Abstract
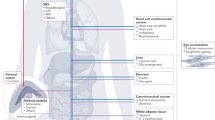
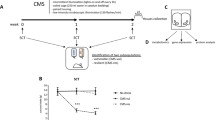
The link between chronic psychosocial and metabolic stress and the pathogenesis of disease has been extensively documented. Nevertheless, the cellular mechanisms by which stressful life experiences and their associated primary neuroendocrine mediators cause biological damage and increase disease risk remain poorly understood. The allostatic load model of chronic stress focuses on glucocorticoid dysregulation. In this Perspectives, we expand upon the metabolic aspects of this model—particularly glucose imbalance—and propose that mitochondrial dysfunction constitutes an early, modifiable target of chronic stress and stress-related health behaviours. Central to this process is mitochondrial regulation of energy metabolism and cellular signalling. Chronically elevated glucose levels damage both mitochondria and mitochondrial DNA, generating toxic products that can promote systemic inflammation, alter gene expression and hasten cell ageing. Consequently, the concept of 'mitochondrial allostatic load' defines the deleterious structural and functional changes that mitochondria undergo in response to elevated glucose levels and stress-related pathophysiology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Emerging Risk Factors Collaboration et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 364, 829–841 (2011).
Olshansky, S. J. et al. A potential decline in life expectancy in the United States in the 21st century. N. Engl. J. Med 352, 1138–1145 (2005).
van Elderen, S. G. et al. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology 75, 997–1002 (2010).
Mattson, M. P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 16, 706–722 (2012).
Dallman, M. F. et al. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 14, 303–347 (1993).
Dickerson, S. S. & Kemeny, M. E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 130, 355–391 (2004).
Testa, R. et al. Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabet. Med. 28, 1388–1394 (2011).
Epel, E. S. et al. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA 101, 17312–17315 (2004).
Shalev, I. et al. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology 38, 1835–1842 (2013).
Picard, M. Pathways to aging: the mitochondrion at the intersection of biological and psychosocial sciences. J. Aging Res. 2011, 814096 (2011).
McEwen, B. S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904 (2007).
McEwen, B. S. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin. Neurosci. 8, 367–381 (2006).
Cohen, S., Janicki-Deverts, D. & Miller, G. E. Psychological stress and disease. JAMA 298, 1685–1687 (2007).
McEwen, B. S. Brain on stress: how the social environment gets under the skin. Proc. Natl Acad. Sci. USA 109 (Suppl. 2), 17180–17185 (2012).
Lupien, S. J., McEwen, B. S., Gunnar, M. R. & Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445 (2009).
Steptoe, A. & Kivimäki, M. Stress and cardiovascular disease: an update on current knowledge. Annu. Rev. Public Health 34, 337–354 (2013).
Sinha, R. & Jastreboff, A. M. Stress as a common risk factor for obesity and addiction. Biol. Psychiatry 73, 827–835 (2013).
Puterman, E. & Epel, E. An intricate dance: life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan. Soc. Personal Psychol. Compass. 6, 807–825 (2012).
Shalev, I. et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol. Psychiatry 18, 576–581 (2013).
Steptoe, A. et al. Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain Behav. Immun. 25, 1292–1298 (2011).
Tomiyama, A. J. et al. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiol. Behav. 106, 40–45 (2012).
Puterman, E. et al. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS ONE 5, e10837 (2010).
Sterling, P. & Eyer, J. in Handbook of Life Stress, Cognition and Health (eds. Fisher, S. & Reason, J.) 629–649 (John Wiley & Sons, New York, 1988).
Stumvoll, M., Tataranni, P. A., Stefan, N., Vozarova, B. & Bogardus, C. Glucose allostasis. Diabetes 52, 903–909 (2003).
McEwen, B. S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 338, 171–179 (1998).
McEwen, B. S. & Stellar, E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 153, 2093–2101 (1993).
Juster, R. P. et al. A transdisciplinary perspective of chronic stress in relation to psychopathology throughout lifespan development. Dev. Psychopathol. 23, 725–776 (2011).
Juster, R. P., McEwen, B. S. & Lupien, S. J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 35, 2–16 (2010).
Seeman, T. E., McEwen, B. S., Rowe, J. W. & Singer, B. H. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc. Natl Acad. Sci. USA 98, 4770–4775 (2001).
Andrews, R. C. & Walker, B. R. Glucocorticoids and insulin resistance: old hormones, new targets. Clin. Sci. (Lond.) 96, 513–523 (1999).
Dinneen, S., Alzaid, A., Miles, J. & Rizza, R. Metabolic effects of the nocturnal rise in cortisol on carbohydrate metabolism in normal humans. J. Clin. Invest. 92, 2283–2290 (1993).
Yuen, K. C., McDaniel, P. A. & Riddle, M. C. Twenty-four-hour profiles of plasma glucose, insulin, C-peptide and free fatty acid in subjects with varying degrees of glucose tolerance following short-term, medium-dose prednisone (20 mg/day) treatment: evidence for differing effects on insulin secretion and action. Clin. Endocrinol. (Oxf.) 77, 224–232 (2012).
Phillips, D. I. et al. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J. Clin. Endocrinol. Metab. 83, 757–760 (1998).
Karatsoreos, I. N. et al. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 151, 2117–2127 (2010).
Chavez, M. et al. Adrenalectomy increases sensitivity to central insulin. Physiol. Behav. 62, 631–634 (1997).
Cannon, W. B. Bodily Changes in Pain, Hunger, Fear, and Rage (Appleton-Century-Crofts, New York, 1929).
Faulenbach, M. et al. Effect of psychological stress on glucose control in patients with type 2 diabetes. Diabet. Med. 29, 128–131 (2012).
Nowotny, B. et al. Effects of acute psychological stress on glucose metabolism and subclinical inflammation in patients with post-traumatic stress disorder. Horm. Metab. Res. 42, 746–753 (2010).
Gonzalez-Bono, E., Rohleder, N., Hellhammer, D. H., Salvador, A. & Kirschbaum, C. Glucose but not protein or fat load amplifies the cortisol response to psychosocial stress. Horm. Behav. 41, 328–333 (2002).
Ismail, K., Winkley, K. & Rabe-Hesketh, S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 363, 1589–1597 (2004).
Kubera, B. et al. The brain's supply and demand in obesity. Front. Neuroenergetics 4, 4 (2012).
Spiegel, K., Leproult, R. & Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 354, 1435–1439 (1999).
Karatsoreos, I. N., Bhagat, S., Bloss, E. B., Morrison, J. H. & McEwen, B. S. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc. Natl Acad. Sci. USA 108, 1657–1662 (2011).
Scheffler, I. E. Mitochondria, 2nd edn (John Wiley & Sons, 2008).
Ballinger, S. W. Beyond retrograde and anterograde signalling: mitochondrial–nuclear interactions as a means for evolutionary adaptation and contemporary disease susceptibility. Biochem. Soc. Trans. 41, 111–117 (2013).
Wallace, D. C. A mitochondrial bioenergetic etiology of disease. J. Clin. Invest. 123, 1405–1412 (2013).
Taylor, R. W. & Turnbull, D. M. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 6, 389–402 (2005).
Gómez-Durán, A. et al. Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Hum. Mol. Genet. 19, 3343–3353 (2010).
Safdar, A. et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc. Natl Acad. Sci. USA 108, 4135–4140 (2011).
Ross, J. M. et al. Germline mitochondrial DNA mutations aggravate ageing and can impair brain development. Nature 501, 412–415 (2013).
Hamilton, M. L. et al. Does oxidative damage to DNA increase with age? Proc. Natl Acad. Sci. USA 98, 10469–10474 (2001).
Meissner, C., Bruse, P. & Oehmichen, M. Tissue-specific deletion patterns of the mitochondrial genome with advancing age. Exp. Gerontol. 41, 518–524 (2006).
Harman, D. The biologic clock: the mitochondria? J. Am. Geriatr. Soc. 20, 145–147 (1972).
Manoli, I. et al. Mitochondria as key components of the stress response. Trends Endocrinol. Metab. 18, 190–198 (2007).
Taivassalo, T. et al. The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain 126, 413–423 (2003).
Jeppesen, T. D., Orngreen, M. C., van Hall, G., Haller, R. G. & Vissing, J. Fat metabolism during exercise in patients with mitochondrial disease. Arch. Neurol. 66, 365–370 (2009).
Morava, E. & Kozicz, T. Mitochondria and the economy of stress (mal)adaptation. Neurosci. Biobehav. Rev. 37, 668–680 (2013).
Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 11, 872–884 (2010).
Picard, M., Shirihai, O. S., Gentil, B. J. & Burelle, Y. Mitochondrial morphology transitions and functions: implications for retrograde signaling? Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R393–R406 (2013).
Liesa, M. & Shirihai, O. S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 17, 491–506 (2013).
Shutt, T. E. & McBride, H. M. Staying cool in difficult times: mitochondrial dynamics, quality control and the stress response. Biochim. Biophys. Acta 1833, 417–424 (2012).
Chen, H. et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141, 280–289 (2010).
Yu, T., Robotham, J. L. & Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl Acad. Sci. USA 103, 2653–2658 (2006).
Picard, M. & Turnbull, D. M. Linking the metabolic state and mitochondrial DNA in chronic disease, health and aging. Diabetes 62, 672–678 (2013).
Psarra, A. M. & Sekeris, C. E. Glucocorticoid receptors and other nuclear transcription factors in mitochondria and possible functions. Biochim. Biophys. Acta 1787, 431–436 (2009).
Psarra, A. M. & Sekeris, C. E. Glucocorticoids induce mitochondrial gene transcription in HepG2 cells: role of the mitochondrial glucocorticoid receptor. Biochim. Biophys. Acta 1813, 1814–1821 (2011).
Sapolsky, R. M. The physiological relevance of glucocorticoid endangerment of the hippocampus. Ann. NY Acad. Sci. 746, 294–304 (1994).
Du, J. et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc. Natl Acad. Sci. USA 106, 3543–3548 (2009).
Tang, V. M., Young, A. H., Tan, H., Beasley, C. & Wang, J. F. Glucocorticoids increase protein carbonylation and mitochondrial dysfunction. Horm. Metab. Res. 45, 709–715 (2013).
Madrigal, J. L. et al. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology 24, 420–429 (2001).
Gong, Y., Chai, Y., Ding, J. H., Sun, X. L. & Hu, G. Chronic mild stress damages mitochondrial ultrastructure and function in mouse brain. Neurosci. Lett. 488, 76–80 (2011).
Rezin, G. T. et al. Inhibition of mitochondrial respiratory chain in brain of rats subjected to an experimental model of depression. Neurochem. Int. 53, 395–400 (2008).
Bennett, M. C., Mlady, G. W., Fleshner, M. & Rose, G. M. Synergy between chronic corticosterone and sodium azide treatments in producing a spatial learning deficit and inhibiting cytochrome oxidase activity. Proc. Natl Acad. Sci. USA 93, 1330–1334 (1996).
Hernández-Alvarez, M. I. et al. Glucocorticoid modulation of mitochondrial function in hepatoma cells requires the mitochondrial fission protein Drp1. Antioxid. Redox Signal. 19, 366–378 (2013).
Medikayala, S., Piteo, B., Zhao, X. & Edwards, J. G. Chronically elevated glucose compromises myocardial mitochondrial DNA integrity by alteration of mitochondrial topoisomerase function. Am. J. Physiol. Cell Physiol. 300, C338–C348 (2011).
Suzuki, S. et al. Oxidative damage to mitochondrial DNA and its relationship to diabetic complications. Diabetes Res. Clin. Pract. 45, 161–168 (1999).
Picard, M. et al. Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am. J. Respir. Crit. Care Med. 186, 1140–1149 (2012).
Lee, Y. J., Jeong, S. Y., Karbowski, M., Smith, C. L. & Youle, R. J. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol. Biol. Cell 15, 5001–5011 (2004).
Gomes, L. C., Di Benedetto, G. & Scorrano, L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 13, 589–598 (2011).
Balaban, R. S., Nemoto, S. & Finkel, T. Mitochondria, oxidants, and aging. Cell 120, 483–495 (2005).
Yakes, F. M. & Van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl Acad. Sci. USA 94, 514–519 (1997).
Kolesar, J. E., Wang, C. Y., Taguchi, Y. V., Chou, S. H. & Kaufman, B. A. Two-dimensional intact mitochondrial DNA agarose electrophoresis reveals the structural complexity of the mammalian mitochondrial genome. Nucleic Acids Res. 41, e58 (2013).
Corral-Debrinski, M. et al. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat. Genet. 2, 324–329 (1992).
Pan, H. Z. et al. The oxidative stress status in diabetes mellitus and diabetic nephropathy. Acta Diabetol. 47 (Suppl. 1), 71–76 (2010).
Aschbacher, K. et al. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology 38, 1698–1708 (2013).
Lin, M. T. & Beal, M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 (2006).
Passos, J. F., Saretzki, G. & von Zglinicki, T. DNA damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res. 35, 7505–7513 (2007).
Passos, J. F. et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 5, e110 (2007).
Oexle, K. & Zwirner, A. Advanced telomere shortening in respiratory chain disorders. Hum. Mol. Genet. 6, 905–908 (1997).
Sahin, E. et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365 (2011).
Malik, A. N. & Czajka, A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 13, 481–492 (2013).
Rasola, A. & Bernardi, P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis 12, 815–833 (2007).
Owusu-Ansah, E., Yavari, A., Mandal, S. & Banerjee, U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 40, 356–361 (2008).
Qian, W. et al. Mitochondrial hyperfusion induced by loss of the fission protein Drp1 causes ATM-dependent G2/M arrest and aneuploidy through DNA replication stress. J. Cell Sci. 125, 5745–5757 (2012).
Escames, G. et al. Mitochondrial DNA and inflammatory diseases. Hum. Genet. 131, 161–173 (2012).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Collins, L. V., Hajizadeh, S., Holme, E., Jonsson, I. M. & Tarkowski, A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J. Leukoc. Biol. 75, 995–1000 (2004).
Oka, T. et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485, 251–255 (2012).
Mathew, A. et al. Degraded mitochondrial DNA is a newly identified subtype of the damage associated molecular pattern (DAMP) family and possible trigger of neurodegeneration. J. Alzheimers Dis. 30, 617–627 (2012).
Komili, S. & Silver, P. A. Coupling and coordination in gene expression processes: a systems biology view. Nat. Rev. Genet. 9, 38–48 (2008).
Hunter, R. G. et al. Acute stress and hippocampal histone H3 lysine 9 trimethylation, a retrotransposon silencing response. Proc. Natl Acad. Sci. USA 109, 17657–17762 (2012).
Nasca, C. et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc. Natl Acad. Sci. USA 110, 4804–4809 (2013).
Guha, M., Pan, H., Fang, J. K. & Avadhani, N. G. Heterogeneous nuclear ribonucleoprotein A2 is a common transcriptional coactivator in the nuclear transcription response to mitochondrial respiratory stress. Mol. Biol. Cell 20, 4107–4119 (2009).
Romanello, V. et al. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 29, 1774–1785 (2010).
Holley, A. K. & St Clair, D. K. Watching the watcher: regulation of p53 by mitochondria. Future Oncol. 5, 117–130 (2009).
Schroeder, E. A., Raimundo, N. & Shadel, G. S. Epigenetic silencing mediates mitochondria stress-induced longevity. Cell Metab. 17, 954–964 (2013).
Wallace, D. C. Bioenergetics and the epigenome: interface between the environment and genes in common diseases. Dev. Disabil. Res. Rev. 16, 114–119 (2010).
Elstner, M. & Turnbull, D. M. Transcriptome analysis in mitochondrial disorders. Brain Res. Bull. 88, 285–293 (2012).
Miller, G. E. et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-κB signaling. Biol. Psychiatry 64, 266–272 (2008).
Slavich, G. M. & Cole, S. W. The emerging field of human social genomics. Clin. Psychol. Sci. 1, 331–348 (2013).
Kuo, L. E. et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 13, 803–811 (2007).
Epel, E. S. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 8, 7–22 (2009).
Andreux, P. A., Houtkooper, R. H. & Auwerx, J. Pharmacological approaches to restore mitochondrial function. Nat. Rev. Drug Discov. 12, 465–483 (2013).
Picard, M. et al. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J. Appl. Physiol. 115, 1562–1571 (2013).
Colberg, S. R. et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care 33, 2692–2696 (2011).
Erickson, K. I. et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl Acad. Sci. USA 108, 3017–3022 (2011).
McManus, M. J., Murphy, M. P. & Franklin, J. L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 31, 15703–15715 (2011).
McEwen, B. S. & Wingfield, J. C. The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15 (2003).
Author information
Authors and Affiliations
Contributions
M.P. researched the data for the article. All authors provided a substantial contribution to discussions of the content, contributed to writing the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Picard, M., Juster, RP. & McEwen, B. Mitochondrial allostatic load puts the 'gluc' back in glucocorticoids. Nat Rev Endocrinol 10, 303–310 (2014). https://doi.org/10.1038/nrendo.2014.22
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2014.22
This article is cited by
-
The cancer-immune dialogue in the context of stress
Nature Reviews Immunology (2024)
-
Systematic review of mitochondrial genetic variation in attention-deficit/hyperactivity disorder
European Child & Adolescent Psychiatry (2022)
-
Mitochondrial oxidative phosphorylation response overrides glucocorticoid-induced stress in a reptile
Journal of Comparative Physiology B (2022)
-
Mitochondrial gene signature in the prefrontal cortex for differential susceptibility to chronic stress
Scientific Reports (2020)
-
Glucose regulation is a repeatable trait affected by successive handling in zebra finches
Journal of Comparative Physiology B (2020)