Key Points
-
DNA-damage-response mechanisms encompass pathways of DNA-repair and signal-transduction processes that regulate cell-cycle-checkpoint arrest and/or apoptosis. The signal-transduction processes can regulate at least some aspects of DNA repair.
-
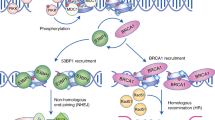
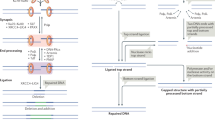
There are two main double-strand break (DSB) repair pathways — homologous recombination and non-homologous end-joining (NHEJ).
-
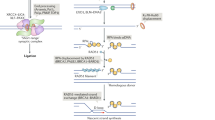
The main signal-transduction pathway that responds to a DSB is regulated by ataxia telangiectasia mutated (ATM), the protein that is defective in the hereditary disorder ataxia telangiectasia. Ataxia telangiectasia and RAD3-related (ATR) probably also has a supporting role in the response to DSBs.
-
Coupling occurs between ATM-dependent signalling and DSB repair. ATM regulates Artemis-dependent processing of a subset of DNA ends, which is required before rejoining by NHEJ can occur.
-
Several human congenital disorders with defects in damage-response mechanisms to DSBs have been described. These include, LIG4 syndrome, severe combined immunodeficiency with sensitivity to ionizing radiation, ataxia telangiectasia, ataxia telangiectasia-like disorder, Nijmegen breakage syndrome, ATR-Seckel syndrome and Fanconi anaemia, as exemplified by FANCD1 (Fanconi anaemia complementation group D1) deficiency.
-
These disorders are associated with a pleiotropic range of clinical features that demonstrate the important role that the damage-response processes have during development. Clinical features include immunodeficiency, development delay and microcephaly, cancer development and premature ageing.
-
The damage-response mechanisms function to maintain genomic stability in somatic cells. During V(D)J recombination and class-switch recombination, the damage-response proteins function to enhance genomic diversity at defined regions. Therefore, immunodeficiency is observed in some of the human DSB-repair-defective disorders.
-
Assays for diagnosis of damage-response disorders are now available and strategies for palliative treatment are beginning to emerge.
Abstract
The efficient repair of DNA double-strand breaks is crucial in safeguarding the genomic integrity of organisms. Responses to double-strand breaks include complex signal-transduction, cell-cycle-checkpoint and repair pathways. Defects in these pathways lead to several human disorders with pleiotropic clinical features. Dissection of the molecular basis that underlies the diverse clinical features is enhancing our understanding of the damage-response mechanisms and their role in development, and might ultimately facilitate treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Cadet, J., Douki, T., Gasparutto, D. & Ravanat, J. L. Oxidative damage to DNA: formation, measurement and biochemical features. Mutat. Res. 531, 5–23 (2003).
Petersen, S. et al. AID is required to initiate Nbs1/γ-H2AX focus formation and mutations at sites of class switching. Nature 414, 660–665 (2001).
d'Adda di Fagagna, F. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003). This study shows that uncapped telomeres trigger activation of the DNA-damage-response pathway, which leads to senescence that is caused by cell-cycle-checkpoint arrest.
d'Adda di Fagagna, F., Teo, S. H. & Jackson, S. P. Functional links between telomeres and proteins of the DNA-damage response. Genes Dev. 18, 1781–1799 (2004).
Jackson, S. Sensing and repairing DNA double strand breaks. Carcinogenesis 23, 687–696 (2002).
Riballo, E. et al. A pathway of double strand break rejoining dependent upon ATM, Artemis and proteins locating to γ-H2AX foci. Mol. Cell 16, 715–724 (2004). The authors show that ATM and other proteins are required for Artemis-dependent processing of a fraction of DNA ends that are induced by ionizing radiation. This further role for ATM partially explains AT radiosensitivity and provides a connection between non-homologous end-joining and the ATM-dependent signalling pathway.
Bolderson, E., Scorah, J., Helleday, T., Smythe, C. & Meuth, M. ATM is required for the cellular response to thymidine induced replication fork stress. Hum. Mol. Genet. 13, 2937–2945 (2004).
Wang, H., Powell, S. N., Iliakis, G. & Wang, Y. ATR affecting cell radiosensitivity is dependent on homologous recombination repair but independent of nonhomologous end joining. Cancer Res. 64, 7139–7143 (2004).
Petrini, J. H. & Stracker, T. H. The cellular response to DNA double-strand breaks: defining the sensors and mediators. Trends Cell Biol. 13, 458–462 (2003).
Falck, J., Coates, J. & Jackson, S. P. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 434, 605–611 (2005).
Stiff, T. et al. ATM and DNA-PK function redundantly to phosphorylate H2AX following exposure to ionizing radiation. Cancer Res. 64, 2390–2396 (2004).
Fernandez-Capetillo, O., Lee, A., Nussenzweig, M. & Nussenzweig, A. H2AX: the histone guardian of the genome. DNA Repair (Amst.) 3, 959–967 (2004).
Paull, T. T. et al. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10, 886–895 (2000). The authors show that H2AFX is a crucial component of the DNA-damage response that facilitates the recruitment of other damage-response proteins to the site of DNA damage.
Huyen, Y. et al. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature 432, 406–411 (2004).
Mochan, T. A., Venere, M., DiTullio, R. A. Jr & Halazonetis, T. D. 53BP1, an activator of ATM in response to DNA damage. DNA Repair (Amst.) 3, 945–952 (2004).
Fernandez-Capetillo, O. et al. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nature Cell Biol. 4, 993–997 (2002).
Lavin, M. F. The Mre11 complex and ATM: a two-way functional interaction in recognising and signaling DNA double strand breaks. DNA Repair (Amst.) 3, 1515–1520 (2004).
Lukas, J., Lukas, C. & Bartek, J. Mammalian cell cycle checkpoints: signalling pathways and their organization in space and time. DNA Repair (Amst.) 3, 997–1007 (2004).
Costanzo, V. et al. An ATR- and Cdc7-dependent DNA damage checkpoint that inhibits initiation of DNA replication. Mol. Cell 11, 203–213 (2003).
O'Driscoll, M., Ruiz-Perez, V. L., Woods, C. G., Jeggo, P. A. & Goodship, J. A. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nature Genet. 33, 497–501 (2003).
Tibbetts, R. S. et al. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 13, 153–157 (1999).
Cliby, W. A. et al. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 17, 159–169 (1998).
Alderton, G. K. et al. Seckel syndrome exhibits cellular features demonstrating defects in the ATR signalling pathway. Hum. Mol. Genet. 13, 3127–3138 (2004).
Cha, R. S. & Kleckner, N. ATR homolog Mec1 promotes fork progression, thus averting breaks in replication slow zones. Science 297, 602–606 (2002). One of several studies that collectively provide evidence that ATR functions not only in response to DNA damage, but also during unperturbed cell growth. This study shows that Mec1, the S. cerevisiae homologue of ATR, is activated during replication of certain DNA regions.
Lucca, C. et al. Checkpoint-mediated control of replisome-fork association and signalling in response to replication pausing. Oncogene 23, 1206–1213 (2004).
Jeggo, P. A. in Eukaryotic DNA Damage Surveillance and Repair (ed. Caldecott, K. W.) 146–158 (Landes Bioscience/Eurekah.com and Kluwer Academic/Plenum, Texas, 2004).
Ma, Y., Lu, H., Schwarz, K. & Lieber, M. R. Repair of double-strand DNA breaks by the human nonhomologous DNA end joining pathway: the iterative processing model. Cell Cycle 4, 1193–1200 (2005).
Espejel, S. et al. Mammalian Ku86 mediates chromosomal fusions and apoptosis caused by critically short telomeres. EMBO J. 21, 2207–2219 (2002).
Espejel, S. et al. Shorter telomeres, accelerated ageing and increased lymphoma in DNA-PKcs-deficient mice. EMBO Rep. 5, 503–509 (2004).
West, S. C. Molecular views of recombination proteins and their control. Nature Rev. Mol. Cell Biol. 4, 435–445 (2003).
Thompson, L. H. & Limoli, C. L. in Eukaryotic DNA Damage Surveillance and Repair (ed. Caldecott, K. W.) 107–145 (Landes Bioscience/Eurekah.Com and Kluwer Academic/Plenum, Texas, 2004).
Pellegrini, L. et al. Insights into DNA recombination from the structure of a RAD51–BRCA2 complex. Nature 420, 287–293 (2002). The authors provide an elegant example of how structural studies can complement biological studies. The structure of a complex between a BRC repeat of BRCA2 and RAD51 shows that the BRC repeat mimics a motif in RAD51 and facilitates oligomerization of RAD51 monomers.
Casper, A. M., Nghiem, P., Arlt, M. F. & Glover, T. W. ATR regulates fragile site stability. Cell 111, 779–789 (2002). A mammalian study that, together with reference 24, provides evidence for ATR function during unperturbed cell growth. The authors show that ATR is involved in maintaining the stability of fragile sites, which are regions of DNA that are hot spots for chromosomal rearrangements.
Thompson, L. H. & Schild, D. Homologous recombinational repair of DNA ensures mammalian chromosome stability. Mutat. Res. 477, 131–153 (2001).
O'Driscoll, M. et al. DNA ligase IV mutations identified in patients exhibiting development delay and immunodeficiency. Mol. Cell 8, 1175–1185 (2001).
Lobrich, M. & Jeggo, P. A. Harmonising the response to DSBs: a new string in the ATM bow. DNA Repair (Amst.) 4, 749–759 (2005).
Kuhne, M. et al. A double-strand break repair defect in ATM-deficient cells contributes to radiosensitivity. Cancer Res. 64, 500–508 (2004).
Moshous, D. et al. Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell 105, 177–186 (2001).
Ma, Y., Pannicke, U., Schwarz, K. & Lieber, M. R. Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell 108, 781–794 (2002). Together with reference 38, this paper shows that the protein Artemis cleaves the hairpin end that is generated during V(D)J recombination, and that its deficiency can lead to human severe combined immunodefiency associated with radiosensitivity.
Celeste, A. et al. Genomic instability in mice lacking histone H2AX. Science 296, 922–927 (2002).
Ward, I. M., Minn, K., van Deursen, J. & Chen, J. p53 Binding protein 53BP1 is required for DNA damage responses and tumor suppression in mice. Mol. Cell. Biol. 23, 2556–2563 (2003).
Zhang, X. et al. Artemis is a phosphorylation target of ATM and ATR and is involved in the G2/M DNA damage checkpoint response. Mol. Cell. Biol. 24, 9207–9220 (2004).
Varon, R. et al. Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell 93, 467–476 (1998).
Venkitaraman, A. R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108, 171–182 (2002).
Howlett, N. G. et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 297, 606–609 (2002).
Wang, X. & D'Andrea, A. D. The interplay of Fanconi anemia proteins in the DNA damage response. DNA Repair (Amst.) 3, 1063–1069 (2004).
Thompson, L. H. Unraveling the Fanconi anemia–DNA repair connection. Nature Genet. 37, 921–922 (2005).
Levitus, M. et al. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nature Genet. 37, 934–935 (2005).
Levran, O. et al. The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nature Genet. 37, 931–933 (2005).
Nakanishi, K. et al. Interaction of FANCD2 and NBS1 in the DNA damage response. Nature Cell Biol. 4, 913–920 (2002).
Taniguchi, T. et al. Convergence of the Fanconi anemia and ataxia telangiectasia signaling pathways. Cell 109, 459–472 (2002).
Saar, K. et al. The gene for the ataxia-telangiectasia variant, Nijmegen breakage syndrome, maps to a 1 cM interval on chromosome 8q21. Am. J. Hum. Genet. 60, 605–610 (1997).
Wegner, R. D., Chrzanowska, K., Sperling, K. & Stumm, M. in Primary Immunodeficiency Diseases, a Molecular and Genetic Approach (eds Ochs, H. D., Smith, C. I. E. & Puck, J. M.) 324–334 (Oxford Univ. Press, New York, 1999).
Stewart, G. S. et al. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell 99, 577–587 (1999).
Stiff, T. et al. Nbs1 is required for ATR-dependent phosphorylation events. EMBO J. 24, 199–208 (2005).
Gennery, A. R. et al. The clinical and biological overlap between Nijmegen breakage syndrome and Fanconi anaemia. Clin. Immunol. 113, 214–219 (2004).
Pichierri, P. & Rosselli, F. The DNA crosslink-induced S-phase checkpoint depends on ATR–CHK1 and ATR–NBS1–FANCD2 pathways. EMBO J. 23, 1178–1187 (2004).
Andreassen, P. R., D'Andrea, A. D. & Taniguchi, T. ATR couples FANCD2 monoubiquitination to the DNA damage response. Genes Dev. 18, 1958–1963 (2004).
O'Driscoll, M. & Jeggo, P. Immunological disorders and DNA Repair. Mutat. Res. 509, 109–126 (2002).
Taccioli, G. E. et al. Impairment of V(D)J recombination in double-strand break repair mutants. Science 260, 207–210 (1993).
Nussenzweig, A. et al. Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature 382, 551–555 (1996).
Riballo, E. et al. Identification of a defect in DNA ligase IV in a radiosensitive leukaemia patient. Curr. Biol. 19, 699–702 (1999).
Girard, P. -M., Kysela, B., Harer, C. J., Doherty, A. J. & Jeggo, P. A. Analysis of DNA ligase IV mutations found in LIG4 syndrome patients: the impact of two linked polymorphisms. Hum. Mol. Genet. 13, 2369–2376 (2004).
Moshous, D. et al. Partial T and B lymphocyte immunodeficiency and predisposition to lymphoma in patients with hypomorphic mutations in Artemis. J. Clin. Invest. 111, 381–387 (2003).
Ege, M. et al. Omenn syndrome due to ARTEMIS mutations. Blood 105, 4179–4186 (2005).
Villa, A. et al. Partial V(D)J recombination activity leads to Omenn syndrome. Cell 93, 885–896 (1998).
Reina-San-Martin, B., Chen, H. T., Nussenzweig, A. & Nussenzweig, M. C. ATM is required for efficient recombination between immunoglobulin switch regions. J. Exp. Med. 200, 1103–1110 (2004). Together with reference 68, this paper demonstrates that ATM is required for class-switch recombination, providing a basis for the immunodeficiency phenotype that is observed in patients with AT.
Lumsden, J. M. et al. Immunoglobulin class switch recombination is impaired in Atm-deficient mice. J. Exp. Med. 200, 1111–1121 (2004).
Lahdesmaki, A., Taylor, A. M., Chrzanowska, K. H. & Pan-Hammarstrom, Q. Delineation of the role of the Mre11 complex in class switch recombination. J. Biol. Chem. 279, 16479–16487 (2004).
Pan-Hammarstrom, Q. et al. ATM is not required in somatic hypermutation of VH, but is involved in the introduction of mutations in the switch μ region. J. Immunol. 170, 3707–3716 (2003).
Reina-San-Martin, B., Nussenzweig, M. C., Nussenzweig, A. & Difilippantonio, S. Genomic instability, endoreduplication, and diminished Ig class-switch recombination in B cells lacking Nbs1. Proc. Natl Acad. Sci. USA 102, 1590–1595 (2005).
Taylor, A. M. R., Metcalfe, J. A., Thick, J. & Mak, Y. F. Leukemia and lymphoma in ataxia telangiectasia. Blood 87, 423–438 (1996).
Liyanage, M. et al. Abnormal rearrangement within the α/δ T-cell receptor locus in lymphomas from Atm-deficient mice. Blood 96, 1940–1946 (2000).
Abner, C. W. & McKinnon, P. J. The DNA double-strand break response in the nervous system. DNA Repair (Amst.) 3, 1141–1147 (2004).
Lee, Y., Chong, M. J. & McKinnon, P. J. Ataxia telangiectasia mutated-dependent apoptosis after genotoxic stress in the developing nervous system is determined by cellular differentiation status. J. Neurosci. 21, 6687–6693 (2001).
Herzog, K. H., Chong, M. J., Kapsetaki, M., Morgan, J. I. & McKinnon, P. J. Requirement for Atm in ionizing radiation-induced cell death in the developing central nervous system. Science 280, 1089–1091 (1998).
Karanjawala, Z. E., Murphy, N., Hinton, D. R., Hsieh, C. L. & Lieber, M. R. Oxygen metabolism causes chromosome breaks and is associated with the neuronal apoptosis observed in DNA double-strand break repair mutants. Curr. Biol. 12, 397–402 (2002).
El-Khamisy, S. F. et al. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature 434, 108–113 (2005).
Clements, P. M. et al. The ataxia-oculomotor apraxia 1 gene product has a role distinct from ATM and interacts with the DNA strand break repair proteins XRCC1 and XRCC4. DNA Repair (Amst.) 3, 1493–1502 (2004).
Bailey, S. M., Cornforth, M. N., Ullrich, R. L. & Goodwin, E. H. Dysfunctional mammalian telomeres join with DNA double-strand breaks. DNA Repair (Amst) 3, 349–57 (2004).
Hayani, A., Suarez, C. R., Molnar, Z., LeBeau, M. & Godwin, J. Acute myeloid leukaemia in a patient with Seckel syndrome. J. Med. Genet. 31, 148–149 (1994).
Butler, M. G., Hall, B. D., Maclean, R. N. & Lozzio, C. B. Do some patients with Seckel syndrome have hematological problems and/or chromosome breakage? Am. J. Med. Genet. 27, 645–649 (1987).
Chanan-Khan, A. et al. T-cell clonality and myelodysplasia without chromosomal fragility in a patient with features of Seckel syndrome. Haematologica 88, ECR14 (2003).
Metcalfe, J. A. et al. Accelerated telomere shortening in ataxia telangiectasia. Nature Genet. 13, 350–353 (1996).
Wong, K. K. et al. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421, 643–648 (2003).
Pandita, T. K. ATM function and telomere stability. Oncogene 21, 611–618 (2002).
Gatei, M. et al. Ataxia-telangiectasia: chronic activation of damage-responsive functions is reduced by α-lipoic acid. Oncogene 20, 289–294 (2001).
Jaco, I., Munoz, P. & Blasco, M. A. Role of human Ku86 in telomere length maintenance and telomere capping. Cancer Res. 64, 7271–7278 (2004).
Gennery, A. R. et al. Bone marrow transplantation for Nijmegan breakage syndrome. J. Pediatr. Hematol. Oncol. 27, 239 (2005).
Bakhshi, A. et al. A DNA insertion/deletion necessitates an aberrant RNA splice accounting for a μ heavy chain disease protein. Proc. Natl Acad. Sci. USA 83, 2689–2693 (1986).
Rogers, P. B., Plowman, P. N., Harris, S. J. & Arlett, C. F. Four radiation hypersensitivity cases and their implications for clinical radiotherapy. Radiother. Oncol. 57, 143–154 (2000).
Zhou, D. et al. D609 inhibits ionizing radiation-induced oxidative damage by acting as a potent antioxidant. J. Pharmacol. Exp. Ther. 298, 103–109 (2001).
Lai, C. H. et al. Correction of ATM gene function by aminoglycoside-induced read-through of premature termination codons. Proc. Natl Acad. Sci. USA 101, 15676–15681 (2004).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Bryant, H. E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005). Together with reference 94, this paper demonstrates how our knowledge of the interplay between damage-response pathways can potentially be exploited in the clinic to treat hereditary breast cancers that are caused by mutations in BRCA1 and BRCA2.
Kawabe, T. G2 checkpoint abrogators as anticancer drugs. Mol. Cancer Ther. 3, 513–519 (2004).
Tenzer, A. & Pruschy, M. Potentiation of DNA-damage-induced cytotoxicity by G2 checkpoint abrogators. Curr. Med. Chem. Anti-Canc. Agents 3, 35–46 (2003).
Helleday, T. Pathways for mitotic homologous recombination in mammalian cells. Mutat. Res. 532, 103–115 (2003).
Richardson, C., Horikoshi, N. & Pandita, T. K. The role of the DNA double-strand break response network in meiosis. DNA Repair (Amst.) 3, 1149–1164 (2004).
Taccioli, G. E. et al. Ku80: product of the XRCC5 gene. Role in DNA repair and V(D)J recombination. Science 265, 1442–1445 (1994). The first identification of a gene that functions in the rejoining step of V(D)J recombination. The paper also represents a milestone in radiation biology, describing the first identification of a gene that has a major role in the response to radiation in mammalian cells. This finding led to the subsequent discovery of further genes in the non-homologous end-joining pathway (see reference 103).
Kemp, L. M., Sedgwick, S. G. & Jeggo, P. A. X-ray sensitive mutants of Chinese hamster ovary cells defective in double-strand break rejoining. Mutat. Res. 132, 189–196 (1984).
Bosma, M. J. & Carroll, A. M. The Scid mouse mutant: definition, characterization, and potential uses. Annu. Rev. Immunol. 9, 323–350 (1991).
Blunt, T. et al. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell 80, 813–823 (1995).
Boulton, S. J. & Jackson, S. P. Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J. 17, 1819–1828 (1998).
Thacker, J. & Zdzienicka, M. Z. The mammalian XRCC genes: their roles in DNA repair and genetic stability. DNA Repair (Amst.) 2, 655–72 (2003).
Thacker, J. & Zdzienicka, M. Z. The XRCC genes: expanding roles in DNA double-strand break repair. DNA Repair (Amst.) 3, 1081–1090 (2004).
Deans, B., Griffin, C. S., Maconochie, M. & Thacker, J. Xrcc2 is required for genetic stability, embryonic neurogenesis and viability in mice. EMBO J. 19, 6675–6685 (2000).
Takata, M. et al. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 17, 5497–5508 (1998).
Savitsky, K. et al. A single ataxia telangiectasia gene with a product similar to PI 3-kinase. Science 268, 1749–1753 (1995). The authors identify the gene that is defective in AT as a phosphatidylinositol 3-kinase. This important milestone paved the way for subsequent molecular analysis of the basis underlying AT.
Usui, T., Ogawa, H. & Petrini, J. H. A DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol. Cell 7, 1255–1266 (2001).
Zhou, B. B. & Elledge, S. J. The DNA damage response: putting checkpoints in perspective. Nature 408, 433–439 (2000).
Caspari, T. & Carr, A. M. DNA structure checkpoint pathways in Schizosaccharomyces pombe. Biochimie 81, 173–181 (1999).
Costanzo, V., Robertson, K. & Gautier, J. Xenopus cell-free extracts to study the DNA damage response. Methods Mol. Biol. 280, 213–27 (2004).
Zhao, H. & Piwnica-Worms, H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol. 21, 4129–4139 (2002).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- V(D)J recombination
-
A specialized form of recombination that assembles the genes that encode lymphocyte antigen receptors from variable (V), diversity (D) and joining (J) gene segments. DNA double-strand breaks are introduced between the V, D and J segments and DNA-repair proteins then join the segments together.
- Class-switch recombination
-
A recombination process that occurs on V(D)J recombined products, generating distinct immunoglobin isotypes.
- Checkpoint arrest
-
Arrest at a specific stage of the cell cycle wherein the cell ensures that the previous stage has been completed normally or detects the presence of DNA damage.
- Resection
-
A process in which a nuclease processes DNA double-strand breaks to create single-stranded overhangs.
- Lymphoblastoid cell lines
-
Cell lines that are obtained from B lymphocytes — a fraction of white cells from blood that can be grown indefinitely in the laboratory after special treatment of the cells with Epstein–Barr virus.
- Holliday junction
-
The cross-over point at which the two complementary strands that are derived from separate DNA molecules form during homologous recombination.
- Pulsed-field gel electrophoresis
-
A technique for separating DNA of high molecular weight by gel electrophoresis in which the current is applied in pulses in alternating directions.
- Confluency
-
A stage of cell growth during which cell division and DNA replication is inhibited by contact inhibition between cells in culture.
- Monoubiquitylation
-
Post-translational modification in which a single ubiquitin moiety is added to lysine residues. Polyubiquitylation, which generates ubiquitin chains, targets proteins for proteasome-mediated degradation.
- Somatic hypermutation
-
A process that occurs after immunoglobulin gene rearrangement, whereby the base sequences of part of the immunoglobulin variable regions are mutated more frequently than the rest of the genome. This sequence variation is subject to a selection process in the immune system that favours those cells that express immunoglobulins with the highest affinity for the antigen.
- Pancytopenia
-
Reduced numbers of all types of blood cell.
- Occipitofrontal circumference
-
The circumference of the head as measured from the forehead to the back of the head (occipital bone).
- Ataxia
-
A failure or irregularity of muscular coordination.
- Purkinje neurons
-
A class of large ganglion neurons in the cerebellum.
- Kyphosis
-
A posterior or backwards orientated curvature of the thoracic region of the spine.
- Psoriasis and squamous patches
-
A generally localized dry scaling or appearance of plate-like layers on the skin.
Rights and permissions
About this article
Cite this article
O'Driscoll, M., Jeggo, P. The role of double-strand break repair — insights from human genetics. Nat Rev Genet 7, 45–54 (2006). https://doi.org/10.1038/nrg1746
Issue Date:
DOI: https://doi.org/10.1038/nrg1746
This article is cited by
-
A critical review on therapeutic approaches of CRISPR-Cas9 in diabetes mellitus
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Cyclophilin A contributes to shikonin-induced glioma cell necroptosis and promotion of chromatinolysis
Scientific Reports (2022)
-
A deep insight into CRISPR/Cas9 application in CAR-T cell-based tumor immunotherapies
Stem Cell Research & Therapy (2021)
-
FK228 sensitizes radioresistant small cell lung cancer cells to radiation
Clinical Epigenetics (2021)
-
Transcriptome analysis of the circadian clock gene BMAL1 deletion with opposite carcinogenic effects
Functional & Integrative Genomics (2021)