Key Points
-
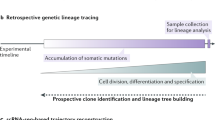
Methods for tracing lineage can be divided into two groups. Prospective methods trace lineage forwards from the application of an experimentally delivered marker, and retrospective methods trace lineage backwards, using endogenous marks that naturally accumulate in the genome.
-
Early methods using sparse retroviral labelling for prospective lineage tracing have given way to retroviral barcodes of essentially unlimited complexity, allowing the labelling and recovery of large populations of cells for lineage tracing experiments.
-
Genetic recombination with Cre-loxP or engineered transposon systems is a popular method for lineage tracing in genetically accessible model organisms. Recent work has demonstrated that CRISPR–Cas9 genome editing is a promising way to track and synthetically reconstruct cell lineage relationships in complex multicellular organisms, and may supplement or supplant older recombination-based systems in the future.
-
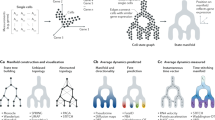
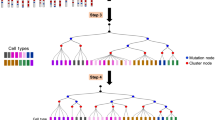
Recent advances in single-cell genome amplification and sequencing make it possible to harness naturally occurring somatic mutations to infer cell lineage information retrospectively. Somatic mutations of many classes, including long interspersed nuclear element 1 (L1; also known as LINE-1) retrotransposition events, copy-number variants, single-nucleotide variants and microsatellite length variants, are appropriate for lineage tracing.
-
Single-cell genome-sequencing experiments require genome amplification, and investigators must consider the frequencies and types of errors that are introduced by different amplification methods to select an approach that best balances signal and noise for the experiment at hand.
-
When designing a lineage tracing experiment, it is important to consider the strengths and weaknesses of either a prospective or a retrospective approach. Prospective approaches require genetic access to the cell population being labelled, but can often be higher throughput and less expensive than retrospective approaches. Retrospective approaches use marks that accumulate in the genome, making any purifiable population accessible to analysis, but can be low-throughput and expensive.
Abstract
Resolving lineage relationships between cells in an organism is a fundamental interest of developmental biology. Furthermore, investigating lineage can drive understanding of pathological states, including cancer, as well as understanding of developmental pathways that are amenable to manipulation by directed differentiation. Although lineage tracking through the injection of retroviral libraries has long been the state of the art, a recent explosion of methodological advances in exogenous labelling and single-cell sequencing have enabled lineage tracking at larger scales, in more detail, and in a wider range of species than was previously considered possible. In this Review, we discuss these techniques for cell lineage tracking, with attention both to those that trace lineage forwards from experimental labelling, and those that trace backwards across the life history of an organism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Deppe, U. et al. Cell lineages of the embryo of the nematode Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 75, 376–380 (1978).
Sulston, J. E., Schierenberg, E., White, J. G. & Thomson, J. N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64–119 (1983).
Mintz, B. Gene control of mammalian pigmentary differentiation. I. Clonal origin of melanocytes. Proc. Natl Acad. Sci. USA 58, 344–351 (1967).
Kelly, S. J. Studies of the developmental potential of 4- and 8-cell stage mouse blastomeres. J. Exp. Zool. 200, 365–376 (1977).
Le Douarin, N. M. & Teillet, M. A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J. Embryol. Exp. Morphol. 30, 31–48 (1973).
Rosenquist, G. C. The location of the pregut endoderm in the chick embryo at the primitive streak stage as determined by radioautographic mapping. Dev. Biol. 26, 323–335 (1971).
Johnson, M. H. & Ziomek, C. A. The foundation of two distinct cell lineages within the mouse morula. Cell 24, 71–80 (1981).
Lawson, K. A., Meneses, J. J. & Pedersen, R. A. Cell fate and cell lineage in the endoderm of the presomite mouse embryo, studied with an intracellular tracer. Dev. Biol. 115, 325–339 (1986).
Pedersen, R. A., Wu, K. & Bałakier, H. Origin of the inner cell mass in mouse embryos: cell lineage analysis by microinjection. Dev. Biol. 117, 581–595 (1986).
Clarke, J. D. & Tickle, C. Fate maps old and new. Nat. Cell Biol. 1, E103–E109 (1999).
Gerrits, A. et al. Cellular barcoding tool for clonal analysis in the hematopoietic system. Blood 115, 2610–2618 (2010).
Cai, D., Cohen, K. B., Luo, T., Lichtman, J. W. & Sanes, J. R. Improved tools for the Brainbow toolbox. Nat. Methods 10, 540–547 (2013).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Navin, N. et al. Tumour evolution inferred by single-cell sequencing. Nature 472, 90–94 (2011). The authors pioneered the sequencing of dividing cancer cells by single-nucleus sequencing (SNS) to construct the lineage of a breast tumour.
Wang, Y. et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature 512, 155–160 (2014).
Behjati, S. et al. Genome sequencing of normal cells reveals developmental lineages and mutational processes. Nature 513, 422–425 (2014). This study uses cell culture as an in vivo DNA-amplification method, sequencing clonal lines of mouse cells to identify lineages in adult tissues.
Lodato, M. A. et al. Somatic mutation in single human neurons tracks developmental and transcriptional history. Science 350, 94–98 (2015). The authors sequence normal human brain tissue and identify SNVs, which they use to place adult neurons in a lineage tree.
Beddington, R. An autoradiographic analysis of the potency of embryonic ectoderm in the 8th day postimplantation mouse embryo. Development 64, 87–104 (1981).
Serbedzija, G. N., Bronner-Fraser, M. & Fraser, S. E. A vital dye analysis of the timing and pathways of avian trunk neural crest cell migration. Development 106, 809–816 (1989).
Turner, D. L. & Cepko, C. L. A common progenitor for neurons and glia persists in rat retina late in development. Nature 328, 131–136 (1987).
Frank, E. & Sanes, J. R. Lineage of neurons and glia in chick dorsal root ganglia: analysis in vivo with a recombinant retrovirus. Development 111, 895–908 (1991).
Brown, K. N. et al. Clonal production and organization of inhibitory interneurons in the neocortex. Science 334, 480–486 (2011).
Noctor, S. C., Flint, A. C., Weissman, T. A., Dammerman, R. S. & Kriegstein, A. R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720 (2001).
Noctor, S. C., Martinez- Cerdeño, V., Ivic, L. & Kriegstein, A. R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136–144 (2004).
Gertz, C. C., Lui, J. H., LaMonica, B. E., Wang, X. & Kriegstein, A. R. Diverse behaviors of outer radial glia in developing ferret and human cortex. J. Neurosci. 34, 2559–2570 (2014).
Betizeau, M. et al. Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron 80, 442–457 (2013).
Walsh, C. A. & Cepko, C. L. Clonally related cortical cells show several migration patterns. Science 241, 1342–1345 (1988).
Walsh, C. A. & Cepko, C. L. Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science 255, 434–440 (1992).
Walsh, C. A. & Cepko, C. L. Clonal dispersion in proliferative layers of developing cerebral cortex. Nature 362, 632–635 (1993).
Reid, C. B., Liang, I. & Walsh, C. Systematic widespread clonal organization in cerebral cortex. Neuron 15, 299–310 (1995).
Golden, J. A., Fields-Berry, S. C. & Cepko, C. L. Construction and characterization of a highly complex retroviral library for lineage analysis. Proc. Natl Acad. Sci. USA 92, 5704–5708 (1995).
Fuentealba, L. C. et al. Embryonic origin of postnatal neural stem cells. Cell 161, 1644–1655 (2015).
Mayer, C. et al. Clonally related forebrain interneurons disperse broadly across both functional areas and structural boundaries. Neuron 87, 989–998 (2015).
Harwell, C. C. et al. Wide dispersion and diversity of clonally related inhibitory interneurons. Neuron 87, 999–1007 (2015).
Lu, R., Neff, N. F., Quake, S. R. & Weissman, I. L. Tracking single hematopoietic stem cells in vivo using high-throughput sequencing in conjunction with viral genetic barcoding. Nat. Biotechnol. 29, 928–933 (2011). The authors demonstrate a method for combining viral genetic barcoding with high-throughput next-generation sequencing, then apply this method to follow the differentiation of single haematopoietic stem cells.
Holt, C. E., Garlick, N. & Cornel, E. Lipofection of cDNAs in the embryonic vertebrate central nervous system. Neuron 4, 203–214 (1990).
Emerson, M. M. & Cepko, C. L. Identification of a retina-specific Otx2 enhancer element active in immature developing photoreceptors. Dev. Biol. 360, 241–255 (2011).
Fukuchi-Shimogori, T. & Grove, E. A. Neocortex patterning by the secreted signaling molecule FGF8. Science 294, 1071–1074 (2001).
LoTurco, J., Manent, J.-B. & Sidiqi, F. New and improved tools for in utero electroporation studies of developing cerebral cortex. Cereb. Cortex 19 (Suppl. 1), i120–i125 (2009).
Wu, S., Meir, Y. & Coates, C. J. piggyBac is a flexible and highly active transposon as compared to sleeping beauty, Tol2, and Mos1 in mammalian cells. Proc. Natl Acad. Sci. USA 103, 15008–15013 (2006).
VandenDriessche, T., Ivics, Z., Izsvák, Z. & Chuah, M. K. L. Emerging potential of transposons for gene therapy and generation of induced pluripotent stem cells. Blood 114, 1461–1468 (2009).
Yoshida, A. et al. Simultaneous expression of different transgenes in neurons and glia by combining in utero electroporation with the Tol2 transposon-mediated gene transfer system. Genes Cells 15, 501–512 (2010).
Chen, F. & LoTurco, J. A method for stable transgenesis of radial glia lineage in rat neocortex by piggyBac mediated transposition. J. Neurosci. Methods 207, 172–180 (2012).
Siddiqi, F. et al. Fate mapping by piggyBac transposase reveals that neocortical GLAST + progenitors generate more astrocytes than nestin + progenitors in rat neocortex. Cereb. Cortex 24, 508–520 (2014).
Ding, S. et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 122, 473–483 (2005). This study demonstrated that the piggyBac transposon could be used for genetic manipulation in human and mouse cells.
Wilson, M. H., Coates, C. J. & George, A. L. PiggyBac transposon-mediated gene transfer in human cells. Mol. Ther. 15, 139–145 (2007).
Woltjen, K. et al. PiggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458, 766–770 (2009).
Orban, P. C., Chui, D. & Marth, J. D. Tissue- and site-specific DNA recombination in transgenic mice. Proc. Natl Acad. Sci. USA 89, 6861–6865 (1992).
Branda, C. S. & Dymecki, S. M. Talking about a revolution: the impact of site-specific recombinases on genetic analyses in mice. Dev. Cell 6, 7–28 (2004).
Greig, L. C., Woodworth, M. B., Greppi, C. & Macklis, J. D. Ctip1 controls acquisition of sensory area identity and establishment of sensory input fields in the developing neocortex. Neuron 90, 261–277 (2016).
Awatramani, R., Soriano, P., Rodriguez, C., Mai, J. J. & Dymecki, S. M. Cryptic boundaries in roof plate and choroid plexus identified by intersectional gene activation. Nat. Genet. 35, 70–75 (2003).
Farago, A. F., Awatramani, R. B. & Dymecki, S. M. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron 50, 205–218 (2006).
Yamamoto, M. et al. A multifunctional reporter mouse line for Cre- and FLP-dependent lineage analysis. Genesis 47, 107–114 (2009).
Zong, H., Espinosa, J. S., Su, H. H., Muzumdar, M. D. & Luo, L. Mosaic analysis with double markers in mice. Cell 121, 479–492 (2005).
Bonaguidi, M. A. et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145, 1142–1155 (2011).
Hippenmeyer, S. et al. Genetic mosaic dissection of Lis1 and Ndel1 in neuronal migration. Neuron 68, 695–709 (2010).
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007). In this work, the authors present the Brainbow method for stochastic multicolour labelling in mice in vivo.
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
Sutherland, K. D. et al. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 19, 754–764 (2011).
Beier, K. T., Samson, M. E. S., Matsuda, T. & Cepko, C. L. Conditional expression of the TVA receptor allows clonal analysis of descendents from Cre-expressing progenitor cells. Dev. Biol. 353, 309–320 (2011).
Cornils, K. et al. Multiplexing clonality: combining RGB marking and genetic barcoding. Nucleic Acids Res. 42, e56 (2014).
Loulier, K. et al. Multiplex cell and lineage tracking with combinatorial labels. Neuron 81, 505–520 (2014).
Kimmerling, R. J. et al. A microfluidic platform enabling single-cell RNA-seq of multigenerational lineages. Nat. Commun. 7, 10220 (2015).
McKenna, A. et al. Whole organism lineage tracing by combinatorial and cumulative genome editing. Science 353, aaf7907 (2016). This study represents a proof of principle for the use of genome editing to simultaneously mark large numbers of lineages in a developing organism, opening the door for the next generation of high-throughput lineage tracing methods.
Junker, J. P. et al. Massively parallel whole-organism lineage tracing using CRISPR/Cas9 induced genetic scars. Preprint at bioRxiv http://dx.doi.org/10.1101/056499 (2016).
Kalhor, R., Mali, P. & Church, G. M. Rapidly evolving homing CRISPR barcodes. Nat. Methods http://dx.doi.org/10.1038/nmeth.4108 (2016).
Frieda, K. L. et al. Synthetic recording and in situ readout of lineage information in single cells. Nature http://dx.doi.org/10.1038/nature20777 (2016).
Schmidt, S. T., Zimmerman, S. M., Wang, J., Kim, S. K. & Quake, S. R. Cell lineage tracing using nuclease barcoding. Preprint at arXiv https://arxiv.org/abs/1606.00786 (2016).
Salipante, S. J., Kas, A., McMonagle, E. & Horwitz, M. S. Phylogenetic analysis of developmental and postnatal mouse cell lineages. Evol. Dev. 12, 84–94 (2010).
Shapiro, E., Biezuner, T. & Linnarsson, S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet. 14, 618–630 (2013).
Grün, D. & van Oudenaarden, A. Design and analysis of single-cell sequencing experiments. Cell 163, 799–810 (2015).
Gawad, C., Koh, W. & Quake, S. R. Single-cell genome sequencing: current state of the science. Nat. Rev. Genet. 17, 175–188 (2016).
Ostertag, E. M. & Kazazian, H. H. Biology of mammalian L1 retrotransposons. Annu. Rev. Genet. 35, 501–538 (2001).
Muotri, A. R. et al. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature 435, 903–910 (2005).
Erwin, J. A., Marchetto, M. C. & Gage, F. H. Mobile DNA elements in the generation of diversity and complexity in the brain. Nat. Rev. Neurosci. 15, 497–506 (2014).
Coufal, N. G. et al. L1 retrotransposition in human neural progenitor cells. Nature 460, 1127–1131 (2009).
Baillie, J. K. et al. Somatic retrotransposition alters the genetic landscape of the human brain. Nature 479, 534–537 (2011).
Evrony, G. D. et al. Single-neuron sequencing analysis of l1 retrotransposition and somatic mutation in the human brain. Cell 151, 483–496 (2012).
Upton, K. R. et al. Ubiquitous L1 mosaicism in hippocampal neurons. Cell 161, 228–239 (2015).
Evrony, G. D., Lee, E., Park, P. J. & Walsh, C. A. Resolving rates of mutation in the brain using single-neuron genomics. eLife 5, e12966 (2016).
Evrony, G. D. et al. Cell lineage analysis in human brain using endogenous retroelements. Neuron 85, 49–59 (2015). This study demonstrated that somatic L1 retrotransposition events could be used to identify distinct lineages in normal human brain.
Abyzov, A. et al. Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature 492, 438–442 (2012).
Knouse, K. A., Wu, J. & Amon, A. Assessment of megabase-scale somatic copy number variation using single-cell sequencing. Genome Res. 26, 376–384 (2016).
McConnell, M. J. et al. Mosaic copy number variation in human neurons. Science 342, 632–637 (2013).
Cai, X. et al. Single-cell, genome-wide sequencing identifies clonal somatic copy-number variation in the human brain. Cell Rep. 8, 1280–1289 (2014).
Baslan, T. et al. Optimizing sparse sequencing of single cells for highly multiplex copy number profiling. Genome Res. 25, 714–724 (2015).
Frank, S. A. Somatic evolutionary genomics: mutations during development cause highly variable genetic mosaicism with risk of cancer & neurodegeneration. Proc. Natl Acad. Sci. USA 107 (Suppl. 1), 1725–1730 (2010).
Hazen, J. L. et al. The complete genome sequences, unique mutational spectra, and developmental potency of adult neurons revealed by cloning. Neuron 89, 1223–1236 (2016).
Zafar, H., Wang, Y., Nakhleh, L., Navin, N. & Chen, K. Monovar: single-nucleotide variant detection in single cells. Nat. Methods 13, 505–507 (2016).
Ellegren, H. Microsatellites: simple sequences with complex evolution. Nat. Rev. Genet. 5, 435–445 (2004).
Frumkin, D., Wasserstrom, A., Kaplan, S., Feige, U. & Shapiro, E. Genomic variability within an organism exposes its cell lineage tree. PLoS Comput. Biol. 1, e50 (2005).
Salipante, S. J. & Horwitz, M. S. Phylogenetic fate mapping. Proc. Natl Acad. Sci. USA 103, 5448–5453 (2006).
Reizel, Y. et al. Colon stem cell and crypt dynamics exposed by cell lineage reconstruction. PLoS Genet. 7, e1002192 (2011).
Reizel, Y. et al. Cell lineage analysis of the mammalian female germline. PLoS Genet. 8, e1002477 (2012).
Thibodeau, S. N., Bren, G. & Schaid, D. Microsatellite instability in cancer of the proximal colon. Science 260, 816–819 (1993).
Ionov, Y., Peinado, M. A., Malkhosyan, S., Shibata, D. & Perucho, M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 363, 558–561 (1993).
Turajlic, S., McGranahan, N. & Swanton, C. Inferring mutational timing and reconstructing tumour evolutionary histories. Biochim. Biophys. Acta 1855, 264–275 (2015).
Ross, E. M. & Markowetz, F. OncoNEM: inferring tumor evolution from single-cell sequencing data. Genome Biol. 17, 69 (2016).
Li, Y. et al. Single-cell sequencing analysis characterizes common and cell-lineage-specific mutations in a muscle-invasive bladder cancer. Gigascience 1, 12 (2012).
Xu, X. et al. Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell 148, 886–895 (2012).
Shibata, D., Navidi, W., Salovaara, R., Li, Z. H. & Aaltonen, L. A. Somatic microsatellite mutations as molecular tumor clocks. Nat. Med. 2, 676–681 (1996).
Naxerova, K. & Jain, R. K. Using tumour phylogenetics to identify the roots of metastasis in humans. Nat. Rev. Clin. Oncol. 12, 258–272 (2015).
Frumkin, D. et al. Cell lineage analysis of a mouse tumor. Cancer Res. 68, 5924–5931 (2008). After previous work by the authors demonstrated the theoretical feasibility of using somatic microsatellite variability to reconstruct lineage, this study carried out the experiments, demonstrating that cells in a mouse tumour could be traced back to their somatic origin.
Shlush, L. I. et al. Cell lineage analysis of acute leukemia relapse uncovers the role of replication-rate heterogeneity and microsatellite instability. Blood 120, 603–612 (2012).
Ding, L. et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 481, 506–510 (2012).
Martincorena, I. et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886 (2015).
Yachida, S. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114–1117 (2010).
Hou, Y. et al. Single-cell exome sequencing and monoclonal evolution of a JAK2-negative myeloproliferative neoplasm. Cell 148, 873–885 (2012).
Gawad, C., Koh, W. & Quake, S. R. Dissecting the clonal origins of childhood acute lymphoblastic leukemia by single-cell genomics. Proc. Natl Acad. Sci. USA 111, 17947–17952 (2014).
Wang, Y. & Navin, N. E. Advances and applications of single-cell sequencing technologies. Mol. Cell 58, 598–609 (2015).
Dean, F. B. et al. Comprehensive human genome amplification using multiple displacement amplification. Proc. Natl Acad. Sci. USA 99, 5261–5266 (2002).
Wang, J., Fan, H. C., Behr, B. & Quake, S. R. Genome-wide single-cell analysis of recombination activity and de novo mutation rates in human sperm. Cell 150, 402–412 (2012).
Fu, Y. et al. Uniform and accurate single-cell sequencing based on emulsion whole-genome amplification. Proc. Natl Acad. Sci. USA 112, 11923–11928 (2015).
Sidore, A. M., Lan, F., Lim, S. W. & Abate, A. R. Enhanced sequencing coverage with digital droplet multiple displacement amplification. Nucleic Acids Res. 44, e66 (2016).
Rhee, M., Light, Y. K., Meagher, R. J. & Singh, A. K. Digital Droplet Multiple Displacement Amplification (ddMDA) for whole genome sequencing of limited dna samples. PLoS ONE 11, e0153699 (2016).
Szulwach, K. E. et al. Single-cell genetic analysis using automated microfluidics to resolve somatic mosaicism. PLoS ONE 10, e0135007 (2015).
Barker, D. L. et al. Two methods of whole-genome amplification enable accurate genotyping across a 2320-SNP linkage panel. Genome Res. 14, 901–907 (2004).
Ning, L. et al. Quantitative assessment of single-cell whole genome amplification methods for detecting copy number variation using hippocampal neurons. Sci. Rep. 5, 11415 (2015).
Zong, C., Lu, S., Chapman, A. R. & Xie, X. S. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science 338, 1622–1626 (2012).
Voet, T. et al. Single-cell paired-end genome sequencing reveals structural variation per cell cycle. Nucleic Acids Res. 41, 6119–6138 (2013).
Leung, M. L. et al. Highly multiplexed targeted DNA sequencing from single nuclei. Nat. Protoc. 11, 214–235 (2016).
Chow, H.-M. & Herrup, K. Genomic integrity and the ageing brain. Nat. Rev. Neurosci. 16, 672–684 (2015).
Spalding, K. L., Bhardwaj, R. D., Buchholz, B. A., Druid, H. & Frisén, J. Retrospective birth dating ofçcells in humans. Cell 122, 133–143 (2005).
Leung, M. L., Wang, Y., Waters, J. & Navin, N. E. SNES: single nucleus exome sequencing. Genome Biol. 16, 55 (2015).
Knouse, K. A., Wu, J., Whittaker, C. A. & Amon, A. Single cell sequencing reveals low levels of aneuploidy across mammalian tissues. Proc. Natl Acad. Sci. USA 111, 13409–13414 (2014).
Espina, V. et al. Laser-capture microdissection. Nat. Protoc. 1, 586–603 (2006).
Gutierrez-Gonzalez, L. et al. Analysis of the clonal architecture of the human small intestinal epithelium establishes a common stem cell for all lineages and reveals a mechanism for the fixation and spread of mutations. J. Pathol. 217, 489–496 (2009).
Crosetto, N., Bienko, M. & van Oudenaarden, A. Spatially resolved transcriptomics and beyond. Nat. Rev. Genet. 16, 57–66 (2015).
Larsson, C., Grundberg, I., Söderberg, O. & Nilsson, M. In situ detection and genotyping of individual mRNA molecules. Nat. Methods 7, 395–397 (2010).
Larsson, C. et al. In situ genotyping individual DNA molecules by target-primed rolling-circle amplification of padlock probes. Nat. Methods 1, 227–232 (2004).
Ke, R. et al. In situ sequencing for RNA analysis in preserved tissue and cells. Nat. Methods 10, 857–860 (2013).
Lee, J. H. et al. Highly multiplexed subcellular RNA sequencing in situ. Science 343, 1360–1363 (2014).
Lee, J. H. et al. Fluorescent in situ sequencing (FISSEQ) of RNA for gene expression profiling in intact cells and tissues. Nat. Protoc. 10, 442–458 (2015).
Janiszewska, M. et al. In situ single-cell analysis identifies heterogeneity for PIK3CA mutation and HER2 amplification in HER2-positive breast cancer. Nat. Genet. 47, 1212–1219 (2015).
Macosko, E. Z. et al. Highly parallel genome- wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Ware, M. L., Tavazoie, S. F., Reid, C. B. & Walsh, C. A. Coexistence of widespread clones and large radial clones in early embryonic ferret cortex. Cereb. Cortex 9, 636–645 (1999).
Reid, C. B., Tavazoie, S. F. & Walsh, C. A. Clonal dispersion and evidence for asymmetric cell division in ferret cortex. Development 124, 2441–2450 (1997).
Acknowledgements
The authors thank members of the Walsh laboratory, especially M. Lodato, for helpful comments. This work was supported by the Manton Center for Orphan Disease Research and grants from the National Institute of Neurological Disorders and Stroke (NINDS) (R01 NS032457, R01 NS079277 and U01 MH106883) to C.A.W. M.B.W. is supported by the Leonard and Isabelle Goldenson Research Fellowship. K.M.G. is supported by US National Institutes of Health (NIH) grant T32 MH20017. C.A.W. is a Distinguished Investigator of the Paul G. Allen Family Foundation and an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Fate-mapping methods
-
Approaches that apply a heritable mark to a given progenitor or class of progenitors, then use the inheritance of the mark to define the progeny of that cell or class.
- Genetic mosaicism
-
The state of containing more than one distinct genome within a single organism, whether achieved by experimental means (by combining early-stage embryos from different individuals or species) or by natural means (by considering differences in DNA from cell to cell).
- Prospective lineage analysis
-
An approach that applies an experimental label to cells, which is then examined at some point in the future to construct a lineage tree looking forwards from development.
- Retrospective lineage analysis
-
An approach that uses naturally occurring labels (for example, somatic mutations) to construct a lineage tree looking backwards at development.
- Intersectional analyses
-
Using two attributes of a cell population (for example, the expression from two different promoters) to select only cells that display both attributes.
- Organotypic slice culture
-
A culture system in which a slice of tissue is cultured, rather than a collection of dissociated cells, to more closely mimic the biological context of an organ.
- Cut-and-paste mechanism
-
Method of mobilization by class II DNA transposable elements, in which the transposon excises itself from its genomic location using transposase protein and integrates into a new target site.
- Cre-loxP
-
A genetic system derived from P1 bacteriophage and adapted for use in genetically modifiable organisms. The site-specific recombinase Cre inverts or recombines any sequence located between 34 bp loxP sites, depending on their orientation.
- FLP-FRT
-
A genetic system derived from Saccharomyces cerevisiae and adapted for use in genetically modifiable organisms. The site-specific recombinase FLP inverts or recombines any sequence located between 34 bp FRT sites, depending on their orientation.
- loxP-STOP-loxP
-
A DNA element containing a transcription termination sequence flanked by loxP sequences, allowing the transcription termination sequence to be removed by the activity of Cre recombinase.
- Pulse
-
An experiment in which a brief bolus of label is followed by a period with no label, allowing events that occurred within a specific time window to be marked.
- Leakiness
-
Activity in the absence of inducing signal.
- Microsatellites
-
(Also known as short tandem repeats). Short genomic repeats consisting of a set of tandem nucleotides, with repeat numbers varying between different alleles.
- Digital droplet PCR
-
A polymerase chain reaction in which the reaction is divided into thousands of small droplets, allowing absolute quantification of PCR products.
- Organoid
-
A three-dimensional culture model of a whole or partial organ or tissue.
- Clade
-
A group on a dendrogram (tree diagram) that is separate from another group.
- Nested
-
To have a set fully contained within a broader set.
- Allelic dropout
-
One of two alleles at a genomic locus fails to amplify and is therefore not recovered in sequencing data. Compare with locus dropout, in which both alleles at a given locus fail to amplify.
- Chimeric amplification
-
In whole-genome amplification, when one amplicon misprimes another locus, leading to a hybrid DNA product with sequences from the original amplicon adjacent to those from the second locus.
- Induced pluripotent stem cell
-
(iPSC). A cell that is capable of giving rise to daughter cells of many or all lineages, derived by reprogramming of an adult cell using pluripotency factors.
- Triturated
-
A homogeneous solution created by mixing or grinding, such as pipetting cells up and down to create a uniform suspension.
Rights and permissions
About this article
Cite this article
Woodworth, M., Girskis, K. & Walsh, C. Building a lineage from single cells: genetic techniques for cell lineage tracking. Nat Rev Genet 18, 230–244 (2017). https://doi.org/10.1038/nrg.2016.159
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg.2016.159
This article is cited by
-
Single-cell lineage tracing with endogenous markers
Biophysical Reviews (2024)
-
The technological landscape and applications of single-cell multi-omics
Nature Reviews Molecular Cell Biology (2023)
-
Grave-to-cradle: human embryonic lineage tracing from the postmortem body
Experimental & Molecular Medicine (2023)
-
Mitochondrial single-cell ATAC-seq for high-throughput multi-omic detection of mitochondrial genotypes and chromatin accessibility
Nature Protocols (2023)
-
Single-cell RNA sequencing to identify cellular heterogeneity and targets in cardiovascular diseases: from bench to bedside
Basic Research in Cardiology (2023)