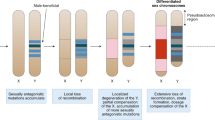
Abstract
In mammals, sex is determined by differential inheritance of a pair of dimorphic chromosomes: the gene-rich X chromosome and the gene-poor Y chromosome. To balance the unequal X-chromosome dosage between the XX female and XY male, mammals have adopted a unique form of dosage compensation in which one of the two X chromosomes is inactivated in the female. This mechanism involves a complex, highly coordinated sequence of events and is a very different strategy from those used by other organisms, such as the fruitfly and the worm. Why did mammals choose an inactivation mechanism when other, perhaps simpler, means could have been used? Recent data offer a compelling link between ontogeny and phylogeny. Here, we propose that X-chromosome inactivation and imprinting might have evolved from an ancient genome-defence mechanism that silences unpaired DNA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Graves, J. A. Mammals that break the rules: genetics of marsupials and monotremes. Annu. Rev. Genet. 30, 233–260 (1996).
Lahn, B. T. & Page, D. C. Functional coherence of the human Y chromosome. Science 278, 675–680 (1997).
Lahn, B. T., Pearson, N. M. & Jegalian, K. The human Y chromosome, in the light of evolution. Nature Rev. Genet. 2, 207–216 (2001).
Vallender, E. J. & Lahn, B. T. How mammalian sex chromosomes acquired their peculiar gene content. Bioessays 26, 159–169 (2004).
Spatz, A., Borg, C. & Feunteun, J. X-chromosome genetics and human cancer. Nature Rev. Cancer 4, 617–629 (2004).
Lyon, M. F. Gene action in the X chromosome of the mouse (Mus musculus). Nature 190, 372–373 (1961).
Avner, P. & Heard, E. X-chromosome inactivation: counting, choice and initiation. Nature Rev. Genet. 2, 59–67 (2001).
Brockdorff, N. et al. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell 71, 515–526 (1992).
Brown, C. J. et al. The human XIST gene: analysis of a 17-kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 71, 527–542 (1992).
Lee, J. T., Davidow, L. S. & Warshawsky, D. Tsix, a gene antisense to Xist at the X-inactivation centre. Nature Genet. 21, 400–404 (1999).
Ogawa, Y. & Lee, J. T. Xite, X-inactivation intergenic transcription elements that regulate the probability of choice. Mol. Cell 11, 731–743 (2003).
Lee, J. T. Disruption of imprinted X inactivation by parent-of-origin effects at Tsix. Cell 103, 17–27 (2000).
Sado, T., Wang, Z., Sasaki, H. & Li, E. Regulation of imprinted X-chromosome inactivation in mice by Tsix. Development 128, 1275–1286 (2001).
Clemson, C. M., McNeil, J. A., Willard, H. F. & Lawrence, J. B. XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J. Cell Biol. 132, 259–275 (1996).
Penny, G. D., Kay, G. F., Sheardown, S. A., Rastan, S. & Brockdorff, N. Requirement for Xist in X chromosome inactivation. Nature 379, 131–137 (1996).
Sharman, G. B. Late DNA replication in the paternally derived X chromosome of female kangaroos. Nature 230, 231–232 (1971).
Takagi, N. & Sasaki, M. Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature 256, 640–642 (1975).
Xue, F. et al. Aberrant patterns of X chromosome inactivation in bovine clones. Nature Genet. 31, 216–220 (2002).
Migeon, B. R. & Do, T. T. In search of non-random X inactivation: studies of fetal membranes heterozygous for glucose-6-phosphate dehydrogenase. Am. J. Hum. Genet. 31, 581–585 (1979).
Ropers, H. H., Wolff, G. & Hitzeroth, H. W. Preferential X inactivation in human placenta membranes: is the paternal X inactive in early embryonic development of female mammals? Hum. Genet. 43, 265–273 (1978).
Zeng, S. M. & Yankowitz, J. X-inactivation patterns in human embryonic and extra-embryonic tissues. Placenta 24, 270–275 (2003).
Cooper, D. W. Directed genetic change model for X chromosome inactivation in eutherian mammals. Nature 230, 292–294 (1971).
Marahrens, Y., Panning, B., Dausman, J., Strauss, W. & Jaenisch, R. Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev. 11, 156–166 (1997).
Cline, T. W. & Meyer, B. J. Vive la difference: males vs females in flies vs worms. Annu. Rev. Genet. 30, 637–702 (1996).
Park, Y. & Kuroda, M. I. Epigenetic aspects of X-chromosome dosage compensation. Science 293, 1083–1085 (2001).
Takagi, N. Imprinted X-chromosome inactivation: enlightenment from embryos in vivo. Semin. Cell Dev. Biol. 14, 319–329 (2003).
Goto, T. & Monk, M. Regulation of X-chromosome inactivation in development in mice and humans. Microbiol. Mol. Biol. Rev. 62, 362–378 (1998).
Heard, E., Clerc, P. & Avner, P. X-chromosome inactivation in mammals. Annu. Rev. Genet. 31, 571–610 (1997).
Lyon, M. F. X-chromosome inactivation and developmental patterns in mammals. Biol. Rev. Camb. Philos. Soc. 47, 1–35 (1972).
Adler, D. A., West, J. D. & Chapman, V. M. Expression of α-galactosidase in preimplantation mouse embryos. Nature 267, 838–839 (1977).
Gardner, R. L. & Lyon, M. F. X chromosome inactivation studied by injection of a single cell into the mouse blastocyst. Nature 231, 385–386 (1971).
Epstein, C. J., Smith, S., Travis, B. & Tucker, G. Both X chromosomes function before visible X-chromosome inactivation in female mouse embryos. Nature 274, 500–503 (1978).
Kratzer, P. G. & Gartler, S. M. HGPRT activity changes in preimplantation mouse embryos. Nature 274, 503–504 (1978).
Monk, M. & Harper, M. I. Sequential X chromosome inactivation coupled with cellular differentiation in early mouse embryos. Nature 281, 311–313 (1979).
Mukherjee, A. B. Cell cycle analysis and X-chromosome inactivation in the developing mouse. Proc. Natl Acad. Sci. USA 73, 1608–1611 (1976).
Sugawara, O., Takagi, N. & Sasaki, M. Correlation between X-chromosome inactivation and cell differentiation in female preimplantation mouse embryos. Cytogenet. Cell Genet. 39, 210–219 (1985).
Latham, K. E. & Rambhatla, L. Expression of X-linked genes in androgenetic, gynogenetic, and normal mouse preimplantation embryos. Dev. Genet. 17, 212–222 (1995).
Matsui, J., Goto, Y. & Takagi, N. Control of Xist expression for imprinted and random X chromosome inactivation in mice. Hum. Mol. Genet. 10, 1393–1401 (2001).
Sheardown, S. A. et al. Stabilization of Xist RNA mediates initiation of X chromosome inactivation. Cell 91, 99–107 (1997).
Nesterova, T. B., Barton, S. C., Surani, M. A. & Brockdorff, N. Loss of Xist imprinting in diploid parthenogenetic preimplantation embryos. Dev. Biol. 235, 343–350 (2001).
Krietsch, W. K. et al. The expression of X-linked phosphoglycerate kinase in the early mouse embryo. Differentiation 23, 141–144 (1982).
Pravtcheva, D. D., Adra, C. N. & Ruddle, F. H. Timing of paternal Pgk-1 expression in embryos of transgenic mice. Development 111, 1109–1120 (1991).
Huynh, K. D. & Lee, J. T. Inheritance of a pre-inactivated paternal X chromosome in early mouse embryos. Nature 426, 857–862 (2003).
Okamoto, I., Otte, A. P., Allis, C. D., Reinberg, D. & Heard, E. Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303, 644–649 (2004).
Brown, C. J. et al. Localization of the X inactivation centre on the human X chromosome in Xq13. Nature 349, 82–84 (1991).
Schultz, R. M. Regulation of zygotic gene activation in the mouse. Bioessays 15, 531–538 (1993).
Reik, W. & Walter, J. Genomic imprinting: parental influence on the genome. Nature Rev. Genet. 2, 21–32 (2001).
Lifschytz, E. & Lindsley, D. L. The role of X-chromosome inactivation during spermatogenesis. Proc. Natl Acad. Sci. USA 69, 182–186 (1972).
Lifschytz, E. & Lindsley, D. I. Sex chromosome activation during spermatogenesis. Genetics 78, 323–331 (1974).
Hoyer-Fender, S. Molecular aspects of XY body formation. Cytogenet. Genome Res. 103, 245–255 (2003).
McKee, B. D. & Handel, M. A. Sex chromosomes, recombination, and chromatin conformation. Chromosoma 102, 71–80 (1993).
Miklos, G. L. Sex-chromosome pairing and male fertility. Cytogenet. Cell Genet. 13, 558–577 (1974).
Lee, J. T. Sex chromosome inactivation: the importance of pairing. Curr. Biol. (in the press).
Hendriksen, P. J. et al. Postmeiotic transcription of X and Y chromosomal genes during spermatogenesis in the mouse. Dev. Biol. 170, 730–733 (1995).
Khalil, A. M., Boyar, F. Z. & Driscoll, D. J. Dynamic histone modifications mark sex chromosome inactivation and reactivation during mammalian spermatogenesis. Proc. Natl Acad. Sci. USA 101, 16583–16587 (2004).
McCarrey, J. R. et al. Differential transcription of Pgk genes during spermatogenesis in the mouse. Dev. Biol. 154, 160–168 (1992).
McCarrey, J. R., Dilworth, D. D. & Sharp, R. M. Semiquantitative analysis of X-linked gene expression during spermatogenesis in the mouse: ethidium-bromide staining of RT-PCR products. Genet. Anal. Tech. Appl. 9, 117–123 (1992).
McCarrey, J. R. et al. X-chromosome inactivation during spermatogenesis is regulated by an Xist/Tsix-independent mechanism in the mouse. Genesis 34, 257–266 (2002).
Singer-Sam, J., Robinson, M. O., Bellve, A. R., Simon, M. I. & Riggs, A. D. Measurement by quantitative PCR of changes in HPRT, PGK-1, PGK-2, APRT, MTase, and Zfy gene transcripts during mouse spermatogenesis. Nucleic Acids Res. 18, 1255–1259 (1990).
Hendriksen, P. J. et al. Testis-specific expression of a functional retroposon encoding glucose-6-phosphate dehydrogenase in the mouse. Genomics 41, 350–359 (1997).
Ashworth, A., Skene, B., Swift, S. & Lovell-Badge, R. Zfa is an expressed retroposon derived from an alternative transcript of the Zfx gene. EMBO J. 9, 1529–1534 (1990).
Bradley, J. et al. An X-to-autosome retrogene is required for spermatogenesis in mice. Nature Genet. 36, 872–876 (2004).
Dahl, H. H., Brown, R. M., Hutchison, W. M., Maragos, C. & Brown, G. K. A testis-specific form of the human pyruvate dehydrogenase E1-α subunit is coded for by an intronless gene on chromosome 4. Genomics 8, 225–232 (1990).
McCarrey, J. R. & Thomas, K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature 326, 501–505 (1987).
Emerson, J. J., Kaessmann, H., Betran, E. & Long, M. Extensive gene traffic on the mammalian X chromosome. Science 303, 537–540 (2004).
Wang, P. J. X chromosomes, retrogenes and their role in male reproduction. Trends Endocrinol. Metab. 15, 79–83 (2004).
Huynh, K. D. & Lee, J. T. Imprinted X inactivation in eutherians: a model of gametic execution and zygotic relaxation. Curr. Opin. Cell Biol. 13, 690–697 (2001).
Grutzner, F. et al. In the platypus a meiotic chain of ten sex chromosomes shares genes with the bird Z and mammal X chromosomes. Nature 432, 913–917 (2004).
Grutzner, F. & Graves, J. A. A platypus' eye view of the mammalian genome. Curr. Opin. Genet. Dev. 14, 642–649 (2004).
VandeBerg, J. L., Johnston, P. G., Cooper, D. W. & Robinson, E. S. X-chromosome inactivation and evolution in marsupials and other mammals. Isozymes Curr. Top. Biol. Med. Res. 9, 201–218 (1983).
Lyon, M. F. Imprinting and X-chromosome inactivation. Results Probl. Cell Differ. 25, 73–90 (1999).
Shiu, P. K., Raju, N. B., Zickler, D. & Metzenberg, R. L. Meiotic silencing by unpaired DNA. Cell 107, 905–916 (2001).
Bean, C. J., Schaner, C. E. & Kelly, W. G. Meiotic pairing and imprinted X chromatin assembly in Caenorhabditis elegans. Nature Genet. 36, 100–105 (2004).
Turner, J. M. et al. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr. Biol. 14, 2135–2142 (2004).
Turner, J. M. et al. Silencing of unsynapsed meiotic chromosomes in the mouse. Nature Genet. 37, 41–47 (2005).
Baarends, W. M. et al. Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol. Cell. Biol. 25, 1041–1053 (2005).
Lee, J. T. Molecular links between X-inactivation and autosomal imprinting: X-inactivation as a driving force for the evolution of imprinting? Curr. Biol. 13, R242–R254 (2003).
Turner, J. M. et al. Meiotic sex chromosome inactivation in male mice with targeted disruptions of Xist. J. Cell Sci. 115, 4097–4105 (2002).
Chadwick, B. P. & Willard, H. F. Multiple spatially distinct types of facultative heterochromatin on the human inactive X chromosome. Proc. Natl Acad. Sci. USA 101, 17450–17455 (2004).
Charlesworth, B. The evolution of sex chromosomes. Science 251, 1030–1033 (1991).
Charlesworth, B. The evolution of chromosomal sex determination and dosage compensation. Curr. Biol. 6, 149–162 (1996).
Kelly, W. G. et al. X-chromosome silencing in the germline of C. elegans. Development 129, 479–492 (2002).
Charlesworth, D. Plant sex determination and sex chromosomes. Heredity 88, 94–101 (2002).
Siroky, J., Castiglione, M. R. & Vyskot, B. DNA methylation patterns of Melandrium album chromosomes. Chromosome Res. 6, 441–446 (1998).
Vyskot, B., Siroky, J., Hladilova, R., Belyaev, N. D. & Turner, B. M. Euchromatic domains in plant chromosomes as revealed by H4 histone acetylation and early DNA replication. Genome 42, 343–350 (1999).
Lengerova, M., Moore, R. C., Grant, S. R. & Vyskot, B. The sex chromosomes of Silene latifolia revisited and revised. Genetics 165, 935–938 (2003).
McQueen, H. A., McBride, D., Miele, G., Bird, A. P. & Clinton, M. Dosage compensation in birds. Curr. Biol. 11, 253–257 (2001).
McCarrey, J. R. & Dilworth, D. D. Expression of Xist in mouse germ cells correlates with X-chromosome inactivation. Nature Genet. 2, 200–203 (1992).
Salido, E. C., Yen, P. H., Mohandas, T. K. & Shapiro, L. J. Expression of the X-inactivation-associated gene XIST during spermatogenesis. Nature Genet. 2, 196–199 (1992).
Richler, C., Soreq, H. & Wahrman, J. X inactivation in mammalian testis is correlated with inactive X-specific transcription. Nature Genet. 2, 192–195 (1992).
Singer-Sam, J., Chapman, V., LeBon, J. M. & Riggs, A. D. Parental imprinting studied by allele-specific primer extension after PCR: paternal X chromosome-linked genes are transcribed prior to preferential paternal X chromosome inactivation. Proc. Natl Acad. Sci. USA 89, 10469–10473 (1992).
Mak, W. et al. Reactivation of the paternal X chromosome in early mouse embryos. Science 303, 666–669 (2004).
Acknowledgements
We thank all the members of our laboratory for insightful discussion and R. Spencer, S. Namekawa and M. Anguera for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- ASYNAPSIS
-
Failure of chromosomes to pair during meiosis.
- BLASTOCYST
-
An early stage of mammalian embryonic development at which the first cell lineages become established.
- COT1 FISH
-
A technique to visualize nascent transcription that uses the Cot1 fraction of DNA that is rich in repetitive elements that often occur in introns and 3′ untranslated regions.
- EPIBLAST
-
An embryonic lineage that is derived from the inner cell mass of the blastocyst, which gives rise to the body of the fetus.
- EUTHERIANS
-
Mammals that give birth to live offspring (that is, they are viviparous) and possess an allantoic placenta — the allantois is the fetal membrane that facilitates nutrient and waste exchange between the fetus and the mother.
- FLUORESCENCE IN SITU HYBRIDIZATION
-
A technique in which a fluorescently labelled DNA probe is used to detect a particular chromosome or gene with the help of fluorescence microscopy.
- MEIOTIC CHECKPOINT
-
A surveillance mechanism specific to meiosis that ensures proper chromosome segregation.
- PACHYTENE
-
The third phase of prophase I in meiosis.
- THERIAN MAMMALS
-
A group of mammals that includes the eutherians and marsupials.
- TROPHECTODERM
-
The outer layer of the blastocyst-stage embryo; the precursor to the bulk of the embryonic part of the placenta.
Rights and permissions
About this article
Cite this article
Huynh, K., Lee, J. X-chromosome inactivation: a hypothesis linking ontogeny and phylogeny. Nat Rev Genet 6, 410–418 (2005). https://doi.org/10.1038/nrg1604
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg1604
This article is cited by
-
Sex at the interface: the origin and impact of sex differences in the developing human placenta
Biology of Sex Differences (2022)
-
Healthy cloned offspring derived from freeze-dried somatic cells
Nature Communications (2022)
-
Dynamic sex chromosome expression in Drosophila male germ cells
Nature Communications (2021)
-
Sex, sex chromosomes and gene expression
BMC Biology (2011)
-
Detection of nascent RNA, single-copy DNA and protein localization by immunoFISH in mouse germ cells and preimplantation embryos
Nature Protocols (2011)