Abstract
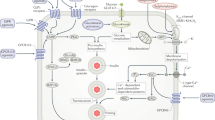
On the basis of data obtained from a prospective cohort of Chinese patients with type 2 diabetes mellitus (T2DM), we discuss cancer subphenotypes (risk factors) in patients with T2DM, which can lead to drug–cancer subphenotype interactions. These subphenotypes include HDL cholesterol levels <1.0 mmol/l, co-occurrence of LDL cholesterol levels <2.8 mmol/l and triglyceride levels <1.7 mmol/l, and co-occurrence of LDL cholesterol levels <2.8 mmol/l and albuminuria. The increased risk of cancer associated with low levels of HDL cholesterol, low LDL cholesterol levels plus low triglyceride levels, and low levels of LDL cholesterol plus albuminuria can be reduced by treatment with metformin, renin–angiotensin system (RAS) inhibitors and statins, respectively. Mechanistic studies support the hypothesis that dysregulation of the 5′-AMP-activated protein kinase pathway and crosstalk between the RAS and insulin-like growth factor 1–cholesterol pathways create a cancer-promoting milieu in patients with T2DM. These findings highlight that in Chinese individuals, multiple pathways are implicated in the link between T2DM and cancer, which can generate multiple subphenotypes as well as drug–subphenotype interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Yang, X. et al. Independent associations between low-density lipoprotein cholesterol and cancer among patients with type 2 diabetes mellitus. CMAJ 179, 427–437 (2008).
Jee, S. H. et al. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 293, 194–202 (2005).
Stattin, P. et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care 30, 561–567 (2007).
Yang, X. et al. Associations of hyperglycemia and insulin usage with the risk of cancer in type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes 59, 1254–1260 (2010).
Saydah, S. H. et al. Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 12, 412–418 (2003).
Johnson, J. A. & Bowker, S. L. Intensive glycaemic control and cancer risk in type 2 diabetes: a meta-analysis of major trials. Diabetologia 54, 25–31 (2011).
Roumie, C. L. et al. Association between intensification of metformin treatment with insulin vs sulfonylureas and cardiovascular events and all-cause mortality among patients with diabetes. JAMA 311, 2288–2296 (2014).
Currie, C. J., Poole, C. D., Evans, M., Peters, J. R. & Morgan, C. L. Mortality and other important diabetes-related outcomes with insulin vs other antihyperglycemic therapies in type 2 diabetes. J. Clin. Endocrinol. Metab. 98, 668–677 (2013).
Lewis, J. D. et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 34, 916–922 (2011).
Yang, X. et al. Use of sulphonylurea and cancer in type 2 diabetes—The Hong Kong Diabetes Registry. Diabetes Res. Clin. Pract 90, 343–351 (2010).
Zhang, P., Li, H., Tan, X., Chen, L. & Wang, S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 37, 207–218 (2013).
Gerstein, H. C. et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 367, 319–328 (2012).
Scirica, B. M. et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369, 1317–1326 (2013).
Home, P. D. et al. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 53, 1838–1845 (2010).
Levin, D. et al. Pioglitazone and bladder cancer risk: a multipopulation pooled, cumulative exposure analysis. Diabetologia 58, 493–504 (2014).
Andersson, T., Alfredsson, L., Kallberg, H., Zdravkovic, S. & Ahlbom, A. Calculating measures of biological interaction. Eur. J. Epidemiol. 20, 575–579 (2005).
Yang, X. et al. Validation of methods to control for immortal time bias in a pharmacoepidemiologic analysis of renin–angiotensin system inhibitors in type 2 diabetes. J. Epidemiol. 24, 267–273 (2014).
Kong, A. P. et al. Additive effects of blood glucose lowering drugs, statins and renin–angiotensin system blockers on all-site cancer risk in patients with type 2 diabetes. BMC Med. 12, 76 (2014).
Yang, X. L. et al. Addressing different biases in analysing drug use on cancer risk in diabetes in non-clinical trial settings—what, why and how? Diabetes Obes. Metab. 14, 579–585 (2012).
Rothman, K. J. & Greenland, S. Causation and causal inference in epidemiology. Am. J. Public Health 95 (Suppl. 1), S144–S150 (2005).
Yang, X. L. et al. Predicting values of lipids and white blood cell count for all-site cancer in type 2 diabetes. Endocr. Relat. Cancer 15, 597–607 (2008).
Yang, X. et al. Low HDL cholesterol, metformin use, and cancer risk in type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes Care 34, 375–380 (2011).
Yang, X. et al. Low triglyceride and nonuse of statins is associated with cancer in type 2 diabetes mellitus: the Hong Kong Diabetes Registry. Cancer 117, 862–871 (2011).
Yang, X. et al. Low LDL cholesterol, albuminuria, and statins for the risk of cancer in type 2 diabetes: the Hong Kong diabetes registry. Diabetes Care 32, 1826–1832 (2009).
Yang, X. et al. Synergistic effects of low LDL cholesterol with other factors for the risk of cancer in type 2 diabetes: the Hong Kong Diabetes Registry. Acta Diabetol. 49 (Suppl. 1), S185–S193 (2012).
Alsheikh-Ali, A. A., Trikalinos, T. A., Kent, D. M. & Karas, R. H. Statins, low-density lipoprotein cholesterol, and risk of cancer. J. Am. Coll. Cardiol. 52, 1141–1147 (2008).
Zhao, W. et al. HDL Cholesterol and cancer risk among patients with type 2 diabetes. Diabetes Care 37, 3196–3203 (2014).
Jafri, H., Alsheikh-Ali, A. A. & Karas, R. H. Baseline and on-treatment high-density lipoprotein cholesterol and the risk of cancer in randomized controlled trials of lipid-altering therapy. J. Am. Coll. Cardiol. 55, 2846–2854 (2010).
Kahn, S. E. The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46, 3–19 (2003).
Yoon, K. H. et al. Selective β-cell loss and α-cell expansion in patients with type 2 diabetes mellitus in Korea. J. Clin. Endocrinol. Metab. 88, 2300–2308 (2003).
Suraamornkul, S., Kwancharoen, R., Ovartlarnporn, M., Rawdaree, P. & Bajaj, M. Insulin clamp-derived measurements of insulin sensitivity and insulin secretion in lean and obese Asian type 2 diabetic patients. Metab. Syndr. Relat. Disord. 8, 113–118 (2010).
Liguori, A. et al. Effect of glycaemic control and age on low-density lipoprotein susceptibility to oxidation in diabetes mellitus type 1. Eur. Heart J. 22, 2075–2084 (2001).
Chen, J. & Mehta, J. L. Interaction of oxidized low-density lipoprotein and the renin–angiotensin system in coronary artery disease. Curr. Hypertens. Rep. 8, 139–143 (2006).
Singh, B. M. & Mehta, J. L. Interactions between the renin–angiotensin system and dyslipidemia: relevance in the therapy of hypertension and coronary heart disease. Arch. Intern. Med. 163, 1296–1304 (2003).
Yang, X. et al. Additive interaction between the renin–angiotensin system and lipid metabolism for cancer in type 2 diabetes. Diabetes 58, 1518–1525 (2009).
Silvente-Poirot, S. & Poirot, M. Cancer. Cholesterol and cancer, in the balance. Science 343, 1445–1446 (2014).
Yang, X. L. & Chan, J. C. Metformin and the risk of cancer in type 2 diabetes: methodological challenges and perspectives. Ann. Transl Med. 2, 52 (2014).
Yang, X. et al. Renin–angiotensin system inhibitors may attenuate low LDL cholesterol-related cancer risk in type 2 diabetes. Diabetes Metab. Res. Rev. 30, 415–423 (2014).
Towler, M. C. & Hardie, D. G. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 100, 328–341 (2007).
Kim, I. & He, Y. Y. Targeting the AMP-activated protein kinase for cancer prevention and therapy. Front. Oncol. 3, 175 (2013).
Hardie, D. G. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes 62, 2164–2172 (2013).
Stephenne, X. et al. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia 54, 3101–3110 (2011).
Dowling, R. J., Zakikhani, M., Fantus, I. G., Pollak, M. & Sonenberg, N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 67, 10804–10812 (2007).
Alimova, I. N. et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 8, 909–915 (2009).
Hofmann, J. Protein kinase C isozymes as potential targets for anticancer therapy. Curr. Cancer Drug Targets 4, 125–146 (2004).
Buzzai, M. et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 67, 6745–6752 (2007).
Murao, K. et al. Effects of glucose and insulin on rat apolipoprotein A-I gene expression. J. Biol. Chem. 273, 18959–18965 (1998).
Han, R. et al. Apolipoprotein A-I stimulates AMP-activated protein kinase and improves glucose metabolism. Diabetologia 50, 1960–1968 (2007).
Kimura, T. et al. Mechanism and role of high density lipoprotein-induced activation of AMP-activated protein kinase in endothelial cells. J. Biol. Chem. 285, 4387–4397 (2010).
Cazzaniga, M., Bonanni, B., Guerrieri-Gonzaga, A. & Decensi, A. Is it time to test metformin in breast cancer clinical trials? Cancer Epidemiol. Biomarkers Prev. 18, 701–705 (2009).
Samani, A. A., Yakar, S., LeRoith, D. & Brodt, P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr. Rev. 28, 20–47 (2007).
Moschos, S. J. & Mantzoros, C. S. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology 63, 317–332 (2002).
Godsland, I. F. Insulin resistance and hyperinsulinaemia in the development and progression of cancer. Clin. Sci. (Lond.) 118, 315–332 (2010).
LeRoith, D. et al. Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp. Clin. Endocrinol. Diabetes 116 (Suppl. 1), S4–S6 (2008).
Hemkens, L. G. et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 52, 1732–1744 (2009).
Andersen, A. S. et al. Identification of determinants that confer ligand specificity on the insulin receptor. J. Biol. Chem. 267, 13681–13686 (1992).
Slaaby, R. et al. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J. Biol. Chem. 281, 25869–25874 (2006).
Felig, P. in Metabolic Control and Disease (eds Bondy, P. K. & Rosenberg, L. E.) 277–301 (Saunders, 1980).
Carlberg, M. et al. Mevalonic acid is limiting for N-linked glycosylation and translocation of the insulin-like growth factor-1 receptor to the cell surface. Evidence for a new link between 3-hydroxy-3-methylglutaryl-coenzyme A reductase and cell growth. J. Biol. Chem. 271, 17453–17462 (1996).
Brown, M. S. & Goldstein, J. L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340 (1997).
Eberle, D., Hegarty, B., Bossard, P., Ferre, P. & Foufelle, F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86, 839–848 (2004).
Shimomura, I. et al. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc. Natl Acad. Sci. USA 96, 13656–13661 (1999).
Bhasker, C. R. & Friedmann, T. Insulin-like growth factor-1 coordinately induces the expression of fatty acid and cholesterol biosynthetic genes in murine C2C12 myoblasts. BMC Genomics 9, 535 (2008).
Du, X., Kristiana, I., Wong, J. & Brown, A. J. Involvement of Akt in ER-to-Golgi transport of SCAP/SREBP: a link between a key cell proliferative pathway and membrane synthesis. Mol. Biol. Cell 17, 2735–2745 (2006).
Luu, W., Sharpe, L. J., Stevenson, J. & Brown, A. J. Akt acutely activates the cholesterogenic transcription factor SREBP-2. Biochim. Biophys. Acta 1823, 458–464 (2012).
Krycer, J. R., Sharpe, L. J., Luu, W. & Brown, A. J. The Akt–SREBP nexus: cell signaling meets lipid metabolism. Trends Endocrinol. Metab. 21, 268–276 (2010).
Eggert, M. L. et al. Cross-sectional and longitudinal relation of IGF1 and IGF-binding protein 3 with lipid metabolism. Eur. J. Endocrinol. 171, 9–19 (2014).
Paolisso, G. et al. Serum levels of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 in healthy centenarians: relationship with plasma leptin and lipid concentrations, insulin action, and cognitive function. J. Clin. Endocrinol. Metab. 82, 2204–2209 (1997).
Vigil, D., Cherfils, J., Rossman, K. L. & Der, C. J. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat. Rev. Cancer 10, 842–857 (2010).
Shimizu, M. et al. Pitavastatin suppresses diethylnitrosamine-induced liver preneoplasms in male C57BL/KsJ-db/db obese mice. BMC Cancer 11, 281 (2011).
Yasuda, Y. et al. Pitavastatin inhibits azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Cancer Sci. 101, 1701–1707 (2010).
Ko, G. T. et al. Effect of interactions between C peptide levels and insulin treatment on clinical outcomes among patients with type 2 diabetes mellitus. CMAJ 180, 919–926 (2009).
Schrijvers, B. F., De Vriese, A. S. & Flyvbjerg, A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr. Rev. 25, 971–1010 (2004).
Sugimoto, M. et al. Influences of chymase and angiotensin I-converting enzyme gene polymorphisms on gastric cancer risks in Japan. Cancer Epidemiol. Biomarkers Prev. 15, 1929–1934 (2006).
George, A. J., Thomas, W. G. & Hannan, R. D. The renin–angiotensin system and cancer: old dog, new tricks. Nat. Rev. Cancer 10, 745–759 (2010).
Jia, G., Aggarwal, A., Yohannes, A., Gangahar, D. M. & Agrawal, D. K. Cross-talk between angiotensin II and IGF-1-induced connexin 43 expression in human saphenous vein smooth muscle cells. J. Cell. Mol. Med. 15, 1695–1702 (2011).
Yasumaru, M. et al. Inhibition of angiotensin II activity enhanced the antitumor effect of cyclooxygenase-2 inhibitors via insulin-like growth factor I receptor pathway. Cancer Res. 63, 6726–6734 (2003).
Yang, X. et al. Low LDL cholesterol, albuminuria, and statins for the risk of cancer in type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes Care 32, 1826–1832 (2009).
Sui, Y. et al. Pancreatic islet β-cell deficit and glucose intolerance in rats with uninephrectomy. Cell. Mol. Life Sci. 64, 3119–3128 (2007).
Zhao, H. L. et al. Fat redistribution and adipocyte transformation in uninephrectomized rats. Kidney Int. 74, 467–477 (2008).
De Mitri, M. S., Cassini, R. & Bernardi, M. Hepatitis B virus-related hepatocarcinogenesis: molecular oncogenic potential of clear or occult infections. Eur. J. Cancer 46, 2178–2186 (2010).
Yamasaki, K., Hayashi, Y., Okamoto, S., Osanai, M. & Lee, G. H. Insulin-independent promotion of chemically induced hepatocellular tumor development in genetically diabetic mice. Cancer Sci. 101, 65–72 (2010).
Tsan, Y. T., Lee, C. H., Wang, J. D. & Chen, P. C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J. Clin. Oncol. 30, 623–630 (2012).
Yang, X. et al. Enhancers and attenuators of risk associations of chronic hepatitis B virus infection with hepatocellular carcinoma in type 2 diabetes. Endocr. Relat. Cancer 20, 161–171 (2013).
Melvin, J. C., Holmberg, L., Rohrmann, S., Loda, M. & Van Hemelrijck, M. Serum lipid profiles and cancer risk in the context of obesity: four meta-analyses. J. Cancer Epidemiol. 2013, 823849 (2013).
Dombrowski, F., Bannasch, P. & Pfeifer, U. Hepatocellular neoplasms induced by low-number pancreatic islet transplants in streptozotocin diabetic rats. Am. J. Pathol. 150, 1071–1087 (1997).
Shafie, S. M. & Grantham, F. H. Role of hormones in the growth and regression of human breast cancer cells (MCF-7) transplanted into athymic nude mice. J. Natl Cancer Inst. 67, 51–56 (1981).
Goodwin, P. J. et al. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J. Clin. Oncol. 30, 164–171 (2012).
Onitilo, A. A. et al. Type 2 diabetes mellitus, glycemic control, and cancer risk. Eur. J. Cancer Prev. 23, 134–140 (2014).
Calle, E. E. & Kaaks, R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579–591 (2004).
Chan, J. C., Zhang, Y. & Ning, G. Diabetes in China: a societal solution for a personal challenge. Lancet Diabetes Endocrinol. 2, 969–979 (2014).
Weinstein, I., Patel, T. B. & Heimberg, M. Secretion of triglyceride and ketogenesis by livers from spontaneous diabetic BB Wistar rats. Biochem. Biophys. Res. Commun. 176, 1157–1162 (1991).
Yoshino, G. et al. Effect of long-term insulin deficiency and insulin treatment on the composition of triglyceride-rich lipoproteins and triglyceride turnover in rats. Atherosclerosis 92, 243–250 (1992).
Kraemer, F. B. Insulin deficiency alters cellular cholesterol metabolism in murine macrophages. Diabetes 35, 764–770 (1986).
Pischon, T. et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 359, 2105–2120 (2008).
Davis-Yadley, A. H. et al. Ethnic disparities in the risk of colorectal adenomas associated with lipid levels: a retrospective multiethnic study. J. Gastrointest. Cancer 46, 29–35 (2015).
Yang, X. et al. White blood cell count and renin–angiotensin system inhibitors for the risk of cancer in type 2 diabetes. Diabetes Res. Clin. Pract. 87, 117–125 (2010).
Ma, R. C. & Chan, J. C. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann. NY Acad. Sci. 1281, 64–91 (2013).
Whiting, D. R., Guariguata, L., Weil, C. & Shaw, J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 94, 311–321 (2011).
Ranc, K., Jorgensen, M. E., Friis, S. & Carstensen, B. Mortality after cancer among patients with diabetes mellitus: effect of diabetes duration and treatment. Diabetologia 57, 927–934 (2014).
Acknowledgements
X.Y., H.M.L. and J.C.N.C. acknowledge H. Gerstein, McMaster University, Canada, for advice regarding this article, and thank all clinical and supporting staff involved in setting up the Hong Kong Diabetes Registry and all patients for donating their anonymized data for research and education purposes.
Author information
Authors and Affiliations
Contributions
X.Y. and J.C.N.C. contributed equally to researching data for the article, to providing substantial contributions to discussions of the content, to writing the article and to reviewing and/or editing of the manuscript before submission. H.M.L. provided a substantial contribution to discussions of the content and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
J.C.N.C. declares that she is a board member of the Asia Diabetes Foundation, has acted as a consultant for AstraZeneca, Bayer, Boehringer Ingelheim, Merck Sharp & Dohme, Pfizer, Sanofi and Qualigenics, and that she has received honoraria, travel expenses and/or payments from AstraZeneca, Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, GlaxoSmithKline, Merck Serono, Merck Sharp & Dohme, Nestle Nutrition Institute, Novo Nordisk, Pfizer, Roche, Sanofi and Takeda for giving lectures. H.M.L. and X.Y. declare no competing interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Yang, X., Lee, H. & Chan, J. Drug–subphenotype interactions for cancer in type 2 diabetes mellitus. Nat Rev Endocrinol 11, 372–379 (2015). https://doi.org/10.1038/nrendo.2015.37
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2015.37
This article is cited by
-
Association Between Statins and Cancer Incidence in Diabetes: a Cohort Study of Japanese Patients with Type 2 Diabetes
Journal of General Internal Medicine (2021)
-
Exendin-4 Exhibits Enhanced Anti-tumor Effects in Diabetic Mice
Scientific Reports (2017)
-
Association between type 2 diabetes and cancer incidence in Taiwan: data from a prospective community-based cohort study
Acta Diabetologica (2017)
-
Hypoglycemia and Comorbidities in Type 2 Diabetes
Current Diabetes Reports (2015)