Key Points
-
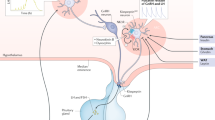
Gonadotropin-releasing hormone (GnRH) neurons exhibit many unique morphological features, including a blended dendrite–axon (dendron) that projects to the median eminence to secrete GnRH into the pituitary portal system
-
Kisspeptin neurons in the arcuate nucleus probably represent a key component of an extrinsic pulse generator that drives pulsatile GnRH secretion in all mammals
-
The preovulatory luteinizing hormone surge is driven by high follicular-phase oestradiol levels that act variably upon the neuronal surge generator and the pituitary gland among spontaneously ovulating mammals
-
Re-emergence of pulsatile GnRH secretion at puberty requires gonadal and non-gonadal mechanisms involving linear maturation of glial and afferent inputs alongside the resurgence of an excitatory kisspeptin input
-
The GnRH neuronal network can be represented by a hierarchical modular design in which functional modules appropriate to the needs of specific mammalian species can be included
-
A variety of genetic mutations affect fertility, either by altering the GnRH neuron migratory pathway or by disrupting the GnRH neuron and its key neuronal regulators
Abstract
The gonadotropin-releasing hormone (GnRH) neuronal network generates pulse and surge modes of gonadotropin secretion critical for puberty and fertility. The arcuate nucleus kisspeptin neurons that innervate the projections of GnRH neurons in and around their neurosecretory zone are key components of the pulse generator in all mammals. By contrast, kisspeptin neurons located in the preoptic area project to GnRH neuron cell bodies and proximal dendrites and are involved in surge generation in female rodents (and possibly other species). The hypothalamic–pituitary–gonadal axis develops embryonically but, apart from short periods of activation immediately after birth, remains suppressed through a combination of gonadal and non-gonadal mechanisms. At puberty onset, the pulse generator reactivates, probably owing to progressive stimulatory influences on GnRH neurons from glial and neurotransmitter signalling, and the re-emergence of stimulatory arcuate kisspeptin input. In females, the development of pulsatile gonadotropin secretion enables final maturation of the surge generator that ultimately triggers the first ovulation. Representation of the GnRH neuronal network as a series of interlocking functional modules could help conceptualization of its functioning in different species. Insights into pulse and surge generation are expected to aid development of therapeutic strategies ameliorating pubertal disorders and infertility in the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Goodman, R. L. in Knobil and Neill's Physiology of Reproduction 4th edn Vol. 2 (eds Plant, T. M. & Zeleznik, A. J.) 1537–1574 (Academic Press, 2015).
Levine, J. E. in Knobil and Neill's Physiology of Reproduction 4th edn Vol. 2 (eds Plant, T. M. & Zeleznik, A. J.) 1199–1257 (Academic Press, 2015).
Herbison, A. E. in Knobil and Neill's Physiology of Reproduction 4th edn Vol. 1 (eds Plant, T. M. & Zeleznik, A. J.) 399–467 (Academic Press, 2015).
Boehm, U. et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism — pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 11, 547–564 (2015).
Sykiotis, G. P., Pitteloud, N., Seminara, S. B., Kaiser, U. B. & Crowley, W. F. Jr. Deciphering genetic disease in the genomic era: the model of GnRH deficiency. Sci. Transl. Med. 2, 32rv2 (2010).
Okubo, K. & Nagahama, Y. Structural and functional evolution of gonadotropin-releasing hormone in vertebrates. Acta Physiol. (Oxf.) 193, 3–15 (2008).
Forni, P. E. & Wray, S. GnRH, anosmia and hypogonadotropic hypogonadism — where are we? Front. Neuroendocrinol. 36, 165–177 (2015).
Abitua, P. B. et al. The pre-vertebrate origins of neurogenic placodes. Nature 524, 462–465 (2015).
Skynner, M. J., Slater, R., Sim, J. A., Allen, N. D. & Herbison, A. E. Promoter transgenics reveal multiple gonadotropin-releasing hormone-1-expressing cell populations of different embryological origin in mouse brain. J. Neurosci. 19, 5955–5966 (1999).
Spergel, D. J., Kruth, U., Hanley, D. F., Sprengel, R. & Seeburg, P. H. GABA-and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurone in transgenic mice. J. Neurosci. 19, 2037–2050 (1999).
Kato, M., Ui-Tei, K., Watanabe, M. & Sakuma, Y. Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology 144, 5118–5125 (2003).
Jasoni, C. L., Todman, M. G., Strumia, M. M. & Herbison, A. E. Cell type-specific expression of a genetically encoded calcium indicator reveals intrinsic calcium oscillations in adult gonadotropin-releasing hormone neurons. J. Neurosci. 27, 860–867 (2007).
Moenter, S. M. Identified GnRH neuron electrophysiology: a decade of study. Brain Res. 1364, 10–24 (2010).
Herbison, A. E., Pape, J. R., Simonian, S. X., Skynner, M. J. & Sim, J. A. Molecular and cellular properties of GnRH neurons revealed through transgenics in the mouse. Mol. Cell. Endocrinol. 185, 185–194 (2001).
Constantin, S., Jasoni, C., Romano, N., Lee, K. & Herbison, A. E. Understanding calcium homeostasis in postnatal gonadotropin-releasing hormone neurons using cell-specific Pericam transgenics. Cell Calcium 51, 267–276 (2012).
Campbell, R. E., Han, S. K. & Herbison, A. E. Biocytin filling of adult gonadotropin-releasing hormone neurons in situ reveals extensive, spiny, dendritic processes. Endocrinology 146, 1163–1169 (2005).
Herde, M. K., Iremonger, K. J., Constantin, S. & Herbison, A. E. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J. Neurosci. 33, 12689–12697 (2013).
Herde, M. K. & Herbison, A. E. Morphological characterization of the action potential initiation segment in GnRH neuron dendrites and axons of male mice. Endocrinology 156, 4174–4186 (2015).
Iremonger, K. J. & Herbison, A. E. Initiation and propagation of action potentials in gonadotropin-releasing hormone neuron dendrites. J. Neurosci. 32, 151–158 (2012).
Campbell, R. E., Gaidamaka, G., Han, S. K. & Herbison, A. E. Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc. Natl Acad. Sci. USA 106, 10835–10840 (2009).
Iremonger, K. J. & Herbison, A. E. Multitasking in gonadotropin-releasing hormone neuron dendrites. Neuroendocrinology 102, 1–7 (2015).
Silverman, A. J., Jhamandas, J. & Renaud, L. P. Localization of luteinizing hormone-releasing hormone (LHRH) neurons that project to the median eminence. J. Neurosci. 7, 2312–2319 (1987).
Merchenthaler, I. et al. Combined retrograde tracing and immunocytochemical identification of luteinizing hormone-releasing hormone- and somatostatin-containing neurons projecting to the median eminence of the rat. Endocrinology 125, 2812–2821 (1989).
Goldsmith, P. C., Thind, K. K., Song, T., Kim, E. J. & Boggan, J. E. Location of the neuroendocrine gonadotropin-releasing hormone neurons in the monkey hypothalamus by retrograde tracing and immunostaining. J. Neuroendocrinol. 2, 157–168 (1990).
Herbison, A. E., Porteous, R., Pape, J. R., Mora, J. M. & Hurst, P. R. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 149, 597–604 (2008).
Kokoris, G. J., Lam, N. Y., Ferin, M., Silverman, A. J. & Gibson, M. J. Transplanted gonadotropin-releasing hormone neurons promote pulsatile luteinizing hormone secretion in congenitally hypogonadal (hpg) male mice. Neuroendocrinology 48, 45–52 (1988).
Campos, P. & Herbison, A. E. Optogenetic activation of GnRH neurons reveals minimal requirements for pulsatile luteinizing hormone secretion. Proc. Natl Acad. Sci. USA 111, 18387–18392 (2014).
Boukhliq, R., Goodman, R. L., Berriman, S. J., Adrian, B. & Lehman, M. N. A subset of gonadotropin-releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion. Endocrinology 140, 5929–5936 (1999).
Funabashi, T., Jinnai, K. & Kimura, F. Fos expression by naloxone in LHRH neurons of the mediobasal hypothalamus and effects of pentobarbital sodium in the proestrous rat. J. Neuroendocrinol. 9, 87–92 (1997).
Weiner, R. I. et al. Gonadotropin-releasing hormone neuronal cell lines. Front. Neuroendocrinol. 13, 95–119 (1992).
Campbell, R. E. et al. Gap junctions between neuronal inputs but not gonadotropin-releasing hormone neurons control estrous cycles in the mouse. Endocrinology 152, 2290–2301 (2011).
Terasawa, E., Schanhofer, W. K., Keen, K. L. & Luchansky, L. Intracellular Ca2+ oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J. Neurosci. 19, 5898–5909 (1999).
Constantin, S., Caraty, A., Wray, S. & Duittoz, A. H. Development of gonadotropin-releasing hormone-1 secretion in mouse nasal explants. Endocrinology 150, 3221–3227 (2009).
Kordon, C., Drouva, S. V., Martinez de la Escalera, G. & Weiner, R. I. in The Physiology of Reproduction 2nd edn Vol. 1 (eds Knobil, E. & Neill, J. D.) 1621–1681 (Raven, 1994).
Iremonger, J. & Herbison, A. E. in Neurophysiology of Neuroendocrine Neurons 1st edn (eds Armstrong, W. E. & Tasker, J. G.) 273–299 (John Wiley & Sons, 2015).
Tenenbaum-Rakover, Y. et al. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J. Clin. Endocrinol. Metab. 92, 1137–1144 (2007).
Seminara, S. B. et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 349, 1614–1627 (2003).
Steyn, F. J. et al. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology 154, 4939–4945 (2013).
Uenoyama, Y. et al. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J. Neuroendocrinol. 27, 187–197 (2015).
Navarro, V. M. et al. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 29, 11859–11866 (2009).
Goodman, R. L. et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148, 5752–5760 (2007).
Lehman, M. N., Coolen, L. M. & Goodman, R. L. Minireview: Kisspeptin/Neurokinin B/Dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151, 3479–3489 (2010).
Yip, S. H., Boehm, U., Herbison, A. E. & Campbell, R. E. Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to GnRH neurons in the mouse. Endocrinology 156, 2582–2594 (2015).
Glanowska, K. M. & Moenter, S. M. Differential regulation of GnRH secretion in the preoptic area (POA) and the median eminence (ME) in male mice. Endocrinology 156, 231–241 (2015).
Han, S. Y., McLennan, T., Czieselsky, K. & Herbison, A. E. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc. Natl Acad. Sci. USA 112, 13109–13114 (2015).
Keen, K. L., Wegner, F. H., Bloom, S. R., Ghatei, M. A. & Terasawa, E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149, 4151–4157 (2008).
Plant, T. M., Ramaswamy, S. & Dipietro, M. J. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology 147, 1007–1013 (2006).
Skrapits, K. et al. Neuropeptide co-expression in hypothalamic kisspeptin neurons of laboratory animals and the human. Front. Neurosci. 9, 29 (2015).
Oakley, A. E., Clifton, D. K. & Steiner, R. A. Kisspeptin signaling in the brain. Endocr. Rev. 30, 713–743 (2009).
Plant, T. M. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front. Neuroendocrinol. 33, 160–168 (2012).
Legan, S. J. & Karsch, F. J. A daily signal for the LH surge in the rat. Endocrinology 96, 57–62 (1975).
Christian, C. A. & Moenter, S. M. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr. Rev. 31, 544–577 (2010).
Herbison, A. E. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res. Rev. 57, 277–287 (2008).
Wintermantel, T. M. et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52, 271–280 (2006).
Glidewell-Kenney, C. et al. Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc. Natl Acad. Sci. USA 104, 8173–8177 (2007).
Micevych, P. & Sinchak, K. The neurosteroid progesterone underlies estrogen positive feedback of the LH surge. Front. Endocrinol. (Lausanne) 2, 90 (2011).
de la Iglesia, H. O. & Schwartz, W. J. Minireview: timely ovulation: circadian regulation of the female hypothalamo–pituitary–gonadal axis. Endocrinology 147, 1148–1153 (2006).
Smith, J. T., Popa, S. M., Clifton, D. K., Hoffman, G. E. & Steiner, R. A. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J. Neurosci. 26, 6687–6694 (2006).
Adachi, S. et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J. Reprod. Dev. 53, 367–378 (2007).
Clarkson, J., d'Anglemont de Tassigny, X., Moreno, A. S., Colledge, W. H. & Herbison, A. E. Kisspeptin–GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J. Neurosci. 28, 8691–8697 (2008).
Liu, X. et al. Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. J. Neurosci. 31, 2421–2430 (2011).
Piet, R., Boehm, U. & Herbison, A. E. Estrous cycle plasticity in the hyperpolarization-activated current ih is mediated by circulating 17β-estradiol in preoptic area kisspeptin neurons. J. Neurosci. 33, 10828–10839 (2013).
Dubois, S. L. et al. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinology 156, 1111–1120 (2015).
Williams, W. P. 3rd, Jarjisian, S. G., Mikkelsen, J. D. & Kriegsfeld, L. J. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology 152, 595–606 (2011).
Vida, B. et al. Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. J. Neuroendocrinol. 22, 1032–1039 (2010).
Robertson, J. L., Clifton, D. K., de la Iglesia, H. O., Steiner, R. A. & Kauffman, A. S. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology 150, 3664–3671 (2009).
Smarr, B. L., Morris, E. & de la Iglesia, H. O. The dorsomedial suprachiasmatic nucleus times circadian expression of Kiss1 and the luteinizing hormone surge. Endocrinology 153, 2839–2850 (2012).
Piet, R., Fraissenon, A., Boehm, U. & Herbison, A. E. Estrogen permits vasopressin signaling in preoptic kisspeptin neurons in the female mouse. J. Neurosci. 35, 6881–6892 (2015).
Cheong, R. Y., Czieselsky, K., Porteous, R. & Herbison, A. E. Expression of ESR1 in glutamatergic and GABAergic neurons is essential for normal puberty onset, estrogen feedback, and fertility in female mice. J. Neurosci. 35, 14533–14543 (2015).
Hoffman, G. E., Smith, M. S. & Verbalis, J. G. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front. Neuroendocrinol. 14, 173–213 (1993).
Herde, M. K., Geist, K., Campbell, R. E. & Herbison, A. E. Gonadotropin-releasing hormone neurons extend complex highly branched dendritic trees outside the blood–brain barrier. Endocrinology 152, 3832–3841 (2011).
Wang, H., Hoffman, G. E. & Smith, M. S. Increased GnRH mRNA in the GnRH neurons expressing cFos during the proestrous LH surge. Endocrinology 136, 3673–3676 (1995).
Chan, H. et al. Dendritic spine plasticity in gonadatropin-releasing hormone (GnRH) neurons activated at the time of the preovulatory surge. Endocrinology 152, 4906–4914 (2011).
Karsch, F. J., Bowen, J. M., Caraty, A., Evans, N. P. & Moenter, S. M. Gonadotropin-releasing hormone requirements for ovulation. Biol. Reprod. 56, 303–309 (1997).
Clarke, I. J. Variable patterns of gonadotropin-releasing hormone secretion during the estrogen-induced luteinizing hormone surge in ovariectomized ewes. Endocrinology 133, 1624–1632 (1993).
Goodman, R. L. & Inskeep, E. K. in Knobil and Neill's Physiology of Reproduction 4th edn Vol. 2 (eds Plant, T. M. & Zeleznik, A. J.) 1259–1305 (Academic Press, 2015).
Caraty, A. et al. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory GnRH surge in the ewe. Endocrinology 139, 1752–1760 (1998).
Moenter, S. M., Karsch, F. J. & Lehman, M. N. Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology 133, 896–903 (1993).
Knobil, E. On the control of gonadotropin secretion in the rhesus monkey. Recent Prog. Horm. Res. 30, 1–46 (1974).
Hall, J. E. et al. Decreased release of gonadotropin-releasing hormone during the preovulatory midcycle luteinizing hormone surge in normal women. Proc. Natl Acad. Sci. USA 91, 6894–6898 (1994).
Ottowitz, W. E., Dougherty, D. D., Fischman, A. J. & Hall, J. E. [18F]2-fluoro-2-deoxy-d-glucose positron emission tomography demonstration of estrogen negative and positive feedback on luteinizing hormone secretion in women. J. Clin. Endocrinol. Metab. 93, 3208–3214 (2008).
Crowley, W. F. Jr & McArthur, J. W. Simulation of the normal menstrual cycle in Kallman's syndrome by pulsatile administration of luteinizing hormone-releasing hormone (LHRH). J. Clin. Endocrinol. Metab. 51, 173–175 (1980).
Knobil, E., Plant, T. M., Wildt, L., Belchetz, P. E. & Marshall, G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science 207, 1371–1373 (1980).
Plant, T. M. et al. Further studies on the effects of lesions in the rostral hypothalamus on gonadotropin secretion in the female rhesus monkey (Macaca mulatta). Endocrinology 105, 465–473 (1979).
Hess, D. L. et al. Estrogen-induced gonadotropin surges in decerebrated female rhesus monkeys with medial basal hypothalamic peninsulae. Endocrinology 101, 1264–1271 (1977).
Karsch, F. J. et al. Positive and negative feedback control by estrogen of luteinizing hormone secretion in the rhesus monkey. Endocrinology 92, 799–804 (1973).
Klingman, K. M., Marsh, E. E., Klerman, E. B., Anderson, E. J. & Hall, J. E. Absence of circadian rhythms of gonadotropin secretion in women. J. Clin. Endocrinol. Metab. 96, 1456–1461 (2011).
Xia, L., Van Vugt, D., Alston, E. J., Luckhaus, J. & Ferin, M. A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology 131, 2812–2820 (1992).
Pau, K. F., Berria, M., Hess, D. L. & Spies, H. G. Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology 133, 1650–1656 (1993).
Skorupskaite, K., George, J. T. & Anderson, R. A. The kisspeptin–GnRH pathway in human reproductive health and disease. Hum. Reprod. Update 20, 485–500 (2014).
Bakker, J. & Baum, M. J. Neuroendocrine regulation of GnRH release in induced ovulators. Front. Neuroendocrinol. 21, 220–262 (2000).
Inoue, N. et al. Kisspeptin neurons mediate reflex ovulation in the musk shrew (Suncus murinus). Proc. Natl Acad. Sci. USA 108, 17527–17532 (2011).
Smith, J. T., Shahab, M., Pereira, A., Pau, K. Y. & Clarke, I. J. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol. Reprod. 83, 568–577 (2010).
Merkley, C. M. et al. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology 153, 5406–5414 (2012).
Norman, R. L., Resko, J. A. & Spies, H. G. The anterior hypothalamus: how it affects gonadotropin secretion in the rhesus monkey. Endocrinology 99, 59–71 (1976).
Watanabe, Y. et al. Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinising hormone surge in male and female Japanese monkeys. J. Neuroendocrinol. 26, 909–917 (2014).
Gibson, M. J. & Silverman, A. J. Effects of gonadectomy and treatment with gonadal steroids and luteinizing hormone secretion in hypogonadal male and female mice with preoptic area implants. Endocrinology 125, 1525–1532 (1989).
Li, X.-F. & O'Byrne, K., T. in Knobil and Neill's Physiology of Reproduction 4th edn Vol. 2 (eds Plant, T. M. & Zeleznik, A. J.) 1637–1660 (Academic Press, 2015).
Clarke, I. J. Interface between metabolic balance and reproduction in ruminants: focus on the hypothalamus and pituitary. Horm. Behav. 66, 15–40 (2014).
Tena-Sempere, M. in Knobil and Neill's Physiology of Reproduction 4th edn Vol. 2 (eds Plant, T. M. & Zeleznik, A. J.) 1605–1636 (Academic Press, 2015).
Turek, F. W. & Campbell, C. S. Photoperiodic regulation of neuroendocrine-gonadal activity. Biol. Reprod. 20, 32–50 (1979).
Hazlerigg, D. & Simonneaux, V. in Knobil and Neill's Physiology of Reproduction (eds Plant, T. M. & Zeleznik, A. J.) 1575–1604 (Academic Press, 2015).
Prevot, V. in Knobil and Neill's Physiology of Reproduction 4th edn Vol. 2 (eds Plant, T. M. & Zeleznik, A. J.) 1395–1439 (Academic Press, 2015).
Foster, D. L. & Hileman, S. M. in Knobil and Neill's Physiology of Reproduction 4th edn Vol. 2 (eds Plant, T. M. & Zeleznik, A. J.) 1441–1486 (Academic Press, 2015).
Plant, T. M., Terasawa, E. & Witchel, S. F. in Knobil and Neill's Physiology of Reproduction 4th edn Vol. 2 (eds Plant, T. M. & Zeleznik, A. J.) 1487–1536 (Academic Press, 2015).
Kaplan, S. L., Grumbach, M. M. & Aubert, M. L. The ontogenesis of pituitary hormones and hypothalamic factors in the human fetus: maturation of central nervous system regulation of anterior pituitary function. Recent Prog. Horm. Res. 32, 161–243 (1976).
Clements, J. A., Reyes, F. I., Winter, J. S. & Faiman, C. Studies on human sexual development. III. Fetal pituitary and serum, and amniotic fluid concentrations of LH, CG, and FSH. J. Clin. Endocrinol. Metab. 42, 9–19 (1976).
Clark, S. J. et al. Hormone ontogeny in the ovine fetus. XVII. Demonstration of pulsatile luteinizing hormone secretion by the fetal pituitary gland. Endocrinology 115, 1774–1779 (1984).
Huhtaniemi, I. T. et al. Stimulation of pituitary-testicular function with gonadotropin-releasing hormone in fetal and infant monkeys. Endocrinology 105, 109–114 (1979).
Polkowska, J. Development of the gonadotrophic and somatotrophic axes of sheep. J. Reprod. Fertil. Suppl. 49, 187–195 (1995).
Duittoz, A. H. & Batailler, M. Pulsatile GnRH secretion from primary cultures of sheep olfactory placode explants. J. Reprod. Fertil. 120, 391–396 (2000).
Terasawa, E., Keen, K. L., Mogi, K. & Claude, P. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology 140, 1432–1441 (1999).
de Zegher, F., Devlieger, H. & Veldhuis, J. D. Pulsatile and sexually dimorphic secretion of luteinizing hormone in the human infant on the day of birth. Pediatr. Res. 32, 605–607 (1992).
Corbier, P. et al. Sex differences in serum luteinizing hormone and testosterone in the human neonate during the first few hours after birth. J. Clin. Endocrinol. Metab. 71, 1344–1348 (1990).
Corbier, P., Kerdelhue, B., Picon, R. & Roffi, J. Changes in testicular weight and serum gonadotropin and testosterone levels before, during, and after birth in the perinatal rat. Endocrinology 103, 1985–1991 (1978).
Thomas, G. B., McNeilly, A. S., Gibson, F. & Brooks, A. N. Effects of pituitary-gonadal suppression with a gonadotrophin-releasing hormone agonist on fetal gonadotrophin secretion, fetal gonadal development and maternal steroid secretion in the sheep. J. Endocrinol. 141, 317–324 (1994).
Clarkson, J. et al. Sexual differentiation of the brain requires perinatal kisspeptin–GnRH neuron signaling. J. Neurosci. 34, 15297–15305 (2014).
Wen, S., Ai, W., Alim, Z. & Boehm, U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc. Natl Acad. Sci. USA 107, 16372–16377 (2010).
Pointis, G. & Mahoudeau, J. A. Demonstration of a pituitary gonadotrophin hormone activity in the male foetal mouse. Acta Endocrinol. (Copenh.) 83, 158–165 (1976).
Liu, L. et al. Effects of pituitary–testicular axis suppression in utero and during the early neonatal period with a long-acting luteinizing hormone-releasing hormone analog on genital development, somatic growth, and bone density in male cynomolgus monkeys in the first 6 months of life. J. Clin. Endocrinol. Metab. 73, 1038–1043 (1991).
Poling, M. C. & Kauffman, A. S. Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin–Kiss1r and GnRH signaling. Endocrinology 153, 782–793 (2012).
Clarkson, J. & Herbison, A. Hypothalamic control of the male neonatal testosterone surge. Phil. Trans. R. Soc. B 371, 20150115 (2016).
Bouvattier, C. et al. Neonatal gonadotropin therapy in male congenital hypogonadotropic hypogonadism. Nat. Rev. Endocrinol. 8, 172–182 (2012).
Bin-Abbas, B., Conte, F. A., Grumbach, M. M. & Kaplan, S. L. Congenital hypogonadotropic hypogonadism and micropenis: effect of testosterone treatment on adult penile size why sex reversal is not indicated. J. Pediatr. 134, 579–583 (1999).
Winter, J. S., Faiman, C., Hobson, W. C., Prasad, A. V. & Reyes, F. I. Pituitary–gonadal relations in infancy. I. Patterns of serum gonadotropin concentrations from birth to four years of age in man and chimpanzee. J. Clin. Endocrinol. Metab. 40, 545–551 (1975).
Grumbach, M. M. The neuroendocrinology of human puberty revisited. Horm. Res. 57 (Suppl. 2), 2–14 (2002).
Schmidt, H. & Schwarz, H. P. Serum concentrations of LH and FSH in the healthy newborn. Eur. J. Endocrinol. 143, 213–215 (2000).
Waldhauser, F., Weissenbacher, G., Frisch, H. & Pollak, A. Pulsatile secretion of gonadotropins in early infancy. Eur. J. Pediatr. 137, 71–74 (1981).
Dohler, K. D. & Wuttke, W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology 97, 898–907 (1975).
Ojeda, S. R. & Ramirez, V. D. Plasma level of LH and FSH in maturing rats: response to hemigonadectomy. Endocrinology 90, 466–472 (1972).
Mann, D. R. et al. Blockade of neonatal activation of the pituitary–testicular axis with continuous administration of a gonadotropin-releasing hormone agonist in male rhesus monkeys. J. Clin. Endocrinol. Metab. 59, 207–211 (1984).
Andrews, W. W., Advis, J. P. & Ojeda, S. R. The maturation of estradiol-negative feedback in female rats: evidence that the resetting of the hypothalamic “gonadostat” does not precede the first preovulatory surge of gonadotropins. Endocrinology 109, 2022–2031 (1981).
Terasawa, E. & Fernandez, D. L. Neurobiological mechanisms of the onset of puberty in primates. Endocr. Rev. 22, 111–151 (2001).
Brann, D. W. Glutamate: a major excitatory transmitter in neuroendocrine regulation. Neuroendocrinology 61, 213–225 (1995).
Plant, T. M., Gay, V. L., Marshall, G. R. & Arslan, M. Puberty in monkeys is triggered by chemical stimulation of the hypothalamus. Proc. Natl Acad. Sci. USA 86, 2506–2510 (1989).
Navarro, V. M. et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J. Physiol. 561, 379–386 (2004).
Glanowska, K. M., Burger, L. L. & Moenter, S. M. Development of gonadotropin-releasing hormone secretion and pituitary response. J. Neurosci. 34, 15060–15069 (2014).
Bourguignon, J. P. & Franchimont, P. Puberty-related increase in episodic LHRH release from rat hypothalamus in vitro. Endocrinology 114, 1941–1943 (1984).
Urbanski, H. F. & Ojeda, S. R. The juvenile-peripubertal transition period in the female rat: establishment of a diurnal pattern of pulsatile luteinizing hormone secretion. Endocrinology 117, 644–649 (1985).
Watanabe, G. & Terasawa, E. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology 125, 92–99 (1989).
Suter, K. J., Pohl, C. R. & Plant, T. M. The pattern and tempo of the pubertal reaugmentation of open-loop pulsatile gonadotropin-releasing hormone release assessed indirectly in the male rhesus monkey (Macaca mulatta). Endocrinology 139, 2774–2783 (1998).
Boyar, R. et al. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N. Engl. J. Med. 287, 582–586 (1972).
Terasawa, E. Developmental changes in the positive feedback effect of estrogen on luteinizing hormone release in ovariectomized female rhesus monkeys. Endocrinology 117, 2490–2497 (1985).
Ybarra, N., Hemond, P. J., O'Boyle, M. P. & Suter, K. J. Spatially selective, testosterone-independent remodeling of dendrites in gonadotropin-releasing hormone (GnRH) neurons prepubertally in male rats. Endocrinology 152, 2011–2019 (2011).
Cottrell, E. C., Campbell, R. E., Han, S. K. & Herbison, A. E. Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology 147, 3652–3661 (2006).
Wray, S. & Hoffman, G. Postnatal morphological changes in rat LHRH neurons correlated with sexual maturation. Neuroendocrinology 43, 93–97 (1986).
Witkin, J. W. & Romero, M. T. Comparison of ultrastructural characteristics of gonadotropin-releasing hormone neurons in prepubertal and adult male rats. Neuroscience 64, 1145–1151 (1995).
Sim, J. A., Skynner, M. J. & Herbison, A. E. Heterogeneity in the basic membrane properties of postnatal gonadotropin-releasing hormone neurons in the mouse. J. Neurosci. 21, 1067–1075 (2001).
Spergel, D. J. Calcium and small-conductance calcium-activated potassium channels in gonadotropin-releasing hormone neurons before, during, and after puberty. Endocrinology 148, 2383–2390 (2007).
Ben-Ari, Y. The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience 279, 187–219 (2014).
Aronica, E., Iyer, A., Zurolo, E. & Gorter, J. A. Ontogenetic modifications of neuronal excitability during brain maturation: developmental changes of neurotransmitter receptors. Epilepsia 52 (Suppl. 8), 3–5 (2011).
Clarkson, J. & Herbison, A. E. Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol. Cell. Endocrinol. 254–255, 32–38 (2006).
Moore, A. M., Prescott, M., Marshall, C. J., Yip, S. H. & Campbell, R. E. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc. Natl Acad. Sci. USA 112, 596–601 (2015).
Herbison, A. E. & Moenter, S. M. Depolarising and hyperpolarising actions of GABAA receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J. Neuroendocrinol. 23, 557–569 (2011).
Iremonger, K. J., Constantin, S., Liu, X. & Herbison, A. E. Glutamate regulation of GnRH neuron excitability. Brain Res. 1364, 35–43 (2010).
Han, S. K., Abraham, I. M. & Herbison, A. E. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology 143, 1459–1466 (2002).
Lee, K., Porteous, R., Campbell, R. E., Luscher, B. & Herbison, A. E. Knockdown of GABAA receptor signaling in GnRH neurons has minimal effects upon fertility. Endocrinology 151, 4428–4436 (2010).
Shimshek, D. R. et al. Impaired reproductive behavior by lack of GluR-B containing AMPA receptors but not of NMDA receptors in hypothalamic and septal neurons. Mol. Endocrinol. 20, 219–231 (2006).
Clasadonte, J. et al. Prostaglandin E2 release from astrocytes triggers gonadotropin-releasing hormone (GnRH) neuron firing via EP2 receptor activation. Proc. Natl Acad. Sci. USA 108, 16104–16109 (2011).
Dziedzic, B. et al. Neuron-to-glia signaling mediated by excitatory amino acid receptors regulates ErbB receptor function in astroglial cells of the neuroendocrine brain. J. Neurosci. 23, 915–926 (2003).
Lomniczi, A., Wright, H., Castellano, J. M., Sonmez, K. & Ojeda, S. R. A system biology approach to identify regulatory pathways underlying the neuroendocrine control of female puberty in rats and nonhuman primates. Horm. Behav. 64, 175–186 (2013).
Kumar, D., Periasamy, V., Freese, M., Voigt, A. & Boehm, U. In utero development of kisspeptin/GnRH neural circuitry in male mice. Endocrinology 156, 3084–3090 (2015).
Kumar, D. et al. Murine arcuate nucleus kisspeptin neurons communicate with GnRH neurons in utero. J. Neurosci. 34, 3756–3766 (2014).
Han, S. K. et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J. Neurosci. 25, 11349–11356 (2005).
Herbison, A. E., de Tassigny, X., Doran, J. & Colledge, W. H. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 151, 312–321 (2010).
Smith, J. T., Cunningham, M. J., Rissman, E. F., Clifton, D. K. & Steiner, R. A. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146, 3686–3692 (2005).
Mayer, C. et al. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc. Natl Acad. Sci. USA 107, 22693–22698 (2010).
Desroziers, E., Mikkelsen, J. D., Duittoz, A. & Franceschini, I. Kisspeptin-immunoreactivity changes in a sex- and hypothalamic-region-specific manner across rat postnatal development. J. Neuroendocrinol. 24, 1154–1165 (2012).
Knoll, J. G. et al. Developmental profile and sexually dimorphic expression of kiss1 and kiss1r in the fetal mouse brain. Front. Endocrinol. (Lausanne) 4, 140 (2013).
Semaan, S. J., Tolson, K. P. & Kauffman, A. S. The development of kisspeptin circuits in the mammalian brain. Adv. Exp. Med. Biol. 784, 221–252 (2013).
Takumi, K., Iijima, N. & Ozawa, H. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J. Mol. Neurosci. 43, 138–145 (2011).
Lehman, M. N., Hileman, S. M. & Goodman, R. L. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv. Exp. Med. Biol. 784, 27–62 (2013).
de Croft, S. et al. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology 153, 5384–5393 (2012).
Guerriero, K. A., Keen, K. L. & Terasawa, E. Developmental increase in kisspeptin-54 release in vivo is independent of the pubertal increase in estradiol in female rhesus monkeys (Macaca mulatta). Endocrinology 153, 1887–1897 (2012).
Guerriero, K. A., Keen, K. L., Millar, R. P. & Terasawa, E. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female rhesus monkeys (Macaca mulatta): implication for the mechanism of puberty. Endocrinology 153, 825–836 (2012).
Shahab, M. et al. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc. Natl Acad. Sci. USA 102, 2129–2134 (2005).
Plant, T. M. Neuroendocrine control of the onset of puberty. Front. Neuroendocrinol. 38, 73–88 (2015).
Abreu, A. P. et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N. Engl. J. Med. 368, 2467–2475 (2013).
Bulcao Macedo, D., Nahime Brito, V. & Latronico, A. C. New causes of central precocious puberty: the role of genetic factors. Neuroendocrinology 100, 1–8 (2014).
Lomniczi, A. et al. Epigenetic control of female puberty. Nat. Neurosci. 16, 281–289 (2013).
Urbanski, H. F. & Ojeda, S. R. The development of afternoon minisurges of luteinizing hormone secretion in prepubertal female rats is ovary dependent. Endocrinology 118, 1187–1193 (1986).
Clarkson, J. & Herbison, A. E. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147, 5817–5825 (2006).
Clarkson, J., Boon, W. C., Simpson, E. R. & Herbison, A. E. Postnatal development of an estradiol–kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology 150, 3214–3220 (2009).
Bronson, F. H. The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology 108, 506–516 (1981).
Simerly, R. B. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 25, 507–536 (2002).
Tsukamura, H., Homma, T., Tomikawa, J., Uenoyama, Y. & Maeda, K. Sexual differentiation of kisspeptin neurons responsible for sex difference in gonadotropin release in rats. Ann. NY Acad. Sci. 1200, 95–103 (2010).
Poling, M. C. & Kauffman, A. S. Organizational and activational effects of sex steroids on kisspeptin neuron development. Front. Neuroendocrinol. 34, 3–17 (2013).
Karsch, F. J., Dierschke, D. J. & Knobil, E. Sexual differentiation of pituitary function: apparent difference bewteen primates and rodents. Science 179, 484–486 (1973).
Steiner, R. A., Clifton, D. K., Spies, H. G. & Resko, J. A. Sexual differentiation and feedback control of luteinizing hormone secretion in the Rhesus monkey. Biol. Reprod. 15, 206–212 (1976).
Matsumoto, S. et al. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc. Natl Acad. Sci. USA 103, 4140–4145 (2006).
de Roux, N. et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl Acad. Sci. USA 100, 10972–10976 (2003).
Topaloglu, A. K. et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat. Genet. 41, 354–358 (2009).
Yang, J. J., Caligioni, C. S., Chan, Y. M. & Seminara, S. B. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology 153, 1498–1508 (2012).
Gill, J. C. et al. Increased neurokinin B (Tac2) expression in the mouse arcuate nucleus is an early marker of pubertal onset with differential sensitivity to sex steroid-negative feedback than kiss1. Endocrinology 153, 4883–4893 (2012).
Frisch, R. E. & Revelle, R. Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science 169, 397–399 (1970).
Ojeda, S. R. & Skinner, M. K. in Knobil and Neill's Physiology of Reproduction 3rd edn Vol. 2 (ed. Neill, J. D.) 2061–2126 (Academic Press, 2006).
Cunningham, M. J., Clifton, D. K. & Steiner, R. A. Leptin's actions on the reproductive axis: perspectives and mechanisms. Biol. Reprod. 60, 216–222 (1999).
Quennell, J. H. et al. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150, 2805–2812 (2009).
Zuure, W. A., Roberts, A. L., Quennell, J. H. & Anderson, G. M. Leptin signaling in GABA Neurons, but not glutamate neurons, is required for reproductive function. J. Neurosci. 33, 17874–17883 (2013).
Martin, C. et al. Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone. J. Neurosci. 34, 6047–6056 (2014).
Donato, J. Jr et al. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J. Clin. Invest. 121, 355–368 (2011).
Franks, S., Hardy, K. & Conway, G. S. in Knobil and Neill's Physiology of Reproduction 4th edn Vol. 2 (eds Plant, T. M. & Zeleznik, A. J.) 1363–1394 (Academic Press, 2015).
Jayasena, C. N. & Franks, S. The management of patients with polycystic ovary syndrome. Nat. Rev. Endocrinol. 10, 624–636 (2014).
Burt Solorzano, C. M. et al. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids 77, 332–337 (2012).
Kauffman, A. S. et al. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol. Reprod. 93, 69 (2015).
Fourman, L. T. & Fazeli, P. K. Neuroendocrine causes of amenorrhea — an update. J. Clin. Endocrinol. Metab. 100, 812–824 (2015).
Bullen, B. A. et al. Induction of menstrual disorders by strenuous exercise in untrained women. N. Engl. J. Med. 312, 1349–1353 (1985).
Caronia, L. M. et al. A genetic basis for functional hypothalamic amenorrhea. N. Engl. J. Med. 364, 215–225 (2011).
Perkins, R. B., Hall, J. E. & Martin, K. A. Aetiology, previous menstrual function and patterns of neuro-endocrine disturbance as prognostic indicators in hypothalamic amenorrhoea. Hum. Reprod. 16, 2198–2205 (2001).
Araujo-Lopes, R. et al. Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology 155, 1010–1020 (2014).
Liu, X., Brown, R. S., Herbison, A. E. & Grattan, D. R. Lactational anovulation in mice results from a selective loss of kisspeptin input to GnRH neurons. Endocrinology 155, 193–203 (2014).
Mitchell, A. L., Dwyer, A., Pitteloud, N. & Quinton, R. Genetic basis and variable phenotypic expression of Kallmann syndrome: towards a unifying theory. Trends Endocrinol. Metab. 22, 249–258 (2011).
Stamou, M. I., Cox, K. H. & Crowley, W. F. Jr. Discovering genes essential to the hypothalamic regulation of human reproduction using a human disease model: adjusting to life in the “-omics” era. Endocr. Rev. 36, 603–621 (2015).
Schwanzel-Fukuda, M., Bick, D. & Pfaff, D. W. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res. Mol. Brain Res. 6, 311–326 (1989).
Pitteloud, N. et al. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J. Clin. Invest. 117, 457–463 (2007).
Clifton, D. K. & Steiner, R. A. Recovery of pulsatile luteinizing hormone secretion following permanent disruption of the ascending noradrenergic fiber tract in the ovariectomized rat. Biol. Reprod. 33, 808–814 (1985).
Popa, S. M. et al. Redundancy in Kiss1 expression safeguards reproduction in the mouse. Endocrinology 154, 2784–2794 (2013).
Bianco, S. D. & Kaiser, U. B. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat. Rev. Endocrinol. 5, 569–576 (2009).
Sidhoum, V. F. et al. Reversal and relapse of hypogonadotropic hypogonadism: resilience and fragility of the reproductive neuroendocrine system. J. Clin. Endocrinol. Metab. 99, 861–870 (2014).
Harris, G. W. Neural control of the pituitary gland (Edward Arnold, 1955).
Schally, A. V. et al. Isolation and properties of the FSH and LH-releasing hormone. Biochem. Biophys. Res. Commun. 43, 393–399 (1971).
Amoss, M. et al. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing hormone factor (LRF) of ovine origin. Biochem. Biophys. Res. Commun. 44, 205–210 (1971).
Millar, R. P. & Newton, C. L. Current and future applications of GnRH, kisspeptin and neurokinin B analogues. Nat. Rev. Endocrinol. 9, 451–466 (2013).
Clarke, H., Dhillo, W. S. & Jayasena, C. N. Comprehensive review on kisspeptin and its role in reproductive disorders. Endocrinol. Metab. (Seoul) 30, 124–141 (2015).
Acknowledgements
Studies in the laboratory of A.E.H. were supported by the New Zealand Health Research Council and the Royal Society Marsden Fund. A.E.H. thanks all past and present members of his laboratory for many stimulating discussions and experimental work that has aimed to advance understanding of the gonadotropin-releasing hormone neuron. Long-standing research collaborations with U. Boehm (University of Saarland, Germany), R. Campbell (University of Otago, New Zealand), B. Colledge (University of Cambridge, UK), D. Grattan (University of Otago, New Zealand) and G. Schütz (Heidelberg University, Germany) have also been invaluable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Herbison, A. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol 12, 452–466 (2016). https://doi.org/10.1038/nrendo.2016.70
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.70
This article is cited by
-
The role of NPY2R/NFATc1/DYRK1A regulatory axis in sebaceous glands for sebum synthesis
Cellular & Molecular Biology Letters (2023)
-
Interplay between hippocampal TACR3 and systemic testosterone in regulating anxiety-associated synaptic plasticity
Molecular Psychiatry (2023)
-
Hypothalamic Kisspeptin Neurons: Integral Elements of the GnRH System
Reproductive Sciences (2023)
-
Intra-pituitary follicle-stimulating hormone signaling regulates hepatic lipid metabolism in mice
Nature Communications (2023)
-
Epigenetic regulation of 5α reductase-1 underlies adaptive plasticity of reproductive function and pubertal timing
BMC Biology (2022)