs and HSCs in Response to Treatment with Gonadotropin and Steroid Hormones, during Pregnancy and Evidence to Support Their Asymmetric/Symmetric Cell Divisions
Stem Cell Reviews and Reports (2018)
-
Concomitant binding of Afadin to LGN and F-actin directs planar spindle orientation
Nature Structural & Molecular Biology (2016)
-
The final cut: cell polarity meets cytokinesis at the bud neck in S. cerevisiae
Cellular and Molecular Life Sciences (2016)