Key Points
-
Disorders of sex development (DSDs) are defined as congenital conditions in which development of chromosomal, gonadal, or anatomic sex is atypical
-
Mutations in genes that encode transcription factors, signalling components and epigenetic modifiers that are involved in sex determination can result in 46,XX and 46,XY DSDs
-
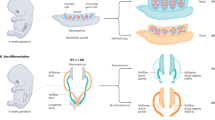
At 6–8 weeks post-conception in human fetal development, upregulated expression of SRY in the bipotential gonad promotes testis determination, whereas activation of WNT4 and RSPO1 signalling promotes ovary determination
-
Gonadal phenotypes in patients with DSDs range from gonadal dysgenesis (in which the gonads are fibrous streak gonads) to varying degrees of ovotesis (in which both ovary and testicular tissue are present)
-
The complexity and interrelatedness of factors that contribute to the aetiology and the medical and psychological outcomes of DSDs demand a multidisciplinary team approach to health care
-
In contrast to gender differences in activities and interests, associations between prenatal exposure to androgens and development of gender identity or sexual orientation are unclear
Abstract
Mammalian sex determination is the unique process whereby a single organ, the bipotential gonad, undergoes a developmental switch that promotes its differentiation into either a testis or an ovary. Disruptions of this complex genetic process during human development can manifest as disorders of sex development (DSDs). Sex development can be divided into two distinct processes: sex determination, in which the bipotential gonads form either testes or ovaries, and sex differentiation, in which the fully formed testes or ovaries secrete local and hormonal factors to drive differentiation of internal and external genitals, as well as extragonadal tissues such as the brain. DSDs can arise from a number of genetic lesions, which manifest as a spectrum of gonadal (gonadal dysgenesis to ovotestis) and genital (mild hypospadias or clitoromegaly to ambiguous genitalia) phenotypes. The physical attributes and medical implications associated with DSDs confront families of affected newborns with decisions, such as gender of rearing or genital surgery, and additional concerns, such as uncertainty over the child's psychosexual development and personal wishes later in life. In this Review, we discuss the underlying genetics of human sex determination and focus on emerging data, genetic classification of DSDs and other considerations that surround gender development and identity in individuals with DSDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
V. A. Arboleda & Vilain, E. in Yen and Jaffe's Reproductive Endocrinology 6th edn Ch. 16 (eds Strauss, J. F. & Barbieri, R. L.) 367–393 (Saunders Elsevier, 2009).
Danon, M. & Sachs, L. Sex chromosomes and human sexual development. Lancet 273, 20–25 (1957).
Sinclair, A. H. et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346, 240–244 (1990).
Koopman, P., Munsterberg, A., Capel, B., Vivian, N. & Lovell-Badge, R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature 348, 450–452 (1990).
Jost, A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Prog. Horm. Res. 8, 379–418 (1953).
Jost, A. Studies on sex differentiation in mammals. Recent Prog. Horm. Res. 29, 1–41 (1973).
Lee, P. A., Houk, C. P., Ahmed, S. F. & Hughes, I. A. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics 118, e488–e500 (2006).
Hughes, I. A., Houk, C., Ahmed, S. F. & Lee, P. A. Consensus statement on management of intersex disorders. J. Pediatr. Urol. 2, 148–162 (2006).
Auchus, R. J. & Miller, W. L. Defects in androgen biosynthesis causing 46, XY disorders of sexual development. Semin. Reprod. Med. 30, 417–426 (2012).
Biason-Lauber, A., Boscaro, M., Mantero, F. & Balercia, G. Defects of steroidogenesis. J. Endocrinol. Invest. 33 (2010).
Lux, A., Kropf, S., Kleinemeier, E., Jurgensen, M. & Thyen, U. Clinical evaluation study of the German network of disorders of sex development (DSD)/intersexuality: study design, description of the study population, and data quality. BMC Public Health 9, 110 (2009).
Baxter, R. M. & Vilain, E. Translational genetics for diagnosis of human disorders of sex development. Annu. Rev. Genomics Hum. Genet. 14, 371–392 (2013).
Disorders of Sex Development Translational Research Network [online] (2014).
I-DSD Registry [online] (2014).
Moriya, K., Mitsui, T., Tanaka, H., Nakamura, M. & Nonomura, K. Long-term outcome of pituitary-gonadal axis and gonadal growth in patients with hypospadias at puberty. J. Urol. 184, 1610–1614 (2010).
Ogata, T., Sano, S., Nagata, E., Kato, F. & Fukami, M. MAMLD1 and 46,XY disorders of sex development. Semin. Reprod. Med. 30, 410–416 (2012).
Rynja, S. P., de Jong, T. P., Bosch, J. L. & de Kort, L. M. Functional, cosmetic and psychosexual results in adult men who underwent hypospadias correction in childhood. J. Pediatr. Urol. 7, 504–515 (2011).
Kazak, A. E. et al. An integrative model of pediatric medical traumatic stress. J. Pediatr. Psychol. 31, 343–355 (2006).
Sandberg, D. E. & Mazur, T. in Gender Dysphoria and Disorders of Sex Development: Progress in Care and Knowledge Focus on Sexuality Research Ch. 5 (eds Kreukels, B. P. C., Steensma, T. D. & de Vries, A. L. C.) 93–114 (Springer Science+Business Media, 2013).
Karkazis, K. & Feder, E. K. Naming the problem: disorders and their meanings. Lancet 372, 2016–2017 (2008).
Reis, E. Divergence or disorder?: The politics of naming intersex. Perspect. Biol. Med. 50, 535–543 (2007).
Tamar-Mattis, A., Baratz, A., Baratz Dalke, K. & Karkazis, K. Emotionally and cognitively informed consent for clinical care for differences of sex development. Psychology & Sexuality 5, 44–55 (2014).
Diamond, M. & Garland, J. Evidence regarding cosmetic and medically unnecessary surgery on infants. J. Pediatr. Urol. 10, 2–6 (2014).
Mouriquand, P., Caldamone, A., Malone, P., Frank, J. D. & Hoebeke, P. The ESPU/SPU standpoint on the surgical management of Disorders of Sex Development (DSD). J. Pediatr. Urol. 10, 8–10 (2014).
Clepet, C. et al. The human SRY transcript. Hum. Mol. Genet. 2, 2007–2012 (1993).
Sadler, T. W. Langman's Medical Embryology 9th edn (Lippincott Williams & Wilkins, 2004).
Poulat, F. et al. Nuclear localization of the testis determining gene product SRY. J. Cell Biol. 128, 737–748 (1995).
Hiramatsu, R. et al. A critical time window of Sry action in gonadal sex determination in mice. Development 136, 129–138 (2009).
Sekido, R. & Lovell-Badge, R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453, 930–934 (2008).
McElreavy, K. et al. XY sex reversal associated with a deletion 5′ to the SRY “HMG box” in the testis-determining region. Proc. Natl Acad. Sci. USA 89, 11016–11020 (1992).
McElreavey, K. et al. Loss of sequences 3′ to the testis-determining gene, SRY, including the Y pseudoautosomal boundary associated with partial testicular determination. Proc. Natl Acad. Sci. USA 93, 8590–8594 (1996).
Berta, P. et al. Genetic evidence equating SRY and the testis-determining factor. Nature 348, 448–450 (1990).
Jager, R. J., Anvret, M., Hall, K. & Scherer, G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature 348 452–454 (1990).
Foster, J. W. et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372, 525–530 (1994).
Wagner, T. et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79, 1111–1120 (1994).
Bagheri-Fam, S. et al. Loss of Fgfr2 leads to partial XY sex reversal. Dev. Biol. 314, 71–83 (2008).
Kim, Y. et al. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc. Natl Acad. Sci. USA 104, 16558–16563 (2007).
Schmahl, J., Kim, Y., Colvin, J. S., Ornitz, D. M. & Capel, B. Fgf9 induces proliferation and nuclear localization of FGFR2 in Sertoli precursors during male sex determination. Development 131, 3627–3636 (2004).
Moniot, B. et al. The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in Sertoli cells during male sexual differentiation. Development 136, 1813–1821 (2009).
Bellus, G. A. et al. Identical mutations in three different fibroblast growth factor receptor genes in autosomal dominant craniosynostosis syndromes. Nat. Genet. 14, 174–176 (1996).
Luo, X., Ikeda, Y. & Parker, K. L. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77, 481–490 (1994).
Wong, M., Ramayya, M. S., Chrousos, G. P., Driggers, P. H. & Parker, K. L. Cloning and sequence analysis of the human gene encoding steroidogenic factor 1. J. Mol. Endocrinol. 17, 139–147 (1996).
Achermann, J. C., Ito, M., Hindmarsh, P. C. & Jameson, J. L. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat. Genet. 22, 125–126 (1999).
Tremblay, J. J. & Viger, R. S. A mutated form of steroidogenic factor 1 (SF-1 G35E) that causes sex reversal in humans fails to synergize with transcription factor GATA-4. J. Biol. Chem. 278, 42637–42642 (2003).
Ikeda, Y., Shen, W. H., Ingraham, H. A. & Parker, K. L. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol. Endocrinol. 8, 654–662 (1994).
Kohler, B. et al. Five novel mutations in steroidogenic factor 1 (SF1, NR5A1) in 46,XY patients with severe underandrogenization but without adrenal insufficiency. Hum. Mutat. 29, 59–64 (2008).
Kohler, B. et al. The spectrum of phenotypes associated with mutations in steroidogenic factor 1 (SF-1, NR5A1, Ad4BP) includes severe penoscrotal hypospadias in 46,XY males without adrenal insufficiency. Eur. J. Endocrinol. 161, 237–242 (2009).
Lourenco, D. et al. Mutations in NR5A1 associated with ovarian insufficiency. N. Engl. J. Med. 360, 1200–1210 (2009).
Bashamboo, A. et al. Human male infertility associated with mutations in NR5A1 encoding steroidogenic factor 1. Am. J. Hum. Genet. 87 (2010).
Tantawy, S. et al. Testosterone production during puberty in two 46, XY patients with disorders of sex development and novel NR5A1 (SF-1) mutations. Eur. J. Endocrinol. 167, 125–130 (2012).
Swain, A., Zanaria, E., Hacker, A., Lovell-Badge, R. & Camerino, G. Mouse Dax1 expression is consistent with a role in sex determination as well as in adrenal and hypothalamus function. Nat. Genet. 12, 404–409 (1996).
Barbaro, M. et al. Isolated 46, XY gonadal dysgenesis in two sisters caused by a Xp21.2 interstitial duplication containing the DAX1 gene. J. Clin. Endocrinol. Metab. 92, 3305–3313 (2007).
Swain, A., Narvaez, V., Burgoyne, P., Camerino, G. & Lovell-Badge, R. Dax1 antagonizes Sry action in mammalian sex determination. Nature 391, 761–767 (1998).
Ludbrook, L. M. et al. Excess DAX1 leads to XY ovotesticular disorder of sex development (DSD) in mice by inhibiting steroidogenic factor-1 (SF1) activation of the testis enhancer of SRY-box-9 (Sox9). Endocrinology 153, 1948–1958 (2012).
Guo, W. et al. Diagnosis of X-linked adrenal hypoplasia congenita by mutation analysis of the DAX1 gene. JAMA 274, 324–330 (1995).
Lourenco, D. et al. Loss-of-function mutation in GATA4 causes anomalies of human testicular development. Proc. Natl Acad. Sci. USA 108, 1597–1602 (2011).
Bashamboo, A. et al. Mutations in the FOG2/ZFPM2 gene are associated with anomalies of human testis determination. Hum. Mol. Genet. 23, 3657–3665 (2014).
Tevosian, S. G. et al. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development 129, 4627–4634 (2002).
Miyamoto, Y., Taniguchi, H., Hamel, F., Silversides, D. W. & Viger, R. S. A GATA4/WT1 cooperation regulates transcription of genes required for mammalian sex determination and differentiation. BMC Mol. Biol. 9, 44 (2008).
Tannour-Louet, M. et al. Identification of de novo copy number variants associated with human disorders of sexual development. PLoS ONE 5, e15392 (2010).
Calvari, V. et al. A new submicroscopic deletion that refines the 9p region for sex reversal. Genomics 65, 203–212 (2000).
Turnbull, C. et al. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nat. Genet. 42, 604–607 (2010).
Kim, S., Bardwell, V. J. & Zarkower, D. Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation. Dev. Biol. 307, 314–327 (2007).
Krentz, A. D. et al. The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc. Natl Acad. Sci. USA 106, 22323–22328 (2009).
Takashima, S. et al. Regulation of pluripotency in male germline stem cells by Dmrt1. Genes Dev. 27, 1949–1958 (2013).
Matson, C. K. et al. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476, 101–104 (2011).
White, S. et al. A multi-exon deletion within WWOX is associated with a 46,XY disorder of sex development. Eur. J. Hum. Genet. 20, 348–351 (2012).
Ludes-Meyers, J. H. et al. WWOX hypomorphic mice display a higher incidence of B-cell lymphomas and develop testicular atrophy. Genes Chromosomes Cancer 46, 1129–1136 (2007).
Nef, S. et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev. Biol. 287, 361–377 (2005).
Vainio, S., Heikkila, M., Kispert, A., Chin, N. & McMahon, A. P. Female development in mammals is regulated by Wnt-4 signalling. Nature 397, 405–409 (1999).
Parma, P. et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat. Genet. 38, 1304–1309 (2006).
Maatouk, D. M. et al. Stabilization of β-catenin in XY gonads causes male-to-female sex-reversal. Hum. Mol. Genet. 17, 2949–2955 (2008).
Tomaselli, S. et al. Human RSPO1/R-spondin1 is expressed during early ovary development and augments β-catenin signaling. PLoS ONE 6, e16366 (2011).
Bowles, J. et al. Retinoid signaling determines germ cell fate in mice. Science 312, 596–600 (2006).
Koubova, J. et al. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl Acad. Sci. USA 103, 2474–2479 (2006).
He, X., Semenov, M., Tamai, K. & Zeng, X. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development 131, 1663–1677 (2004).
Uhlenhaut, N. H. et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139, 1130–1142 (2009).
Couse, J. F. et al. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors α and β. Science 286, 2328–2331 (1999).
Dupont, S. et al. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127, 4277–4291 (2000).
Coulam, C. B. Premature gonadal failure. Fertil. Steril. 38, 645–655 (1982).
Seminara, S. B., Oliveira, L. M., Beranova, M., Hayes, F. J. & Crowley, W. F. Jr. Genetics of hypogonadotropic hypogonadism. J. Endocrinol. Invest. 23, 560–565 (2000).
Persani, L., Rossetti, R. & Cacciatore, C. Genes involved in human premature ovarian failure. J. Mol. Endocrinol. 45, 257–279 (2010).
Bachelot, A. et al. Phenotyping and genetic studies of 357 consecutive patients presenting with premature ovarian failure. Eur. J. Endocrinol. 161, 179–187 (2009).
Spiller, C. M., Bowles, J. & Koopman, P. Regulation of germ cell meiosis in the fetal ovary. Int. J. Dev. Biol. 56, 779–787 (2012).
Stochholm, K., Juul, S., Juel, K., Naeraa, R. W. & Gravholt, C. H. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J. Clin. Endocrinol. Metab. 91, 3897–3902 (2006).
Crisponi, L. et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat. Genet. 27, 159–166 (2001).
Pailhoux, E. et al. A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat. Genet. 29, 453–458 (2001).
De Baere, E. et al. Spectrum of FOXL2 gene mutations in blepharophimosis-ptosis-epicanthus inversus (BPES) families demonstrates a genotype–phenotype correlation. Hum. Mol. Genet. 10, 1591–1600 (2001).
Voican, A. et al. NR5A1 (SF-1) mutations are not a major cause of primary ovarian insufficiency. J. Clin. Endocrinol. Metab. 98, E1017–E1021 (2013).
Philibert, P. et al. NR5A1 (SF-1) gene variants in a group of 26 young women with XX primary ovarian insufficiency. Fertil. Steril. 99, 484–489 (2013).
Ottolenghi, C. et al. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum. Mol. Genet. 16, 2795–2804 (2007).
Biason-Lauber, A., Konrad, D., Navratil, F. & Schoenle, E. J. A WNT4 mutation associated with Müllerian-duct regression and virilization in a 46,XX woman. N. Engl. J. Med. 351, 792–798 (2004).
Biason-Lauber, A. et al. WNT4 deficiency--a clinical phenotype distinct from the classic Mayer–Rokitansky–Kuster–Hauser syndrome: a case report. Hum. Reprod. 22 (2007).
Gao, X. et al. Clinical, cytogenetic, and molecular analysis with 46,XX male sex reversal syndrome: case reports. J. Assist Reprod. Genet. 30, 431–435 (2013).
Huang, B., Wang, S., Ning, Y., Lamb, A. N. & Bartley, J. Autosomal XX sex reversal caused by duplication of SOX9. Am. J. Med. Genet. 87, 349–353 (1999).
Cox, J. J., Willatt, L., Homfray, T. & Woods, C. G. A SOX9 duplication and familial 46,XX developmental testicular disorder. N. Engl. J. Med. 364, 91–93 (2011).
Xiao, B., Ji, X., Xing, Y., Chen, Y. W. & Tao, J. A rare case of 46,XX SRY-negative male with approximately 74-kb duplication in a region upstream of SOX9. Eur. J. Med. Genet. 56, 695–698 (2013).
Benko, S. et al. Disruption of a long distance regulatory region upstream of SOX9 in isolated disorders of sex development. J. Med. Genet. 48, 825–830 (2011).
Moalem, S. et al. XX male sex reversal with genital abnormalities associated with a de novo SOX3 gene duplication. Am. J. Med. Genet. A 158A, 1759–1764 (2012).
Sutton, E. et al. Identification of SOX3 as an XX male sex reversal gene in mice and humans. J. Clin. Invest. 121, 328–341 (2011).
White, S. et al. Copy number variation in patients with disorders of sex development due to 46,XY gonadal dysgenesis. PLoS ONE 6, e17793 (2011).
Polanco, J. C., Wilhelm, D., Davidson, T. L., Knight, D. & Koopman, P. Sox10 gain-of-function causes XX sex reversal in mice: implications for human 22q-linked disorders of sex development. Hum. Mol. Genet. 19, 506–516 (2010).
Pearlman, A. et al. Mutations in MAP3K1 cause 46,XY disorders of sex development and implicate a common signal transduction pathway in human testis determination. Am. J. Hum. Genet. 87, 898–904 (2010).
Das, D. K., Rahate, S. G., Mehta, B. P., Gawde, H. M. & Tamhankar, P. M. Mutation analysis of mitogen activated protein kinase 1 gene in Indian cases of 46, XY disorder of sex development. Indian J. Hum. Genet. 19, 437–442 (2013).
Loke, J. et al. Mutations in MAP3K1 tilt the balance from SOX9/FGF9 to WNT/β-catenin signaling. Hum. Mol. Genet. 23 (2014).
Warr, N. et al. Gadd45γ and Map3k4 interactions regulate mouse testis determination via p38 MAPK-mediated control of Sry expression. Dev. Cell 23, 1020–1031 (2012).
Canto, P., Soderlund, D., Reyes, E. & Mendez, J. P. Mutations in the desert hedgehog (DHH) gene in patients with 46, XY complete pure gonadal dysgenesis. J. Clin. Endocrinol. Metab. 89, 4480–4483 (2004).
Umehara, F. et al. A novel mutation of desert hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am. J. Hum. Genet. 67, 1302–1305 (2000).
Callier, P. et al. Loss of function mutation in the palmitoyl-transferase HHAT leads to syndromic 46,XY disorder of sex development by impeding Hedgehog protein palmitoylation and signaling. PLoS Genet. 10, e1004340 (2014).
Mandel, H. et al. SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am. J. Hum. Genet. 82, 39–47 (2008).
Chassot, A. A. et al. WNT4 and RSPO1 together are required for cell proliferation in the early mouse gonad. Development 139, 4461–4472 (2012).
Biason-Lauber, A., Konrad, D., Meyer, M., DeBeaufort, C. & Schoenle, E. J. Ovaries and female phenotype in a girl with 46,XY karyotype and mutations in the CBX2 gene. Am. J. Hum. Genet. 84, 658–663 (2009).
Norling, A., Hirschberg, A. L., Iwarsson, E., Wedell, A. & Barbaro, M. CBX2 gene analysis in patients with 46,XY and 46,XX gonadal disorders of sex development. Fertil. Steril. 99, 819–826 e3 (2013).
Volkel, P., Le Faou, P., Vandamme, J., Pira, D. & Angrand, P. O. A human Polycomb isoform lacking the Pc box does not participate to PRC1 complexes but forms protein assemblies and represses transcription. Epigenetics 7, 482–491 (2012).
Milne, T. A., Sinclair, D. A. & Brock, H. W. The Additional sex combs gene of Drosophila is required for activation and repression of homeotic loci, and interacts specifically with Polycomb and super sex combs. Mol. Gen. Genet. 261, 753–761 (1999).
Shirai, M. et al. The Polycomb-group gene Rae28 sustains Nkx2.5/Csx expression and is essential for cardiac morphogenesis. J. Clin. Invest. 110, 177–184 (2002).
Katoh-Fukui, Y. et al. Cbx2, a polycomb group gene, is required for Sry gene expression in mice. Endocrinology 153, 913–924 (2012).
Irving, M. et al. Deletion of the distal long arm of chromosome 10; is there a characteristic phenotype? A report of 15 de novo and familial cases. Am. J. Med. Genet. A 123A, 153–163 (2003).
Andresen, J. H., Aftimos, S., Doherty, E., Love, D. R. & Battin, M. 13q33.2 deletion: a rare cause of ambiguous genitalia in a male newborn with growth restriction. Acta Paediatr. 99, 784–786 (2010).
Dravis, C. et al. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev. Biol. 271, 272–290 (2004).
Chowdhury, S. et al. Phenotypic and molecular characterization of 19q12q13.1 deletions: a report of five patients. Am. J. Med. Genet. A 164A, 62–69 (2014).
Beysen, D. et al. Deletions involving long-range conserved nongenic sequences upstream and downstream of FOXL2 as a novel disease-causing mechanism in blepharophimosis syndrome. Am. J. Hum. Genet. 77, 205–218 (2005).
Fatica, A. & Bozzoni, I. Long non-coding RNAs: new players in cell differentiation and development. Nat. Rev. Genet. 15, 7–21 (2014).
Fluck, C. E. et al. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am. J. Hum. Genet. 89, 201–218 (2011).
Katsanis, N. et al. Triallelic inheritance in Bardet–Biedl syndrome, a Mendelian recessive disorder. Science 293, 2256–2259 (2001).
Mykytyn, K. et al. Identification of the gene (BBS1) most commonly involved in Bardet–-Biedl syndrome, a complex human obesity syndrome. Nat. Genet. 31, 435–438 (2002).
Jensen, M. S. et al. Cryptorchidism and hypospadias in a cohort of 934,538 Danish boys: the role of birth weight, gestational age, body dimensions, and fetal growth. Am. J. Epidemiol. 175, 917–925 (2012).
Yinon, Y. et al. Hypospadias in males with intrauterine growth restriction due to placental insufficiency: the placental role in the embryogenesis of male external genitalia. Am. J. Med. Genet. A 152A, 75–83 (2010).
Kalfa, N., Philibert, P., Baskin, L. S. & Sultan, C. Hypospadias: interactions between environment and genetics. Mol. Cell Endocrinol. 335, 89–95 (2011).
Meijer, L. et al. Influence of prenatal organohalogen levels on infant male sexual development: sex hormone levels, testes volume and penile length. Hum. Reprod. 27, 867–872 (2012).
Fisch, H., Hyun, G. & Hensle, T. W. Rising hypospadias rates: disproving a myth. J. Pediatr. Urol. 6, 37–39 (2010).
Arboleda, V. A. et al. Targeted massively parallel sequencing provides comprehensive genetic diagnosis for patients with disorders of sex development. Clin. Genet. 83, 35–43 (2013).
Yang, Y. et al. Clinical whole-exome sequencing for the diagnosis of Mendelian disorders. N. Engl. J. Med. 369, 1502–1511 (2013).
Wizemann, T. M. & Pardue, M.-L. (Eds) Exploring the Biological Contributions to Human Health: Does Sex Matter? (National Academy Press, 2001).
Stein, M. T., Sandberg, D. E., Mazur, T., Eugster, E. & Daaboul, J. A newborn infant with a disorder of sexual differentiation. J. Dev. Behav. Pediatr. 24, 115–119 (2003).
Dessens, A. B., Slijper, F. M. & Drop, S. L. Gender dysphoria and gender change in chromosomal females with congenital adrenal hyperplasia. Arch. Sex Behav. 34, 389–397 (2005).
Mazur, T. Gender dysphoria and gender change in androgen insensitivity or micropenis. Arch. Sex Behav. 34, 411–421 (2005).
Cohen-Kettenis, P. T. Gender change in 46,XY persons with 5α-reductase-2 deficiency and 17β-hydroxysteroid dehydrogenase-3 deficiency. Arch. Sex Behav. 34, 399–410 (2005).
Maimoun, L. et al. Phenotypical, biological, and molecular heterogeneity of 5α-reductase deficiency: an extensive international experience of 55 patients. J. Clin. Endocrinol. Metab. 96, 296–307 (2011).
Chuang, J. et al. Complexities of gender assignment in 17β-hydroxysteroid dehydrogenase type 3 deficiency: is there a role for early orchiectomy? Int. J. Pediatr. Endocrinol. 2013, 15 (2013).
Zucker, K. J. Intersexuality and gender identity differentiation. Annu. Rev. Sex Res. 10, 1–69 (1999).
Reiner, W. G. & Gearhart, J. P. Discordant sexual identity in some genetic males with cloacal exstrophy assigned to female sex at birth. N. Engl. J. Med. 350, 333–341 (2004).
Diamond, D. A. et al. Sex assignment for newborns with ambiguous genitalia and exposure to fetal testosterone: attitudes and practices of pediatric urologists. J. Pediatr. 148, 445–449 (2006).
Diamond, D. A., Burns, J. P., Huang, L., Rosoklija, I. & Retik, A. B. Gender assignment for newborns with 46XY cloacal exstrophy: a 6-year followup survey of pediatric urologists. J. Urol. 186, 1642–1648 (2011).
Meyer-Bahlburg, H. F. Gender identity outcome in female-raised 46,XY persons with penile agenesis, cloacal exstrophy of the bladder, or penile ablation. Arch. Sex Behav. 34, 423–438 (2005).
Ruble, D., Martin, C. & Berenbaum, S. in Handbook of child psychology: Social, emotional, and personality 6th edn Vol. 3 Ch. 14 (Eds Damon, W., Lerner, R. & Eisenberg, N.) 858–932 (New York: Wiley, 2006).
Stout, S. A., Litvak, M., Robbins, N. M. & Sandberg, D. E. Congenital adrenal hyperplasia: classification of studies employing psychological endpoints. Int. J. Pediatr. Endocrinol. 2010, 191520 (2010).
Jordan-Young, R. M. Hormones, context, and “brain gender”: a review of evidence from congenital adrenal hyperplasia. Soc. Sci. Med. 74, 1738–1744 (2012).
Hines, M. in Multiple origins of sex differences in brain (eds Pfaff, D. W. & Christen, Y.) 59–69 (Springer, 2013). [Series Ed. Christen, Y. Research and Perspectives in Endocrine Interactions].
Mueller, S. C. et al. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH). Psychoneuroendocrinology 33, 973–980 (2008).
Frisen, L. et al. Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. J. Clin. Endocrinol. Metab. 94, 3432–3439 (2009).
Meyer-Bahlburg, H. F., Dolezal, C., Baker, S. W. & New, M. I. Sexual orientation in women with classical or non-classical congenital adrenal hyperplasia as a function of degree of prenatal androgen excess. Arch. Sex Behav. 37, 85–99 (2008).
Arnold, A. P. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 55, 570–578 (2009).
Arnold, A. P. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J. Neuroendocrinol. 21, 377–386 (2009).
Arnold, A. P. & Chen, X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 30, 1–9 (2009).
Smith-Bouvier, D. L. et al. A role for sex chromosome complement in the female bias in autoimmune disease. J. Exp. Med. 205, 1099–1108 (2008).
Quinn, J. J., Hitchcott, P. K., Umeda, E. A., Arnold, A. P. & Taylor, J. R. Sex chromosome complement regulates habit formation. Nat. Neurosci. 10, 1398–1400 (2007).
Chung, Y. P. et al. Prenatal diagnosis of monosomy 10q25 associated with single umbilical artery and sex reversal: report of a case. Prenat. Diagn. 18, 73–77 (1998).
Courtens, W., Wuyts, W., Rooms, L., Pera, S. B. & Wauters, J. A subterminal deletion of the long arm of chromosome 10: a clinical report and review. Am. J. Med. Genet. A 140, 402–409 (2006).
Battaglia, A. et al. Further delineation of deletion 1p36 syndrome in 60 patients: a recognizable phenotype and common cause of developmental delay and mental retardation. Pediatrics 121, 404–410 (2008).
Jordan, B. K. et al. Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am. J. Hum. Genet. 68, 1102–1109 (2001).
Acknowledgements
Funding for this project was from the Doris Duke Foundation and the National Institute of Child Health and Human Development RO1HD06138 DSD-TRN (Platform for Basic and Translational Research) grant to E.V. and D.E.S, University of California Los Angeles institutional funds to V.A.A. and Patient-Centered Outcomes Research Institute contract funds to D.E.S.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Arboleda, V., Sandberg, D. & Vilain, E. DSDs: genetics, underlying pathologies and psychosexual differentiation. Nat Rev Endocrinol 10, 603–615 (2014). https://doi.org/10.1038/nrendo.2014.130
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2014.130
This article is cited by
-
Genetic control of typical and atypical sex development
Nature Reviews Urology (2023)
-
A new era: improving use of sociodemographic constructs in the analysis of pediatric cohort study data
Pediatric Research (2021)