Key Points
-
Thyroid hormone signalling is required for skeletal muscle development, contractile function and muscle regeneration
-
As skeletal muscle comprises 30–40% of body mass, the altered basal metabolic rate in patients with thyroid hormone excess or deficiency is largely due to changes in skeletal muscle energy turnover
-
Functional studies indicate that the active thyroid hormone isoform T3 signals predominantly through the thyroid-hormone receptor α1 (THRA1) isoform in skeletal muscle
-
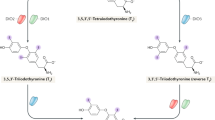
Expression of the type 2 iodothyronine deiodinase (DIO2), which converts the prohormone T4 to the active thyroid hormone isoform T3, is increased in developing or injured muscles
-
In the absence of DIO2, the muscle-specific thyroid hormone-dependent gene expression programme fails to be induced in the stem-cell-like satellite cells of skeletal muscle, resulting in impaired muscle regeneration
-
Current studies suggest that the dynamic control of thyroid hormone activity through the regulation of deiodinase expression can be harnessed to optimize myogenesis in patients with muscle diseases or injury
Abstract
Thyroid hormone signalling regulates crucial biological functions, including energy expenditure, thermogenesis, development and growth. The skeletal muscle is a major target of thyroid hormone signalling. The type 2 and 3 iodothyronine deiodinases (DIO2 and DIO3, respectively) have been identified in skeletal muscle. DIO2 expression is tightly regulated and catalyses outer-ring monodeiodination of the secreted prohormone tetraiodothyronine (T4) to generate the active hormone tri-iodothyronine (T3). T3 can remain in the myocyte to signal through nuclear receptors or exit the cell to mix with the extracellular pool. By contrast, DIO3 inactivates T3 through removal of an inner-ring iodine. Regulation of the expression and activity of deiodinases constitutes a cell-autonomous, pre-receptor mechanism for controlling the intracellular concentration of T3. This local control of T3 activity is crucial during the various phases of myogenesis. Here, we review the roles of T3 in skeletal muscle development and homeostasis, with a focus on the emerging local deiodinase-mediated control of T3 signalling. Moreover, we discuss these novel findings in the context of both muscle homeostasis and pathology, and examine how skeletal muscle deiodinase activity might be therapeutically harnessed to improve satellite-cell-mediated muscle repair in patients with skeletal muscle disorders, muscle atrophy or injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Simonides, W. S. & van Hardeveld, C. Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid 18, 205–216 (2008).
Schiaffino, S. & Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447–1531 (2011).
Murphy, R. M., Larkins, N. T., Mollica, J. P., Beard, N. A. & Lamb, G. D. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J. Physiol. 587, 443–460 (2009).
Novák, P. & Soukup, T. Calsequestrin distribution, structure and function, its role in normal and pathological situations and the effect of thyroid hormones. Physiol. Res. 60, 439–452 (2011).
Bianco, A. C., Salvatore, D., Gereben, B., Berry, M. J. & Larsen, P. R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 23, 38–89 (2002).
Gereben, B. et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 29, 898–938 (2008).
Croteau, W., Davey, J. C., Galton, V. A. & St Germain, D. L. Cloning of the mammalian type II iodothyronine deiodinase. A selenoprotein differentially expressed and regulated in human and rat brain and other tissues. J. Clin. Invest. 98, 405–417 (1996).
Salvatore, D., Bartha, T., Harney, J. W. & Larsen, P. R. Molecular biological and biochemical characterization of the human type 2 selenodeiodinase. Endocrinology 137, 3308–3315 (1996).
Peeters, R. P. et al. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J. Clin. Endocrinol. Metab. 88, 3202–3211 (2003).
Brack, A. S. & Rando, T. A. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell 10, 504–514 (2012).
Yin, H., Price, F. & Rudnicki, M. A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 93, 23–67 (2013).
Relaix, F. & Zammit, P. S. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 139, 2845–2856 (2012).
Yu, F. et al. Effects of thyroid hormone receptor gene disruption on myosin isoform expression in mouse skeletal muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R1545–R1554 (2000).
Brent, G. A. Mechanisms of thyroid hormone action. J. Clin. Invest. 122, 3035–3043 (2012).
Mebis, L. et al. Expression of thyroid hormone transporters during critical illness. Eur. J. Endocrinol. 161, 243–250 (2009).
Friesema, E. C. et al. Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol. Endocrinol. 22, 1357–1369 (2008).
Marsili, A. et al. Type 2 iodothyronine deiodinase levels are higher in slow-twitch than fast-twitch mouse skeletal muscle and are increased in hypothyroidism. Endocrinology 151, 5952–5960 (2010).
Marsili, A. et al. Type II iodothyronine deiodinase provides intracellular 3,5,3′-triiodothyronine to normal and regenerating mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 301, E818–E824 (2011).
Dentice, M. et al. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J. Clin. Invest. 120, 4021–4030 (2010).
Hosoi, Y. et al. Expression and regulation of type II iodothyronine deiodinase in cultured human skeletal muscle cells. J. Clin. Endocrinol. Metab. 84, 3293–3300 (1999).
Maia, A. L., Kim, B. W., Huang, S. A., Harney, J. W. & Larsen, P. R. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J. Clin. Invest. 115, 2524–2533 (2005).
Grozovsky, R. et al. Type 2 deiodinase expression is induced by peroxisomal proliferator-activated receptor-gamma agonists in skeletal myocytes. Endocrinology 150, 1976–1983 (2009).
Mebis, L., Langouche, L., Visser, T. J. & Van den Berghe, G. The type II iodothyronine deiodinase is up-regulated in skeletal muscle during prolonged critical illness. J. Clin. Endocrinol. Metab. 92, 3330–3333 (2007).
Heemstra, K. A. et al. Type 2 iodothyronine deiodinase in skeletal muscle: effects of hypothyroidism and fasting. J. Clin. Endocrinol. Metab. 94, 2144–2150 (2009).
Steinsapir, J., Harney, J. & Larsen, P. R. Type 2 iodothyronine deiodinase in rat pituitary tumor cells is inactivated in proteasomes. J. Clin. Invest. 102, 1895–1899 (1998).
Mills, I., Barge, R. M., Silva, J. E. & Larsen, P. R. Insulin stimulation of iodothyronine 5′-deiodinase in rat brown adipocytes. Biochem. Biophys. Res. Commun. 143, 81–86 (1987).
Silva, J. E. & Larsen, P. R. Hormonal regulation of iodothyronine 5′-deiodinase in rat brown adipose tissue. Am. J. Physiol. 251, E639–E643 (1986).
Boelen, A., Kwakkel, J., Wiersinga, W. M. & Fliers, E. Chronic local inflammation in mice results in decreased TRH and type 3 deiodinase mRNA expression in the hypothalamic paraventricular nucleus independently of diminished food intake. J. Endocrinol. 191, 707–714 (2006).
Peeters, R. P. et al. Serum 3,3′,5′-triiodothyronine (rT3) and 3,5,3′-triiodothyronine/rT3 are prognostic markers in critically ill patients and are associated with postmortem tissue deiodinase activities. J. Clin. Endocrinol. Metab. 90, 4559–4565 (2005).
Yen, P. M. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 81, 1097–1142 (2001).
Simonides, W. S. et al. Characterization of the promoter of the rat sarcoplasmic endoplasmic reticulum Ca2+-ATPase 1 gene and analysis of thyroid hormone responsiveness. J. Biol. Chem. 271, 32048–32056 (1996).
Hartong, R. et al. Delineation of three different thyroid hormone-response elements in promoter of rat sarcoplasmic reticulum Ca2+ATPase gene. Demonstration that retinoid X receptor binds 5′ to thyroid hormone receptor in response element 1. J. Biol. Chem. 269, 13021–13029 (1994).
Solanes, G. et al. Thyroid hormones directly activate the expression of the human and mouse uncoupling protein-3 genes through a thyroid response element in the proximal promoter region. Biochem. J. 386, 505–513 (2005).
Zorzano, A., Palacin, M. & Guma, A. Mechanisms regulating GLUT4 glucose transporter expression and glucose transport in skeletal muscle. Acta Physiol. Scand. 183, 43–58 (2005).
Desvergne, B., Petty, K. J. & Nikodem, V. M. Functional characterization and receptor binding studies of the malic enzyme thyroid hormone response element. J. Biol. Chem. 266, 1008–1013 (1991).
Dümmler, K., Müller, S. & Seitz, H. J. Regulation of adenine nucleotide translocase and glycerol 3-phosphate dehydrogenase expression by thyroid hormones in different rat tissues. Biochem. J. 317 (Pt 3), 913–918 (1996).
Morkin, E. Control of cardiac myosin heavy chain gene expression. Microsc. Res. Tech. 50, 522–531 (2000).
Muscat, G. E., Mynett-Johnson, L., Dowhan, D., Downes, M. & Griggs, R. Activation of myoD gene transcription by 3,5,3′-triiodo-L-thyronine: a direct role for the thyroid hormone and retinoid X receptors. Nucleic Acids Res. 22, 583–591 (1994).
Downes, M., Griggs, R., Atkins, A., Olson, E. N. & Muscat, G. E. Identification of a thyroid hormone response element in the mouse myogenin gene: characterization of the thyroid hormone and retinoid X receptor heterodimeric binding site. Cell Growth Differ. 4, 901–909 (1993).
Muller, A., Thelen, M. H., Zuidwijk, M. J., Simonides, W. S. & van Hardeveld, C. Expression of MyoD in cultured primary myotubes is dependent on contractile activity: correlation with phenotype-specific expression of a sarcoplasmic reticulum Ca2+-ATPase isoform. Biochem. Biophys. Res. Commun. 229, 198–204 (1996).
Kraus, B. & Pette, D. Quantification of MyoD, myogenin, MRF4 and Id-1 by reverse-transcriptase polymerase chain reaction in rat muscles—effects of hypothyroidism and chronic low-frequency stimulation. Eur. J. Biochem. 247, 98–106 (1997).
Wheeler, M. T., Snyder, E. C., Patterson, M. N. & Swoap, S. J. An E-box within the MHC IIB gene is bound by MyoD and is required for gene expression in fast muscle. Am. J. Physiol. 276, C1069–C1078 (1999).
Allen, D. L., Sartorius, C. A., Sycuro, L. K. & Leinwand, L. A. Different pathways regulate expression of the skeletal myosin heavy chain genes. J. Biol. Chem. 276, 43524–43533 (2001).
Weitzel, J. M., Iwen, K. A. & Seitz, H. J. Regulation of mitochondrial biogenesis by thyroid hormone. Exp. Physiol. 88, 121–128 (2003).
Irrcher, I., Adhihetty, P. J., Joseph, A. M., Ljubicic, V. & Hood, D. A. Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports Med. 33, 783–793 (2003).
Psarra, A. M., Solakidi, S. & Sekeris, C. E. The mitochondrion as a primary site of action of steroid and thyroid hormones: presence and action of steroid and thyroid hormone receptors in mitochondria of animal cells. Mol. Cell Endocrinol. 246, 21–33 (2006).
Muscat, G. E., Downes, M. & Dowhan, D. H. Regulation of vertebrate muscle differentiation by thyroid hormone: the role of the myoD gene family. Bioessays 17, 211–218 (1995).
White, R. B., Bierinx, A. S., Gnocchi, V. F. & Zammit, P. S. Dynamics of muscle fibre growth during postnatal mouse development. BMC Dev. Biol. 10, 21 (2010).
Braverman, L. E. & Cooper, D. S. Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text, 10th edn (Lippincott, Williams & Wilkins, Philadelphia, 2012).
de Lange, P. et al. Uncoupling protein-3 is a molecular determinant for the regulation of resting metabolic rate by thyroid hormone. Endocrinology 142, 3414–3420 (2001).
Silva, J. E. Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 86, 435–464 (2006).
Visser, W. E. et al. Physiological thyroid hormone levels regulate numerous skeletal muscle transcripts. J. Clin. Endocrinol. Metab. 94, 3487–3496 (2009).
Clement, K. et al. In vivo regulation of human skeletal muscle gene expression by thyroid hormone. Genome Res. 12, 281–291 (2002).
Lebon, V. et al. Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J. Clin. Invest. 108, 733–737 (2001).
Mitchell, C. S. et al. Resistance to thyroid hormone is associated with raised energy expenditure, muscle mitochondrial uncoupling, and hyperphagia. J. Clin. Invest. 120, 1345–1354 (2010).
Johannsen, D. L. et al. Effect of short-term thyroxine administration on energy metabolism and mitochondrial efficiency in humans. PLoS ONE 7, e40837 (2012).
Queiroz, M. S., Shao, Y. & Ismail-Beigi, F. Effect of thyroid hormone on uncoupling protein-3 mRNA expression in rat heart and skeletal muscle. Thyroid 14, 177–185 (2004).
Ramadan, W., Marsili, A., Larsen, P. R., Zavacki, A. M. & Silva, J. E. Type-2 iodothyronine 5′deiodinase (D2) in skeletal muscle of C57Bl/6 mice. II. Evidence for a role of D2 in the hypermetabolism of thyroid hormone receptor alpha-deficient mice. Endocrinology 152, 3093–102 (2011).
van Marken Lichtenbelt, W. D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009).
Cypess, A. M. et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc. Natl Acad. Sci. USA 109, 10001–10005 (2012).
van der Lans, A. A. et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 123, 3395–3403 (2013).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Boström, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012).
Chidakel, A., Mentuccia, D. & Celi, F. S. Peripheral metabolism of thyroid hormone and glucose homeostasis. Thyroid 15, 899–903 (2005).
Klieverik, L. P. et al. Thyroid hormone effects on whole-body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology 150, 5639–5648 (2009).
Harrison, S. A., Buxton, J. M., Clancy, B. M. & Czech, M. P. Insulin regulation of hexose transport in mouse 3T3-L1 cells expressing the human HepG2 glucose transporter. J. Biol. Chem. 265, 20106–20116 (1990).
Marsili, A. et al. Mice with a targeted deletion of the type 2 deiodinase are insulin resistant and susceptible to diet induced obesity. PLoS ONE 6, e20832 (2011).
Mentuccia, D. et al. Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance: evidence of interaction with the Trp64Arg variant of the β-3-adrenergic receptor. Diabetes 51, 880–883 (2002).
Canani, L. H. et al. The type 2 deiodinase A/G. (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 90, 3472–3478 (2005).
Meulenbelt, I. et al. Identification of DIO2 as a new susceptibility locus for symptomatic osteoarthritis. Hum. Mol. Genet. 17, 1867–1875 (2008).
Heemstra, K. A. et al. Thr92Ala polymorphism in the type 2 deiodinase is not associated with T4 dose in athyroid patients or patients with Hashimoto thyroiditis. Clin. Endocrinol. (Oxf.) 71, 279–283 (2009).
Charge, S. B. & Rudnicki, M. A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84, 209–238 (2004).
Schultz, E. & McCormick, K. M. Skeletal muscle satellite cells. Rev. Physiol. Biochem. Pharmacol. 123, 213–257 (1994).
Yablonka-Reuveni, Z. Development and postnatal regulation of adult myoblasts. Microsc. Res. Tech. 30, 366–380 (1995).
Olson, E. N. Interplay between proliferation and differentiation within the myogenic lineage. Dev. Biol. 154, 261–272 (1992).
Rudnicki, M. A. & Jaenisch, R. The MyoD family of transcription factors and skeletal myogenesis. Bioessays 17, 203–209 (1995).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96, 857–868 (1999).
Bois, P. R., Brochard, V. F., Salin-Cantegrel, A. V., Cleveland, J. L. & Grosveld, G. C. FoxO1a-cyclic GMP-dependent kinase I interactions orchestrate myoblast fusion. Mol. Cell Biol. 25, 7645–7656 (2005).
Gross, D. N., van den Heuvel, A. P. & Birnbaum, M. J. The role of FoxO in the regulation of metabolism. Oncogene 27, 2320–2336 (2008).
Mammucari, C., Schiaffino, S. & Sandri, M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy 4, 524–526 (2008).
Zhao, J. et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 6, 472–483 (2007).
Hu, P., Geles, K. G., Paik, J. H., DePinho, R. A. & Tjian, R. Codependent activators direct myoblast-specific MyoD transcription. Dev. Cell 15, 534–546 (2008).
Dentice, M. et al. Type 3 deiodinase is highly expressed in proliferating myoblasts and during the early phase of muscle regeneration. Presented at the 35th Annual Meeting of the European Thyroid Association (Krakow, Poland, 2011).
Koenig, M., Monaco, A. P. & Kunkel, L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 53, 219–228 (1988).
Partridge, T. Pathophysiology of muscular dystrophy. Br. J. Hosp. Med. 49, 26–36 (1993).
England, S. B. et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature 343, 180–182 (1990).
Anderson, J. E., Liu, L. & Kardami, E. The effects of hyperthyroidism on muscular dystrophy in the mdx mouse: greater dystrophy in cardiac and soleus muscle. Muscle Nerve 17, 64–73 (1994).
McIntosh, L. M. & Anderson, J. E. Hypothyroidism prolongs and increases mdx muscle precursor proliferation and delays myotube formation in normal and dystrophic limb muscle. Biochem. Cell Biol. 73, 181–190 (1995).
Pernitsky, A. N., McIntosh, L. M. & Anderson, J. E. Hyperthyroidism impairs early repair in normal but not dystrophic mdx mouse tibialis anterior muscle. An in vivo study. Biochem. Cell Biol. 74, 315–324 (1996).
Acknowledgements
Work described in this Review was supported by Telethon grant GGP00185 to D. Salvatore and NIH grant DK044128 to P. R. Larsen and D. Salvatore.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Salvatore, D., Simonides, W., Dentice, M. et al. Thyroid hormones and skeletal muscle—new insights and potential implications. Nat Rev Endocrinol 10, 206–214 (2014). https://doi.org/10.1038/nrendo.2013.238
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2013.238
This article is cited by
-
Sensitivity to thyroid hormones is associated with sleep duration in the euthyroid population with depression degree lower than moderate
Scientific Reports (2024)
-
Lower serum FT3 within the reference range is associated with mortality for older adults over 80 years of age with sarcopenia
BMC Geriatrics (2023)
-
Effect of Soy Protein Supplementation on Muscle Adaptations, Metabolic and Antioxidant Status, Hormonal Response, and Exercise Performance of Active Individuals and Athletes: A Systematic Review of Randomised Controlled Trials
Sports Medicine (2023)
-
Correlation of subclinical hypothyroidism with sarcopenia and its components in the Chinese older adults
Endocrine (2023)
-
Temporary hypothyroidism in severe crush syndrome: a potential novel entity
Hormones (2023)