Abstract
ATP-sensitive potassium channels (KATP channels) link cell metabolism to electrical activity by controlling the cell membrane potential. They participate in many physiological processes but have a particularly important role in systemic glucose homeostasis by regulating hormone secretion from pancreatic islet cells. Glucose-induced closure of KATP channels is crucial for insulin secretion. Emerging data suggest that KATP channels also play a key part in glucagon secretion, although precisely how they do so remains controversial. This Review highlights the role of KATP channels in insulin and glucagon secretion. We discuss how KATP channels might contribute not only to the initiation of insulin release but also to the graded stimulation of insulin secretion that occurs with increasing glucose concentrations. The various hypotheses concerning the role of KATP channels in glucagon release are also reviewed. Furthermore, we illustrate how mutations in KATP channel genes can cause hyposecretion or hypersecretion of insulin, as in neonatal diabetes mellitus and congenital hyperinsulinism, and how defective metabolic regulation of the channel may underlie the hypoinsulinaemia and the hyperglucagonaemia that characterize type 2 diabetes mellitus. Finally, we outline how sulphonylureas, which inhibit KATP channels, stimulate insulin secretion in patients with neonatal diabetes mellitus or type 2 diabetes mellitus, and suggest their potential use to target the glucagon secretory defects found in diabetes mellitus.
Key Points
-
Closure of ATP-sensitive potassium channels (KATP channels) stimulates insulin secretion but inhibits glucagon release
-
The role of KATP channels in insulin secretion is well understood, but precisely how glucose inhibits glucagon release remains controversial
-
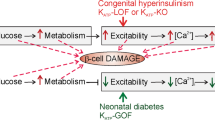
Activating mutations in KATP channel genes cause neonatal diabetes mellitus, whereas loss-of-function mutations cause congenital hyperinsulinism
-
Both insufficient insulin secretion and dysregulation of glucagon release contribute to impaired glucose homeostasis in type 2 diabetes mellitus (T2DM)
-
A common KATP channel haplotype predisposes to T2DM
-
An age-dependent decline in metabolism may also contribute to T2DM via mechanisms that are both dependent on, and independent of, KATP channels
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
09 October 2013
In the version of this article initially published online in Figure 3, part c was incorrectly labelled as part b. The error has been corrected for the print, HTML and PDF versions of the article.
References
Ashcroft, F. M. & Rorsman, P. Diabetes mellitus and the β cell: the last ten years. Cell 148, 1160–1171 (2012).
Nichols, C. G. KATP channels as molecular sensors of cellular metabolism. Nature 440, 470–476 (2006).
Ashcroft, F. M., Harrison, D. E. & Ashcroft, S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 312, 446–448 (1984).
Zhang, Q. et al. R-type Ca2+-channel-evoked CICR regulates glucose-induced somatostatin secretion. Nat. Cell Biol. 9, 453–460 (2007).
Gromada, J. et al. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse α-cells. Diabetes 53 (Suppl. 3), S181–S189 (2004).
Shiota, C., Rocheleau, J. V., Shiota, M., Piston, D. W. & Magnuson, M. A. Impaired glucagon secretory responses in mice lacking the type 1 sulfonylurea receptor. Am. J. Physiol. Endocrinol. Metab. 289, E570–E577 (2005).
Aguilar-Bryan, L. et al. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science 268, 423–426 (1995).
Inagaki, N. et al. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science 270, 1166–1170 (1995).
Sakura, H., Ammala, C., Smith, P. A., Gribble, F. M. & Ashcroft, F. M. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic β-cells, brain, heart and skeletal muscle. FEBS Lett. 377, 338–344 (1995).
Tucker, S. J., Gribble, F. M., Zhao, C., Trapp, S. & Ashcroft, F. M. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature 387, 179–183 (1997).
Shyng, S., Ferrigni, T. & Nichols, C. G. Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J. Gen. Physiol. 110, 643–654 (1997).
Gribble, F. M., Tucker, S. J. & Ashcroft, F. M. The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. EMBO J. 16, 1145–1152 (1997).
Nichols, C. G. et al. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science 272, 1785–1787 (1996).
Trube, G., Rorsman, P. & Ohno-Shosaku, T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic β-cells. Pflugers Arch. 407, 493–499 (1986).
Gribble, F. M. & Reimann, F. Sulphonylurea action revisited: the post-cloning era. Diabetologia 46, 875–891 (2003).
Sturgess, N. C., Ashford, M. L., Cook, D. L. & Hales, C. N. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet 2, 474–475 (1985).
Ashcroft, F. M., Ashcroft, S. J. & Harrison, D. E. Properties of single potassium channels modulated by glucose in rat pancreatic β-cells. J. Physiol. 400, 501–527 (1988).
Ashcroft, F. & Rorsman, P. Type 2 diabetes mellitus: not quite exciting enough? Hum. Mol. Genet. 13 (Suppl. 1), R21–R31 (2004).
Rorsman, P., Eliasson, L., Kanno, T., Zhang, Q. & Gopel, S. Electrophysiology of pancreatic β-cells in intact mouse islets of Langerhans. Prog. Biophys. Mol. Biol. 107, 224–235 (2011).
Schulla, V. et al. Impaired insulin secretion and glucose tolerance in beta cell-selective CaV1.2 Ca2+ channel null mice. EMBO J. 22, 3844–3854 (2003).
Braun, M. et al. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes 57, 1618–1628 (2008).
Rorsman, P. & Braun, M. Regulation of insulin secretion in human pancreatic islets. Annu. Rev. Physiol. 75, 155–179 (2013).
Henquin, J. C. & Meissner, H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic β-cells. Experientia 40, 1043–1052 (1984).
Atwater, I., Ribalet, B. & Rojas, E. Mouse pancreatic beta-cells: tetraethylammonium blockage of the potassium permeability increase induced by depolarization. J. Physiol. 288, 561–574 (1979).
Dean, P. M. & Matthews, E. K. Electrical activity in pancreatic islet cells. Nature 219, 389–390 (1968).
Meissner, H. P. Electrical characteristics of the β-cells in pancreatic islets. J. Physiol. (Paris) 72, 757–767 (1976).
Atwater, I., Ribalet, B. & Rojas, E. Cyclic changes in potential and resistance of the beta-cell membrane induced by glucose in islets of Langerhans from mouse. J. Physiol. 278, 117–139 (1978).
Cook, D. L., Crill, W. E. & Porte, D., Jr. Plateau potentials in pancreatic islet cells are voltage-dependent action potentials. Nature 286, 404–406 (1980).
Kanno, T., Rorsman, P. & Gopel, S. O. Glucose-dependent regulation of rhythmic action potential firing in pancreatic beta-cells by K(ATP)-channel modulation. J. Physiol. 545, 501–507 (2002).
Larsson, O., Kindmark, H., Brandstrom, R., Fredholm, B. & Berggren, P. O. Oscillations in KATP channel activity promote oscillations in cytoplasmic free Ca2+ concentration in the pancreatic beta cell. Proc. Natl Acad. Sci. USA 93, 5161–5165 (1996).
Ammala, C. et al. Inositol trisphosphate-dependent periodic activation of a Ca2+-activated K+ conductance in glucose-stimulated pancreatic β-cells. Nature 353, 849–852 (1991).
Worley, J. F., 3rd . et al. Endoplasmic reticulum calcium store regulates membrane potential in mouse islet β-cells. J. Biol. Chem. 269, 14359–14362 (1994).
Satin, L. S., Tavalin, S. J. & Smolen, P. D. Inactivation of HIT cell Ca2+ current by a simulated burst of Ca2+ action potentials. Biophys. J. 66, 141–148 (1994).
Sherman, A., Rinzel, J. & Keizer, J. Emergence of organized bursting in clusters of pancreatic β-cells by channel sharing. Biophys. J. 54, 411–425 (1988).
Smith, P. A., Ashcroft, F. M. & Rorsman, P. Simultaneous recordings of glucose dependent electrical activity and ATP-regulated K+-currents in isolated mouse pancreatic β-cells. FEBS Lett. 261, 187–190 (1990).
Gopel, S. O. et al. Activation of Ca2+-dependent K+ channels contributes to rhythmic firing of action potentials in mouse pancreatic β cells. J. Gen. Physiol. 114, 759–770 (1999).
Goforth, P. B. et al. Calcium-activated K+ channels of mouse β-cells are controlled by both store and cytoplasmic Ca2+: experimental and theoretical studies. J. Gen. Physiol. 120, 307–322 (2002).
Tarasov, A. I. et al. The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic β-cells. PLoS ONE 7, e39722 (2012).
Detimary, P., Gilon, P. & Henquin, J. C. Interplay between cytoplasmic Ca2+ and the ATP/ADP ratio: a feedback control mechanism in mouse pancreatic islets. Biochem. J. 333, 269–274 (1998).
Rolland, J. F., Henquin, J. C. & Gilon, P. Feedback control of the ATP-sensitive K+ current by cytosolic Ca2+ contributes to oscillations of the membrane potential in pancreatic β-cells. Diabetes 51, 376–384 (2002).
Henquin, J. C. ATP-sensitive K+ channels may control glucose-induced electrical activity in pancreatic β-cells. Biochem. Biophys. Res. Commun. 156, 769–775 (1988).
Henquin, J. C. The fiftieth anniversary of hypoglycaemic sulphonamides. How did the mother compound work? Diabetologia 35, 907–912 (1992).
Zunkler, B. J., Lins, S., Ohno-Shosaku, T., Trube, G. & Panten, U. Cytosolic ADP enhances the sensitivity to tolbutamide of ATP-dependent K+ channels from pancreatic β-cells. FEBS Lett. 239, 241–244 (1988).
Gribble, F. M., Tucker, S. J. & Ashcroft, F. M. The interaction of nucleotides with the tolbutamide block of cloned ATP-sensitive K+ channel currents expressed in Xenopus oocytes: a reinterpretation. J. Physiol. 504, 35–45 (1997).
Proks, P., Reimann, F., Green, N., Gribble, F. & Ashcroft, F. Sulfonylurea stimulation of insulin secretion. Diabetes 51 (Suppl. 3), S368–S376 (2002).
Masia, R. et al. An ATP-binding mutation (G334D) in KCNJ11 is associated with a sulfonylurea-insensitive form of developmental delay, epilepsy, and neonatal diabetes. Diabetes 56, 328–336 (2007).
Gromada, J., Franklin, I. & Wollheim, C. B. α-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr. Rev. 28, 84–116 (2007).
Rorsman, P., Salehi, S. A., Abdulkader, F., Braun, M. & MacDonald, P. E. KATP-channels and glucose-regulated glucagon secretion. Trends Endocrinol. Metab. 19, 277–284 (2008).
Miki, T. et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat. Neurosci. 4, 507–512 (2001).
Munoz, A. et al. Regulation of glucagon secretion at low glucose concentrations: evidence for adenosine triphosphate-sensitive potassium channel involvement. Endocrinology 146, 5514–5521 (2005).
Kawamori, D. et al. Insulin signaling in α cells modulates glucagon secretion in vivo. Cell Metab. 9, 350–361 (2009).
Ishihara, H., Maechler, P., Gjinovci, A., Herrera, P. L. & Wollheim, C. B. Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat. Cell Biol. 5, 330–335 (2003).
Rorsman, P. et al. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 341, 233–236 (1989).
Franklin, I., Gromada, J., Gjinovci, A., Theander, S. & Wollheim, C. B. β-cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54, 1808–1815 (2005).
Prost, A. L., Bloc, A., Hussy, N., Derand, R. & Vivaudou, M. Zinc is both an intracellular and extracellular regulator of KATP channel function. J. Physiol. 559, 157–167 (2004).
Hardy, A. B., Serino, A. S., Wijesekara, N., Chimienti, F. & Wheeler, M. B. Regulation of glucagon secretion by zinc: lessons from the β cell-specific Znt8 knockout mouse model. Diabetes Obes. Metab. 13 (Suppl. 1), 112–117 (2011).
Cheng-Xue, R. et al. Tolbutamide controls glucagon release from mouse islets differently than glucose: involvement of KATP channels from both α-cells and δ-cells. Diabetes 62, 1612–1622 (2013).
Hauge-Evans, A. C. et al. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 58, 403–411 (2009).
Walker, J. N. et al. Regulation of glucagon secretion by glucose: paracrine, intrinsic or both? Diabetes Obes. Metab. 13 (Suppl. 1), 95–105 (2011).
MacDonald, P. E. et al. A KATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. 5, e143 (2007).
Gopel, S. O., Kanno, T., Barg, S. & Rorsman, P. Patch-clamp characterisation of somatostatin-secreting -cells in intact mouse pancreatic islets. J. Physiol. 528, 497–507 (2000).
Rorsman, P. & Hellman, B. Voltage-activated currents in guinea pig pancreatic α2 cells. Evidence for Ca2+-dependent action potentials. J. Gen. Physiol. 91, 223–242 (1988).
Manning Fox, J. E., Gyulkhandanyan, A. V., Satin, L. S. & Wheeler, M. B. Oscillatory membrane potential response to glucose in islet β-cells: a comparison of islet-cell electrical activity in mouse and rat. Endocrinology 147, 4655–4663 (2006).
Quoix, N. et al. Glucose and pharmacological modulators of ATP-sensitive K+ channels control [Ca2+]c by different mechanisms in isolated mouse α-cells. Diabetes 58, 412–421 (2009).
Barg, S., Galvanovskis, J., Gopel, S. O., Rorsman, P. & Eliasson, L. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting α-cells. Diabetes 49, 1500–1510 (2000).
Gopel, S. O. et al. Regulation of glucagon release in mouse-cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J. Physiol. 528, 509–520 (2000).
Bokvist, K. et al. Characterisation of sulphonylurea and ATP-regulated K+ channels in rat pancreatic α-cells. Pflugers Arch. 438, 428–436 (1999).
Huang, Y. C., Rupnik, M. & Gaisano, H. Y. Unperturbed islet α-cell function examined in mouse pancreas tissue slices. J. Physiol. 589, 395–408 (2011).
Tarasov, A. I., Girard, C. A. & Ashcroft, F. M. ATP sensitivity of the ATP-sensitive K+ channel in intact and permeabilized pancreatic β-cells. Diabetes 55, 2446–2454 (2006).
Leung, Y. M. et al. Electrophysiological characterization of pancreatic islet cells in the mouse insulin promoter-green fluorescent protein mouse. Endocrinology 146, 4766–4775 (2005).
Ramracheya, R. et al. Membrane potential-dependent inactivation of voltage-gated ion channels in α-cells inhibits glucagon secretion from human islets. Diabetes 59, 2198–2208 (2010).
Gopel, S. et al. Capacitance measurements of exocytosis in mouse pancreatic α-, β- and δ-cells within intact islets of Langerhans. J. Physiol. 556, 711–726 (2004).
Olsen, H. L. et al. Glucose stimulates glucagon release in single rat α-cells by mechanisms that mirror the stimulus-secretion coupling in beta-cells. Endocrinology 146, 4861–4870 (2005).
Thorel, F. et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 464, 1149–1154 (2010).
Li, C. et al. Regulation of glucagon secretion in normal and diabetic human islets by γ-hydroxybutyrate and glycine. J. Biol. Chem. 288, 3938–3951 (2013).
Geng, X., Li, L., Watkins, S., Robbins, P. D. & Drain, P. The insulin secretory granule is the major site of KATP channels of the endocrine pancreas. Diabetes 52, 767–776 (2003).
Ozanne, S. E., Guest, P. C., Hutton, J. C. & Hales, C. N. Intracellular localization and molecular heterogeneity of the sulphonylurea receptor in insulin-secreting cells. Diabetologia 38, 277–282 (1995).
Guiot, Y. et al. Morphological localisation of sulfonylurea receptor 1 in endocrine cells of human, mouse and rat pancreas. Diabetologia 50, 1889–1899 (2007).
Yang, S. N. et al. Glucose recruits KATP channels via non-insulin-containing dense-core granules. Cell Metab. 6, 217–228 (2007).
Shibasaki, T., Sunaga, Y., Fujimoto, K., Kashima, Y. & Seino, S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J. Biol. Chem. 279, 7956–7961 (2004).
Eliasson, L. et al. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic β-cells. J. Gen. Physiol. 121, 181–197 (2003).
Nakazaki, M. et al. cAMP-activated protein kinase-independent potentiation of insulin secretion by cAMP is impaired in SUR1 null islets. Diabetes 51, 3440–3449 (2002).
Kang, G., Leech, C. A., Chepurny, O. G., Coetzee, W. A. & Holz, G. G. Role of the cAMP sensor Epac as a determinant of KATP channel ATP sensitivity in human pancreatic β-cells and rat INS-1 cells. J. Physiol. 586, 1307–1319 (2008).
Kang, Y. et al. ATP modulates interaction of syntaxin-1A with sulfonylurea receptor 1 to regulate pancreatic β-cell KATP channels. J. Biol. Chem. 286, 5876–5883 (2011).
Eliasson, L. et al. PKC-dependent stimulation of exocytosis by sulfonylureas in pancreatic β cells. Science 271, 813–815 (1996).
Hoy, M. et al. Tolbutamide stimulates exocytosis of glucagon by inhibition of a mitochondrial-like ATP-sensitive K+ (KATP) conductance in rat pancreatic A-cells. J. Physiol. 527 Pt 1, 109–120 (2000).
Mariot, P., Gilon, P., Nenquin, M. & Henquin, J. C. Tolbutamide and diazoxide influence insulin secretion by changing the concentration but not the action of cytoplasmic Ca2+ in β-cells. Diabetes 47, 365–373 (1998).
Zhang, C. L. et al. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 325, 607–610 (2009).
Gloyn, A. L. et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N. Engl. J. Med. 350, 1838–1849 (2004).
Hattersley, A. T. & Ashcroft, F. M. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes 54, 2503–2513 (2005).
Rubio-Cabezas, O., Flanagan, S. E., Damhuis, A., Hattersley, A. T. & Ellard, S. KATP channel mutations in infants with permanent diabetes diagnosed after 6 months of life. Pediatr. Diabetes 13, 322–325 (2012).
Flanagan, S. E. et al. Update of mutations in the genes encoding the pancreatic β-cell KATP channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum. Mutat. 30, 170–180 (2009).
Clark, R. H. et al. Muscle dysfunction caused by a KATP channel mutation in neonatal diabetes is neuronal in origin. Science 329, 458–461 (2010).
Proks, P. et al. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc. Natl Acad. Sci. USA 101, 17539–17544 (2004).
McTaggart, J. S., Clark, R. H. & Ashcroft, F. M. The role of the KATP channel in glucose homeostasis in health and disease: more than meets the islet. J. Physiol. 588, 3201–3209 (2010).
Ellard, S. et al. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am. J. Hum. Genet. 81, 375–382 (2007).
Babenko, A. P. et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N. Engl. J. Med. 355, 456–466 (2006).
Proks, P., Girard, C. & Ashcroft, F. M. Functional effects of KCNJ11 mutations causing neonatal diabetes: enhanced activation by MgATP. Hum. Mol. Genet. 14, 2717–2726 (2005).
Koster, J. C., Marshall, B. A., Ensor, N., Corbett, J. A. & Nichols, C. G. Targeted overactivity of β cell KATP channels induces profound neonatal diabetes. Cell 100, 645–654 (2000).
Girard, C. A. et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic β cells recapitulates neonatal diabetes. J. Clin. Invest. 119, 80–90 (2009).
Remedi, M. S. et al. Secondary consequences of β cell inexcitability: identification and prevention in a murine model of KATP-induced neonatal diabetes mellitus. Cell Metab. 9, 140–151 (2009).
Pearson, E. R. et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N. Engl. J. Med. 355, 467–477 (2006).
Ashcroft, F. M. New uses for old drugs: neonatal diabetes and sulphonylureas. Cell Metab. 11, 179–181 (2010).
Zung, A., Glaser, B., Nimri, R. & Zadik, Z. Glibenclamide treatment in permanent neonatal diabetes mellitus due to an activating mutation in Kir6.2. J. Clin. Endocrinol. Metab. 89, 5504–5507 (2004).
Remedi, M. S., Agapova, S. E., Vyas, A. K., Hruz, P. W. & Nichols, C. G. Acute sulfonylurea therapy at disease onset can cause permanent remission of KATP-induced diabetes. Diabetes 60, 2515–2522 (2011).
Dunne, M. J., Cosgrove, K. E., Shepherd, R. M., Aynsley-Green, A. & Lindley, K. J. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol. Rev. 84, 239–275 (2004).
Thomas, P. M. et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science 268, 426–429 (1995).
Huopio, H. et al. A new subtype of autosomal dominant diabetes attributable to a mutation in the gene for sulfonylurea receptor 1. Lancet 361, 301–307 (2003).
Ocal, G. et al. Clinical characteristics of recessive and dominant congenital hyperinsulinism due to mutation(s) in the ABCC8/KCNJ11 genes encoding the ATP-sensitive potassium channel in the pancreatic β cell. J. Pediatr. Endocrinol. Metab. 24, 1019–1023 (2011).
Hussain, K., Bryan, J., Christesen, H. T., Brusgaard, K. & Aguilar-Bryan, L. Serum glucagon counterregulatory hormonal response to hypoglycemia is blunted in congenital hyperinsulinism. Diabetes 54, 2946–2951 (2005).
Hardy, O. T. et al. Accuracy of [18F]fluorodopa positron emission tomography for diagnosing and localizing focal congenital hyperinsulinism. J. Clin. Endocrinol. Metab. 92, 4706–4711 (2007).
Blomberg, B. A., Moghbel, M. C., Saboury, B., Stanley, C. A. & Alavi, A. The value of radiologic interventions and 18F-DOPA PET in diagnosing and localizing focal congenital hyperinsulinism: systematic review and meta-analysis. Mol. Imaging Biol. 15, 97–105 (2013).
Kane, C. et al. Loss of functional KATP channels in pancreatic β-cells causes persistent hyperinsulinemic hypoglycemia of infancy. Nat. Med. 2, 1344–1347 (1996).
Henquin, J. C. et al. In vitro insulin secretion by pancreatic tissue from infants with diazoxide-resistant congenital hyperinsulinism deviates from model predictions. J. Clin. Invest. 121, 3932–3942 (2011).
Henquin, J. C. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in β-cells. Diabetes Res. Clin. Pract. 93 (Suppl. 1), S27–S31 (2011).
Hugill, A., Shimomura, K., Ashcroft, F. M. & Cox, R. D. A mutation in KCNJ11 causing human hyperinsulinism (Y12X) results in a glucose-intolerant phenotype in the mouse. Diabetologia 53, 2352–2356 (2010).
Miki, T. et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc. Natl Acad. Sci. USA 95, 10402–10406 (1998).
Seghers, V., Nakazaki, M., DeMayo, F., Aguilar-Bryan, L. & Bryan, J. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J. Biol. Chem. 275, 9270–9277 (2000).
Remedi, M. S. et al. Hyperinsulinism in mice with heterozygous loss of KATP channels. Diabetologia 49, 2368–2378 (2006).
Szollosi, A., Nenquin, M. & Henquin, J. C. Overnight culture unmasks glucose-induced insulin secretion in mouse islets lacking ATP-sensitive K+ channels by improving the triggering Ca2+ signal. J. Biol. Chem. 282, 14768–14776 (2007).
Rodriguez-Diaz, R. et al. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming β cell function in humans. Nat. Med. 17, 888–892 (2011).
De Marinis, Y. Z. et al. GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis. Cell Metab. 11, 543–553 (2010).
Cryer, P. E. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 45, 937–948 (2002).
Shah, P. et al. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 85, 4053–4059 (2000).
Lee, Y., Wang, M. Y., Du, X. Q., Charron, M. J. & Unger, R. H. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 60, 391–397 (2011).
Thorel, F. et al. Normal glucagon signaling and β-cell function after near-total α-cell ablation in adult mice. Diabetes 60, 2872–2882 (2011).
Sakura, H. et al. Sequence variations in the human Kir6.2 gene, a subunit of the β-cell ATP-sensitive K-channel: no association with NIDDM in while Caucasian subjects or evidence of abnormal function when expressed in vitro. Diabetologia 39, 1233–1236 (1996).
Gloyn, A. L. et al. Large-scale association studies of variants in genes encoding the pancreatic β-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 52, 568–572 (2003).
Tschritter, O. et al. The prevalent Glu23Lys polymorphism in the potassium inward rectifier 6.2 (KIR6.2) gene is associated with impaired glucagon suppression in response to hyperglycemia. Diabetes 51, 2854–2860 (2002).
Riedel, M. J., Boora, P., Steckley, D., de Vries, G. & Light, P. E. Kir6.2 polymorphisms sensitize β-cell ATP-sensitive potassium channels to activation by acyl CoAs: a possible cellular mechanism for increased susceptibility to type 2 diabetes? Diabetes 52, 2630–2635 (2003).
Schwanstecher, C., Meyer, U. & Schwanstecher, M. K(IR)6.2 polymorphism predisposes to type 2 diabetes by inducing overactivity of pancreatic β-cell ATP-sensitive K+ channels. Diabetes 51, 875–879 (2002).
Villareal, D. T. et al. Kir6.2 variant E23K increases ATP-sensitive K+ channel activity and is associated with impaired insulin release and enhanced insulin sensitivity in adults with normal glucose tolerance. Diabetes 58, 1869–1878 (2009).
Hamming, K. S. et al. Coexpression of the type 2 diabetes susceptibility gene variants KCNJ11 E23K and ABCC8 S1369A alter the ATP and sulfonylurea sensitivities of the ATP-sensitive K+ channel. Diabetes 58, 2419–2424 (2009).
Fatehi, M. et al. The ATP-sensitive K+ channel ABCC8 S1369A type 2 diabetes risk variant increases MgATPase activity. Diabetes 61, 241–249 (2012).
U. K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U. K. Prospective Diabetes Study Group. Diabetes 44, 1249–1258 (1995).
Doliba, N. M. et al. Glucokinase activation repairs defective bioenergetics of islets of Langerhans isolated from type 2 diabetics. Am. J. Physiol. Endocrinol. Metab. 302, E87–E102 (2012).
Iafusco, D. et al. No beta cell desensitisation after a median of 68 months on glibenclamide therapy in patients with KCNJ11-associated permanent neonatal diabetes. Diabetologia 54, 2736–2738 (2011).
Njolstad, P. R. et al. Neonatal diabetes mellitus due to complete glucokinase deficiency. N. Engl. J. Med. 344, 1588–1592 (2001).
Gloyn, A. L. Glucokinase (GCK) mutations in hyper- and hypoglycemia: maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy. Hum. Mutat. 22, 353–362 (2003).
Rosengren, A. H. et al. Reduced insulin exocytosis in human pancreatic β-cells with gene variants linked to type 2 diabetes. Diabetes 61, 1726–1733 (2012).
Acknowledgements
Work in the authors' laboratories is funded by the Wellcome Trust, the European Union, Diabetes UK, the Medical Research Council and the Royal Society. F. M. Ashcroft holds the Wolfson-Royal Society merit award.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ashcroft, F., Rorsman, P. KATP channels and islet hormone secretion: new insights and controversies. Nat Rev Endocrinol 9, 660–669 (2013). https://doi.org/10.1038/nrendo.2013.166
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2013.166
This article is cited by
-
Sugar signals from oral glucose transporters elicit cephalic-phase insulin release in mice
The Journal of Physiological Sciences (2023)
-
Applications of synthetic biology in medical and pharmaceutical fields
Signal Transduction and Targeted Therapy (2023)
-
Sensory Systems for Sugar-Induced Cephalic Phase Insulin Release
Current Oral Health Reports (2023)
-
Engineering living therapeutics with synthetic biology
Nature Reviews Drug Discovery (2021)
-
The effects of beta-cell mass and function, intercellular coupling, and islet synchrony on \({\text {Ca}}^{2+}\) dynamics
Scientific Reports (2021)