Key Points
-
Adipocytes have an important role in sensing and managing energy status
-
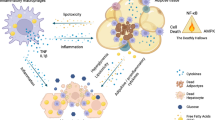
Adipose tissue responds to overnutrition by mounting an immune response; however, the initial inflammatory trigger in adipose tissue is unknown
-
Inflammation induces insulin resistance through a variety of molecular mechanisms
-
The maladaptive responses that occur in long-term obesity are a result of chronic inflammation, particularly catecholamine resistance
-
Inflammatory pathways are intriguing therapeutic targets for metabolic disease; however, the clinical efficacy of drugs targeting these pathways has been disappointing
Abstract
Adipose tissue not only has an important role in the storage of excess nutrients but also senses nutrient status and regulates energy mobilization. An overall positive energy balance is associated with overnutrition and leads to excessive accumulation of fat in adipocytes. These cells respond by initiating an inflammatory response that, although maladaptive in the long run, might initially be a physiological response to the stresses obesity places on adipose tissue. In this Review, we characterize adipose tissue inflammation and review the current knowledge of what triggers obesity-associated inflammation in adipose tissue. We examine the connection between adipose tissue inflammation and the development of insulin resistance and catecholamine resistance and discuss the ensuing state of metabolic inflexibility. Finally, we review the current and potential new anti-inflammatory treatments for obesity-associated metabolic disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Lumeng, C. N. & Saltiel, A. R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 (2011).
Olefsky, J. M. & Glass, C. K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 (2010).
Kotas, M. E. & Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 (2015).
Buettner, C. et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat. Med. 14, 667–675 (2008).
Scarpace, P. J. & Matheny, M. Leptin induction of UCP1 gene expression is dependent on sympathetic innervation. Am. J. Physiol. 275, E259–E264 (1998).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Frederich, R. C. et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1, 1311–1314 (1995).
Considine, R. V. et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334, 292–295 (1996).
Shen, J., Tanida, M., Niijima, A. & Nagai, K. In vivo effects of leptin on autonomic nerve activity and lipolysis in rats. Neurosci. Lett. 416, 193–197 (2007).
Friedman, J. M. Obesity in the new millennium. Nature 404, 632–634 (2000).
Saltiel, A. R. & Kahn, C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (2001).
Fisher, F. M. et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281 (2012).
Emanuelli, B. et al. Interplay between FGF21 and insulin action in the liver regulates metabolism. J. Clin. Invest. 124, 515–527 (2014).
Hondares, E. et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 286, 12983–12990 (2011).
Yamauchi, T. et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769 (2003).
Trujillo, M. E. & Scherer, P. E. Adiponectin — journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J. Intern. Med. 257, 167–175 (2005).
Fruebis, J. et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl Acad. Sci. USA 98, 2005–2010 (2001).
Yamauchi, T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7, 941–946 (2001).
Berg, A. H., Combs, T. P., Du, X., Brownlee, M. & Scherer, P. E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 7, 947–953 (2001).
Mora, S. & Pessin, J. E. An adipocentric view of signaling and intracellular trafficking. Diabetes Metab. Res. Rev. 18, 345–356 (2002).
Lidell, M. E. et al. Evidence for two types of brown adipose tissue in humans. Nat. Med. 19, 631–634 (2013).
Tews, D. et al. Comparative gene array analysis of progenitor cells from human paired deep neck and subcutaneous adipose tissue. Mol. Cell. Endocrinol. 395, 41–50 (2014).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Hu, H. H. et al. MRI detection of brown adipose tissue with low fat content in newborns with hypothermia. Magn. Reson. Imaging 32, 107–117 (2014).
Young, P., Arch, J. R. & Ashwell, M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 167, 10–14 (1984).
Liu, W. et al. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. J. Cell Sci. 126, 3527–3532 (2013).
Wang, W. & Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 17, 691–702 (2016).
Sidossis, L. & Kajimura, S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Invest. 125, 478–486 (2015).
Brestoff, J. R. & Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 161, 146–160 (2015).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Lizcano, F. & Vargas, D. Biology of beige adipocyte and possible therapy for type 2 diabetes and obesity. Int. J. Endocrinol. 2016, 9542061 (2016).
van den Berg, S. M., van Dam, A. D., Rensen, P. C., de Winther, M. P. & Lutgens, E. Immune modulation of brown(ing) adipose tissue in obesity. Endocr. Rev. 38, 46–68 (2017).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
Saad, M. J., Santos, A. & Prada, P. O. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology (Bethesda) 31, 283–293 (2016).
Kim, J. Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 (2007).
Wernstedt Asterholm, I. et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 20, 103–118 (2014).
Jayashree, B. et al. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell. Biochem. 388, 203–210 (2014).
Nguyen, M. T. et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 282, 35279–35292 (2007).
Lee, J. Y. et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J. Biol. Chem. 279, 16971–16979 (2004).
Ghanim, H. et al. Acute modulation of Toll-like receptors by insulin. Diabetes Care 31, 1827–1831 (2008).
Vitseva, O. I. et al. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity (Silver Spring) 16, 932–937 (2008).
Makowski, L., Brittingham, K. C., Reynolds, J. M., Suttles, J. & Hotamisligil, G. S. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J. Biol. Chem. 280, 12888–12895 (2005).
Cranmer-Byng, M. M., Liddle, D. M., De Boer, A. A., Monk, J. M. & Robinson, L. E. Proinflammatory effects of arachidonic acid in a lipopolysaccharide-induced inflammatory microenvironment in 3T3-L1 adipocytes in vitro. Appl. Physiol. Nutr. Metab. 40, 142–154 (2015).
Rocha, D. M., Bressan, J. & Hermsdorff, H. H. The role of dietary fatty acid intake in inflammatory gene expression: a critical review. Sao Paulo Med. J. 135, 157–168 (2017).
Chilton, L. et al. Metabolism of gammalinolenic acid in human neutrophils. J. Immunol. 156, 2941–2947 (1996).
Simopoulos, A. P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 8, 128 (2016).
Khan, S. A. et al. Unraveling the complex relationship triad between lipids, obesity, and inflammation. Mediators Inflamm. 2014, 502749 (2014).
Oliveira, V. et al. Diets containing alpha-linolenic (omega3) or oleic (omega9) fatty acids rescues obese mice from insulin resistance. Endocrinology 156, 4033–4046 (2015).
Finucane, O. M. et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1beta secretion and insulin resistance despite obesity. Diabetes 64, 2116–2128 (2015).
Scoditti, E. et al. Additive regulation of adiponectin expression by the mediterranean diet olive oil components oleic acid and hydroxytyrosol in human adipocytes. PLoS ONE 10, e0128218 (2015).
Estruch, R. et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 368, 1279–1290 (2013).
Cinti, S. et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 (2005).
Fischer-Posovszky, P., Wang, Q. A., Asterholm, I. W., Rutkowski, J. M. & Scherer, P. E. Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology 152, 3074–3081 (2011).
Strissel, K. J. et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56, 2910–2918 (2007).
Lumeng, C. N., Deyoung, S. M., Bodzin, J. L. & Saltiel, A. R. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56, 16–23 (2007).
Haase, J. et al. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia 57, 562–571 (2014).
Jin, C. & Flavell, R. A. Innate sensors of pathogen and stress: linking inflammation to obesity. J. Allergy Clin. Immunol. 132, 287–294 (2013).
Shi, Y., Evans, J. E. & Rock, K. L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425, 516–521 (2003).
Lamkanfi, M. & Dixit, V. M. Inflammasomes: guardians of cytosolic sanctity. Immunol. Rev. 227, 95–105 (2009).
Vandanmagsar, B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 (2011).
Schroder, K., Zhou, R. & Tschopp, J. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327, 296–300 (2010).
Lee, Y. S. et al. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352 (2014).
Gonzalez-Muniesa, P. et al. Effects of hyperoxia on oxygen-related inflammation with a focus on obesity. Oxid. Med. Cell. Longev. 2015, 8957827 (2015).
Ye, J., Gao, Z., Yin, J. & He, Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 293, E1118–E1128 (2007).
Quintero, P., Gonzalez-Muniesa, P., Garcia-Diaz, D. F. & Martinez, J. A. Effects of hyperoxia exposure on metabolic markers and gene expression in 3T3-L1 adipocytes. J. Physiol. Biochem. 68, 663–669 (2012).
Trayhurn, P., Wang, B. & Wood, I. S. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br. J. Nutr. 100, 227–235 (2008).
He, Q. et al. Regulation of HIF-1α activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am. J. Physiol. Endocrinol. Metab. 300, E877–E885 (2011).
Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 93, 1–21 (2013).
Rausch, M. E., Weisberg, S., Vardhana, P. & Tortoriello, D. V. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. (Lond.) 32, 451–463 (2008).
Rius, J. et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453, 807–811 (2008).
Skinner, B. M. & Johnson, E. E. Nuclear morphologies: their diversity and functional relevance. Chromosoma 126, 195–212 (2017).
Williams, A. S., Kang, L. & Wasserman, D. H. The extracellular matrix and insulin resistance. Trends Endocrinol. Metab. 26, 357–366 (2015).
Khan, T. et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 29, 1575–1591 (2009).
Chun, T. H. et al. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell 125, 577–591 (2006).
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K. & Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 (2004).
Hara, Y. et al. Rho and Rho-kinase activity in adipocytes contributes to a vicious cycle in obesity that may involve mechanical stretch. Sci. Signal. 4, ra3 (2011).
Li, Q., Hata, A., Kosugi, C., Kataoka, N. & Funaki, M. The density of extracellular matrix proteins regulates inflammation and insulin signaling in adipocytes. FEBS Lett. 584, 4145–4150 (2010).
Doherty, T. A. At the bench: understanding group 2 innate lymphoid cells in disease. J. Leukoc. Biol. 97, 455–467 (2015).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Molofsky, A. B. et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 210, 535–549 (2013).
Lee, M. W. et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160, 74–87 (2015).
Brestoff, J. R. et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015).
Odegaard, J. I. et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
Kang, K. et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 7, 485–495 (2008).
Fujisaka, S. et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 58, 2574–2582 (2009).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Lackey, D. E. & Olefsky, J. M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 12, 15–28 (2016).
Xu, X. et al. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 18, 816–830 (2013).
Kratz, M. et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 20, 614–625 (2014).
Odegaard, J. I. et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 7, 496–507 (2008).
McLaughlin, T. et al. T-Cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler. Thromb. Vasc. Biol. 34, 2637–2643 (2014).
Nishimura, S. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 (2009).
Yang, H. et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J. Immunol. 185, 1836–1845 (2010).
Travers, R. L., Motta, A. C., Betts, J. A., Bouloumie, A. & Thompson, D. The impact of adiposity on adipose tissue-resident lymphocyte activation in humans. Int. J. Obes. (Lond.) 39, 762–769 (2015).
Winer, S. et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 15, 921–929 (2009).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009).
Bapat, S. P. et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 528, 137–141 (2015).
Duffaut, C., Galitzky, J., Lafontan, M. & Bouloumie, A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem. Biophys. Res. Commun. 384, 482–485 (2009).
Kitamura, D., Roes, J., Kuhn, R. & Rajewsky, K. A. B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350, 423–426 (1991).
Winer, D. A. et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17, 610–617 (2011).
DeFuria, J. et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl Acad. Sci. USA 110, 5133–5138 (2013).
Shen, L. et al. B-1a lymphocytes attenuate insulin resistance. Diabetes 64, 593–603 (2015).
Deng, T. et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 17, 411–422 (2013).
Huh, J. Y. et al. Deletion of CD1d in adipocytes aggravates adipose tissue inflammation and insulin resistance in obesity. Diabetes 66, 835–847 (2017).
Schmitz, J. et al. Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Mol. Metab. 5, 328–339 (2016).
Mayoral Monibas, R., Johnson, A. M., Osborn, O., Traves, P. G. & Mahata, S. K. Distinct hepatic macrophage populations in lean and obese mice. Front. Endocrinol. (Lausanne) 7, 152 (2016).
Winer, D. A., Luck, H., Tsai, S. & Winer, S. The intestinal immune system in obesity and insulin resistance. Cell Metab. 23, 413–426 (2016).
Yu, E. et al. Weight history and all-cause and cause-specific mortality in three prospective cohort studies. Ann. Intern. Med. 166, 613–620 (2017).
Hardy, O. T. et al. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg. Obes. Relat. Dis. 7, 60–67 (2011).
Kloting, N. et al. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 299, E506–E515 (2010).
Arkan, M. C. et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 11, 191–198 (2005).
Hirosumi, J. et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002).
Holland, W. L. et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 (2007).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 (2006).
Nakamura, T. et al. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell 140, 338–348 (2010).
Hotamisligil, G. S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 (2010).
Summers, S. A. Sphingolipids and insulin resistance: the five Ws. Curr. Opin. Lipidol. 21, 128–135 (2010).
Saberi, M. et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 10, 419–429 (2009).
Wellen, K. E. et al. Coordinated regulation of nutrient and inflammatory responses by STAMP2 is essential for metabolic homeostasis. Cell 129, 537–548 (2007).
Lesniewski, L. A. et al. Bone marrow-specific Cap gene deletion protects against high-fat diet-induced insulin resistance. Nat. Med. 13, 455–462 (2007).
Li, P. et al. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat. Med. 21, 239–247 (2015).
Li, P. et al. Hematopoietic-derived galectin-3 causes cellular and systemic insulin resistance. Cell 167, 973–984.e12 (2016).
Himes, R. W. & Smith, C. W. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 24, 731–739 (2010).
Avota, E., Gulbins, E. & Schneider-Schaulies, S. DC-SIGN mediated sphingomyelinase-activation and ceramide generation is essential for enhancement of viral uptake in dendritic cells. PLoS Pathog. 7, e1001290 (2011).
Summers, S. A., Garza, L. A., Zhou, H. & Birnbaum, M. J. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 18, 5457–5464 (1998).
Teruel, T., Hernandez, R. & Lorenzo, M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes 50, 2563–2571 (2001).
Stratford, S., Hoehn, K. L., Liu, F. & Summers, S. A. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 279, 36608–36615 (2004).
Solinas, G. et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 6, 386–397 (2007).
Witczak, C. A. et al. JNK1 deficiency does not enhance muscle glucose metabolism in lean mice. Biochem. Biophys. Res. Commun. 350, 1063–1068 (2006).
Chiang, S. H. et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell 138, 961–975 (2009).
Baker, R. G., Hayden, M. S. & Ghosh, S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 13, 11–22 (2011).
Zick, Y. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci. STKE 2005, pe4 (2005).
Arner, P., Arner, E., Hammarstedt, A. & Smith, U. Genetic predisposition for type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS ONE 6, e18284 (2011).
Tchkonia, T. et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 17, 644–656 (2013).
Chung, S. et al. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology 147, 5340–5351 (2006).
Saltiel, A. R. Insulin resistance in the defense against obesity. Cell Metab. 15, 798–804 (2012).
Lu, Q., Li, M., Zou, Y. & Cao, T. Induction of adipocyte hyperplasia in subcutaneous fat depot alleviated type 2 diabetes symptoms in obese mice. Obesity (Silver Spring) 22, 1623–1631 (2014).
Nov, O. et al. Interleukin-1beta regulates fat-liver crosstalk in obesity by auto-paracrine modulation of adipose tissue inflammation and expandability. PLoS ONE 8, e53626 (2013).
Arner, P. Catecholamine-induced lipolysis in obesity. Int. J. Obes. Relat. Metab. Disord. 23 (Suppl. 1), 10–13 (1999).
Knight, Z. A., Hannan, K. S., Greenberg, M. L. & Friedman, J. M. Hyperleptinemia is required for the development of leptin resistance. PLoS ONE 5, e11376 (2010).
Caro, J. F. et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348, 159–161 (1996).
Diano, S., Kalra, S. P. & Horvath, T. L. Leptin receptor immunoreactivity is associated with the Golgi apparatus of hypothalamic neurons and glial cells. J. Neuroendocrinol. 10, 647–650 (1998).
Ozcan, L. et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 9, 35–51 (2009).
Zhang, X. et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008).
Collins, S., Daniel, K. W., Petro, A. E. & Surwit, R. S. Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology 138, 405–413 (1997).
Gettys, T. W. et al. Age-dependent changes in beta-adrenergic receptor subtypes and adenylyl cyclase activation in adipocytes from Fischer 344 rats. Endocrinology 136, 2022–2032 (1995).
Bougneres, P. et al. In vivo resistance of lipolysis to epinephrine. A new feature of childhood onset obesity. J. Clin. Invest. 99, 2568–2573 (1997).
Reynisdottir, S., Ellerfeldt, K., Wahrenberg, H., Lithell, H. & Arner, P. Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. J. Clin. Invest. 93, 2590–2599 (1994).
Horowitz, J. F. & Klein, S. Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. Am. J. Physiol. Endocrinol. Metab. 278, E1144–E1152 (2000).
Lowell, B. B. & Bachman, E. S. Beta-adrenergic receptors, diet-induced thermogenesis, and obesity. J. Biol. Chem. 278, 29385–29388 (2003).
Sakamoto, T. et al. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. Am. J. Physiol. Endocrinol. Metab. 310, E676–E687 (2016).
Guo, T. et al. Adipocyte ALK7 links nutrient overload to catecholamine resistance in obesity. eLife 3, e03245 (2014).
Mowers, J. et al. Inflammation produces catecholamine resistance in obesity via activation of PDE3B by the protein kinases IKKε and TBK1. eLife 2, e01119 (2013).
Reilly, S. M. et al. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nat. Med. 19, 313–321 (2013).
Feldmann, M. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2, 364–371 (2002).
Ofei, F., Hurel, S., Newkirk, J., Sopwith, M. & Taylor, R. Effects of an engineered human anti-TNFa antibody (SDP571) on insulin sensitivity and glycemic control in pateints with NIDDM. Diabetes 45, 881–885 (1996).
Solomon, D. H. et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA 305, 2525–2531 (2011).
Moller, D. E. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol. Metab. 11, 212–217 (2000).
Ofei, F., Hurel, S., Newkirk, J., Sopwith, M. & Taylor, R. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 45, 881–885 (1996).
Sloan-Lancaster, J. et al. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1beta antibody, in patients with type 2 diabetes. Diabetes Care 36, 2239–2246 (2013).
Goldfine, A. B. et al. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann. Intern. Med. 152, 346–357 (2010).
Hawley, S. A. et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science 336, 918–922 (2012).
Goldfine, A. B. et al. A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia 56, 714–723 (2013).
Penesova, A. et al. Salsalate has no effect on insulin secretion but decreases insulin clearance: a randomized, placebo-controlled trial in subjects without diabetes. Diabetes Obes. Metab. 17, 608–612 (2015).
Cipolletta, D. et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 486, 549–553 (2012).
Reilly, S. M. et al. A subcutaneous adipose tissue-liver signalling axis controls hepatic gluconeogenesis. Nat. Commun. 6, 6047 (2015).
Oral, E. A. et al. Inhibition of IKKε and TBK1 improves glucose control in a subset of patients with type 2 diabetes. Cell Metab. 26 157–170 (2017).
Wheaton, W. W. et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 3, e02242 (2014).
Isoda, K. et al. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler. Thromb. Vasc. Biol. 26, 611–617 (2006).
Koppaka, S. et al. Reduced adipose tissue macrophage content is associated with improved insulin sensitivity in thiazolidinedione-treated diabetic humans. Diabetes 62, 1843–1854 (2013).
Peraldi, P., Xu, M. & Spiegelman, B. M. Thiazolidinediones block tumor necrosis factor-alpha-induced inhibition of insulin signaling. J. Clin. Invest. 100, 1863–1869 (1997).
Chawla, A. Control of macrophage activation and function by PPARs. Circ. Res. 106, 1559–1569 (2010).
Kirwan, J. P. et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes 51, 2207–2213 (2002).
Ategbo, J. M. et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J. Clin. Endocrinol. Metab. 91, 4137–4143 (2006).
Xu, J. et al. Maternal circulating concentrations of tumor necrosis factor-alpha, leptin, and adiponectin in gestational diabetes mellitus: a systematic review and meta-analysis. ScientificWorldJournal 2014, 926932 (2014).
Author information
Authors and Affiliations
Contributions
S.M.R. and A.R.S. researched data for the article, contributed to discussion of the content, wrote the article and reviewed and/or edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Biogenic amines
-
Endogenous molecules with one or more amine groups; five important neurotransmitters are biogenic amines, including three catecholamines (dopamine, noradrenaline and adrenaline) as well as histamine and serotonin.
- Mesenteric adipose tissue
-
A small membrane-like visceral fat depot connected to the intestine that expands into a large adipose depot surrounding the intestine during obesity.
- Crown-like structures
-
These histological structures are observed in adipose tissue and are the result of immune cells surrounding dead adipocytes; as the immune cells pack into the spaces around the dead adipocytes and between the surrounding live adipocytes, they form a shape reminiscent of a crown.
- Oxygen tension
-
The partial pressure of oxygen within a tissue, which is a measure of the amount of oxygen within the tissue.
- Adipogenesis
-
The differentiation of pre-adipocytes (or other fibroblast-like cells) to make new adipocytes.
- Type 2 or T helper 2 (TH2)
-
Type 2 cytokines, such as IL-4 and IL-13, are classified based on their secretion from TH2 cells (however, they are secreted from many other immune cells) and are typically anti-inflammatory and promote M2 macrophage differentiation; type 2 immunity is characterized by antibody-mediated immune responses.
- Type 1 or T helper 1 (TH1)
-
Type 1 cytokines, such as IL-1β and tumour necrosis factor, are classified based on their secretion from TH1 cells (however, they are secreted from many other immune cells) and are typically pro-inflammatory; type 1 immunity is characterized by a phagocytic immune response.
- Angiogenesis
-
The proliferation and migration of endothelial cells to form new blood vessels.
- Metabolic inflexibility
-
A state in which responsiveness to catabolic and anabolic signals is reduced in adipocytes, leaving them unable to efficiently contract (mobilize nutrients) or expand (store nutrients).
Rights and permissions
About this article
Cite this article
Reilly, S., Saltiel, A. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol 13, 633–643 (2017). https://doi.org/10.1038/nrendo.2017.90
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2017.90
This article is cited by
-
Regulation of inflammatory diseases via the control of mRNA decay
Inflammation and Regeneration (2024)
-
Predictive value of insulin resistance surrogates for the development of diabetes in individuals with baseline normoglycemia: findings from two independent cohort studies in China and Japan
Diabetology & Metabolic Syndrome (2024)
-
Pathophysiology of obesity-related infertility and its prevention and treatment by potential phytotherapeutics
International Journal of Obesity (2024)
-
Myeloid-derived grancalcin instigates obesity-induced insulin resistance and metabolic inflammation in male mice
Nature Communications (2024)
-
Prognosis of a malignant phenotype of obesity defined by a cardiac biomarker in hypertension: the Japan Morning Surge-Home Blood Pressure study
Hypertension Research (2024)