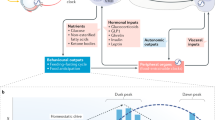
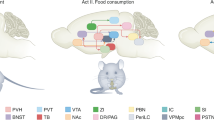
Abstract
Many questions must be considered with regard to consuming food, including when to eat, what to eat and how much to eat. Although eating is often thought to be a homeostatic behaviour, little evidence exists to suggest that eating is an automatic response to an acute shortage of energy. Instead, food intake can be considered as an integrated response over a prolonged period of time that maintains the levels of energy stored in adipocytes. When we eat is generally determined by habit, convenience or opportunity rather than need, and meals are preceded by a neurally-controlled coordinated secretion of numerous hormones that prime the digestive system for the anticipated caloric load. How much we eat is determined by satiation hormones that are secreted in response to ingested nutrients, and these signals are in turn modified by adiposity hormones that indicate the fat content of the body. In addition, many nonhomeostatic factors, including stress, learning, palatability and social influences, interact with other controllers of food intake. If a choice of food is available, what we eat is based on pleasure and past experience. This article reviews the hormones that mediate and influence these processes.
Key Points
-
Endocrine influences over eating are complex and include factors related to the body's energy needs (homeostatic factors) and to experience, habits and opportunity (nonhomeostatic factors)
-
Meal-anticipatory endocrine responses that are initiated by food cues before eating start the digestive process and reduce the incidence of meal-associated hyperglycaemia and other metabolic challenges
-
Although satiation hormones such as cholecystokinin and glucagon-like peptide 1 (GLP-1) determine how much a person eats, they have not proven efficacious as clinical treatments for reducing body weight
-
Adiposity hormones, such as leptin and insulin, change the sensitivity of the brain to satiation signals, thereby helping maintain stable levels of body fat and body weight over time
-
Stress, reproductive regulatory hormones and cardiovascular regulatory hormones interact with adiposity signals in complex ways to influence food intake
-
Although chronically altered levels of GLP-1, ghrelin and other gastrointestinal hormones are thought to underlie metabolic improvements following bariatric surgery, supporting evidence is lacking
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Woods, S. C. The control of food intake: behavioral versus molecular perspectives. Cell Metab. 9, 489–498 (2009).
Stanley, S., Wynne, K., McGowan, B. & Bloom, S. Hormonal regulation of food intake. Physiol. Rev. 85, 1131–1158 (2005).
Woods, S. C. & Ramsay, D. S. Food intake, metabolism and homeostasis. Physiol. Behav. 104, 4–7 (2011).
Day, F. R. & Loos, R. J. Developments in obesity genetics in the era of genome-wide association studies. J. Nutrigenet. Nutrigenomics 4, 222–238 (2011).
Fall, T. & Ingelsson, E. Genome-wide association studies of obesity and metabolic syndrome. Mol. Cell. Endocrinol. http://dx.doi.org/10.1016/j.mce.2012.08.018.
Sandholt, C. H., Hansen, T. & Pedersen, O. Beyond the fourth wave of genome-wide obesity association studies. Nutr. Diabetes 2, e37 (2012).
Yeo, G. S. & Heisler, L. K. Unraveling the brain regulation of appetite: lessons from genetics. Nat. Neurosci. 15, 1343–1349 (2012).
Rucker, D., Padwal, R., Li, S. K., Curioni, C. & Lau, D. C. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ 335, 1194–1199 (2007).
Bradley, D., Magkos, F. & Klein, S. Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology 143, 897–912 (2012).
Wing, R. R. & Phelan, S. Long-term weight loss maintenance. Am. J. Clin. Nutr. 82, 222S–225S (2005).
Woods, S. C., Decke, E. & Vasselli, J. R. Metabolic hormones and regulation of body weight. Psychol. Rev. 81, 26–43 (1974).
Pavlov, I. P. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex (Ed. Anrep, G. V.) (Oxford University Press, Humphrey Milford, 1927).
Teff, K. L. How neural mediation of anticipatory and compensatory insulin release helps us tolerate food. Physiol. Behav. 103, 44–50 (2011).
Woods, S. C. The eating paradox: how we tolerate food. Psychol. Rev. 98, 488–505 (1991).
Ahren, B. & Holst, J. J. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes 50, 1030–1038 (2001).
Powley, T. L. The ventromedial hypothalamic syndrome, satiety, and a cephalic phase hypothesis. Psychol. Rev. 84, 89–126 (1977).
Power, M. L. & Schulkin, J. Anticipatory physiological regulation in feeding biology: cephalic phase responses. Appetite 50, 194–206 (2008).
Woods, S. C. Conditioned hypoglycemia: effect of vagotomy and pharmacological blockade. Am. J. Physiol. 223, 1424–1427 (1972).
Berthoud, H. R., Bereiter, D. A., Trimble, E. R., Siegel, E. G. & Jeanrenaud, B. Cephalic phase, reflex insulin secretion. Neuroanatomical and physiological characterization. Diabetologia 20 (Suppl.), 393–401 (1981).
Teff, K. L., Mattes, R. D. & Engelman, K. Cephalic phase insulin release in normal weight males: verification and reliability. Am. J. Physiol. 261, E430–E436 (1991).
Just, T., Pau, H. W., Engel, U. & Hummel, T. Cephalic phase insulin release in healthy humans after taste stimulation? Appetite 51, 622–627 (2008).
Woods, S. C. et al. Conditioned insulin secretion and meal feeding in rats. J. Comp. Physiol. Psychol. 91, 128–133 (1977).
Strubbe, J. H. Parasympathetic involvement in rapid meal-associated conditioned insulin secretion in the rat. Am. J. Physiol. 263, R615–R618 (1992).
Cohn, C. & Joseph, D. Effects of caloric intake and feeding frequency on carbohydrate metabolism of the rat. J. Nutr. 100, 78–84 (1970).
Sugino, T. et al. A transient surge of ghrelin secretion before feeding is modified by different feeding regimens in sheep. Biochem. Biophys. Res. Commun. 298, 785–788 (2002).
Drazen, D. L., Vahl, T. P., D'Alessio, D. A., Seeley, R. J. & Woods, S. C. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 147, 23–30 (2006).
Shin, Y. K. et al. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS ONE 5, e12729 (2010).
Tong, J. et al. Ghrelin enhances olfactory sensitivity and exploratory sniffing in rodents and humans. J. Neurosci. 31, 5841–5846 (2011).
Tschöp, M., Smiley, D. L. & Heiman, M. L. Ghrelin induces adiposity in rodents. Nature 407, 908–913 (2000).
Vahl, T. P., Drazen, D. L., Seeley, R. J., D'Alessio, D. A. & Woods, S. C. Meal-anticipatory glucagon-like peptide-1 secretion in rats. Endocrinology 151, 569–575 (2010).
Martin, B. et al. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann. NY Acad. Sci. 1170, 98–101 (2009).
Schafmayer, A., Nustede, R., Pompino, A. & Kohler, H. Vagal influence on cholecystokinin and neurotensin release in conscious dogs. Scand. J. Gastroenterol. 23, 315–320 (1988).
Secchi, A. et al. Cephalic-phase insulin and glucagon release in normal subjects and in patients receiving pancreas transplantation. Metabolism 44, 1153–1158 (1995).
Davidson, A. J. & Stephan, F. K. Plasma glucagon, glucose, insulin, and motilin in rats anticipating daily meals. Physiol. Behav. 1966, 309–315 (1999).
Taylor, I. L., Feldman, M., Richardson, C. T. & Walsh, J. H. Gastric and cephalic stimulation of human pancreatic polypeptide release. Gastroenterology 75, 432–437 (1978).
Teff, K. L. Cephalic phase pancreatic polypeptide responses to liquid and solid stimuli in humans. Physiol. Behav. 99, 317–323 (2010).
Morricone, L. et al. Food-related sensory stimuli are able to promote pancreatic polypeptide elevation without evident cephalic phase insulin secretion in human obesity. Horm. Metab. Res. 32, 240–245 (2000).
Katschinski, M. et al. Cephalic stimulation of gastrointestinal secretory and motor responses in humans. Gastroenterology 103, 383–391 (1992).
Liu, M. et al. Diurnal rhythm of apolipoprotein A-IV in rat hypothalamus and its relation to food intake and corticosterone. Endocrinology 145, 3232–3238 (2004).
Leahy, J. L. & Fineman, M. S. Impaired phasic insulin and amylin secretion in diabetic rats. Am. J. Physiol. 275, E457–E462 (1998).
LeBlanc, J. Nutritional implications of cephalic phase thermogenic responses. Appetite 34, 214–216 (2000).
de Vries, J., Strubbe, J. H., Wildering, W. C., Gorter, J. A. & Prins, A. J. Patterns of body temperature during feeding in rats under varying ambient temperatures. Physiol. Behav. 53, 229–235 (1993).
Katschinski, M. Nutritional implications of cephalic phase gastrointestinal responses. Appetite 34, 189–196 (2000).
Konturek, S. J. et al. Brain-gut axis in pancreatic secretion and appetite control. J. Physiol. Pharmacol. 54, 293–317 (2003).
Weingarten, H. P. & Powley, T. L. Pavlovian conditioning of the cephalic phase of gastric acid secretion in the rat. Physiol. Behav. 27, 217–221 (1981).
Feldman, M. & Richardson, C. T. Role of thought, sight, smell, and taste of food in the cephalic phase of gastric acid secretion in humans. Gastroenterology 90, 428–433 (1986).
Carneiro, B. T. & Araujo, J. F. Food entrainment: major and recent findings. Front. Behav. Neurosci. 6, 83 (2012).
Gibbs, J., Young, R. C. & Smith, G. P. Cholecystokinin decreases food intake in rats. J. Comp. Physiol. Psychol. 84, 488–495 (1973).
Muurahainenn, N., Kissileff, H. R., Derogatis, A. J. & Pi-Sunyer, F. X. Effects of cholecystokinin-octapeptide (CCK-8) on food intake and gastric emptying in man. Physiol. Behav. 44, 644–649 (1988).
Lieverse, R. J. et al. Effects of a physiological dose of cholecystokinin on food intake and postprandial satiation in man. Regul. Pept. 43, 83–89 (1993).
Beglinger, C., Degen, L., Matzinger, D., D'Amato, M. & Drewe, J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am. J. Physiol. 280, R1149–R1154 (2001).
Moran, T. H., Smith, G. P., Hostetler, A. M. & McHugh, P. R. Transport of cholecystokinin (CCK) binding sites in subdiaphragmatic vagal branches. Brain Res. 415, 149–152 (1987).
Abbott, C. R. et al. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 1044, 127–131 (2005).
Weatherford, S. C. & Ritter, S. Lesion of vagal afferent terminals impairs glucagon-induced suppression of food intake. Physiol. Behav. 43, 645–650 (1988).
Lo, C. C. et al. Apolipoprotein AIV requires cholecystokinin and vagal nerves to suppress food intake. Endocrinology 153, 5857–5865 (2012).
Koda, S. et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology 146, 2369–2375 (2005).
le Roux, C. W. et al. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J. Clin. Endocrinol. Metab. 90, 4521–4524 (2005).
Date, Y. et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123, 1120–1128 (2002).
Lutz, T. A. et al. Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene-related peptide (CGRP) in rats. Peptides 19, 309–317 (1998).
Hayes, M. R. et al. The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1479–R1485 (2011).
Kanoski, S. E., Fortin, S. M., Arnold, M., Grill, H. J. & Hayes, M. R. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152, 3103–3112 (2011).
Arnold, M., Mura, A., Langhans, W. & Geary, N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J. Neurosci. 26, 11052–11060 (2006).
Asakawa, A. et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120, 337–345 (2001).
Wren, A. M. et al. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 86, 5992 (2001).
Lo, C. M. et al. Characterization of mice lacking the gene for cholecystokinin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R803–R810 (2008).
Scrocchi, L. A., Hill, M. E., Saleh, J., Perkins, B. & Drucker, D. J. Elimination of glucagon-like peptide 1R signaling does not modify weight gain and islet adaptation in mice with combined disruption of leptin and GLP-1 action. Diabetes 49, 1552–1560 (2000).
Sun, Y., Ahmed, S. & Smith, R. G. Deletion of ghrelin impairs neither growth nor appetite. Mol. Cell Biol. 23, 7973–7981 (2003).
Weinstock, P. H. et al. Decreased HDL cholesterol levels but normal lipid absorption, growth, and feeding behavior in apolipoprotein A-IV knockout mice. J. Lipid Res. 38, 1782–1794 (1997).
Ladenheim, E. E. et al. Disruptions in feeding and body weight control in gastrin-releasing peptide receptor deficient mice. J. Endocrinol. 174, 273–281 (2002).
Goodison, T. & Siegel, S. Learning and tolerance to the intake suppressive effect of cholecystokinin in rats. Behav. Neurosci. 109, 62–70 (1995).
Duncan, E. A., Davita, G. & Woods, S. C. Changes in the satiating effect of cholecystokinin over repeated trials. Physiol. Behav. 85, 387–393 (2005).
Woods, S. C. & Langhans, W. Inconsistencies in the assessment of food intake. Am. J. Physiol. Endocrinol. Metab. 303, E1408–E1418 (2012).
Sandoval, D. A., Bagnol, D., Woods, S. C., D'Alessio, D. A. & Seeley, R. J. Arcuate GLP-1 receptors regulate glucose homeostasis but not food intake. Diabetes 57, 2046–2054 (2008).
Williams, D. L., Baskin, D. G. & Schwartz, M. W. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150, 1680–1687 (2009).
Flint, A., Raben, A., Astrup, A. & Holst, J. J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Invest. 101, 515–520 (1998).
Morley, J. E. & Levine, A. S. Involvement of dynorphin and the κ opioid receptor in feeding. Peptides 4, 797–800 (1983).
Sweet, D. C., Levine, A. S., Billington, C. J. & Kotz, C. M. Feeding response to central orexins. Brain Res. 821, 535–538 (1999).
Fujimoto, K., Fukagawa, K., Sakata, T. & Tso, P. Suppression of food intake by apolioprotein A-IV is mediated through the central nervous system in rats. J. Clin. Invest. 91, 1830–1833 (1993).
Yamada-Goto, N. et al. Intracerebroventricular administration of c-type natriuretic peptide suppresses food intake via activation of the melanocortin system in mice. Diabetes 62, 1500–1504 (2013).
Hoebel, B. G. Integrative peptides. Brain Res. Bull. 14, 525–528 (1985).
Antin, J., Gibbs, J., Holt, J., Young, R. C. & Smith, G. P. Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J. Comp. Physiol. Psychol. 89, 784–790 (1975).
West, D. B. et al. Infusion of cholecystokinin between meals into free-feeding rats fails to prolong the intermeal interval. Physiol. Behav. 39, 111–115 (1987).
West, D. B., Fey, D. & Woods, S. C. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am. J. Physiol. 246, R776–R787 (1984).
Bagdade, J. D., Bierman, E. L. & Porte, D. Jr. The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J. Clin. Invest. 46, 1549–1557 (1967).
Lonnqvist, F., Arner, P., Nordfors, L. & Schalling, M. Overexpression of the obest (ob) gene in adipose tissue of human obese subjects. Nat. Med. 1, 950–953 (1995).
Baura, G. et al. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo: a mechanism for regulated insulin delivery to the brain. J. Clin. Invest. 92, 1824–1830 (1993).
Banks, W. A., Kastin, A. J., Huang, W., Jaspan, J. B. & Maness, L. M. Leptin enters the brain by a saturable system independent of insulin. Peptides 17, 305–311 (1996).
Maresh, G. A., Maness, L. M., Zadina, J. E. & Kastin, A. J. In vitro demonstration of a saturable transport system for leptin across the blood–brain barrier. Life Sci. 69, 67–73 (2001).
Woods, S. C., Lotter, E. C., McKay, L. D. & Porte, D. Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282, 503–505 (1979).
Vogt, M. C. & Bruning, J. C. CNS insulin signaling in the control of energy homeostasis and glucose metabolism—from embryo to old age. Trends Endocrinol. Metab. 24, 76–84 (2013).
Campfield, L. A., Smith, F. J., Guisez, Y., Devos, R. & Burn, P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269, 546–549 (1995).
Sindelar, D. K., Mystkowski, P., Marsh, D. J., Palmiter, R. D. & Schwartz, M. W. Attenuation of diabetic hyperphagia in neuropeptide Y-deficient mice. Diabetes 51, 778–783 (2002).
Sipols, A. J., Baskin, D. G. & Schwartz, M. W. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes 44, 147–151 (1995).
Weigle, D. S. et al. Recombinant ob protein reduces feeding and body weight in the ob/ob mouse. J. Clin. Invest. 96, 2065–2070 (1995).
Benoit, S. C. et al. The catabolic action of insulin in the brain is mediated by melanocortins. J. Neurosci. 22, 9048–9052 (2002).
Woods, S. C., Seeley, R. J., Porte, D. Jr & Schwartz, M. W. Signals that regulate food intake and energy homeostasis. Science 280, 1378–1383 (1998).
Schwartz, M. W., Woods, S. C., Porte, D. Jr, Seeley, R. J. & Baskin, D. G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Begg, D. P. et al. Reversal of diet-induced obesity increases insulin transport into cerebrospinal fluid and restores sensitivity to the anorexic action of central insulin in male rats. Endocrinology 154, 1047–1054 (2013).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Bruning, J. C. et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 (2000).
Morley, J. E. & Flood, J. F. Amylin decreases food intake in mice. Peptides 12, 865–869 (1991).
Lutz, T. A. The interaction of amylin with other hormones in the control of eating. Diabetes Obes. Metab. 15, 99–111 (2013).
Langhans, W. & Hrupka, B. Interleukins and tumor necrosis factor as inhibitors of food intake. Neuropeptides 33, 415–424 (1999).
de Kloet, A. D., Pacheco-Lopez, G., Langhans, W. & Brown, L. M. The effect of TNFα on food intake and central insulin sensitivity in rats. Physiol. Behav. 103, 17–20 (2011).
Qi, Y. et al. Adiponectin acts in the brain to decrease body weight. Nat. Med. 10, 524–529 (2004).
Hansen, T. K. et al. Weight loss increases circulating levels of ghrelin in human obesity. Clin. Endocrinol. (Oxf.) 56, 203–206 (2002).
Dietrich, M. O. & Horvath, T. L. Feeding signals and brain circuitry. Eur. J. Neurosci. 30, 1688–1696 (2009).
Kirchner, H., Heppner, K. M. & Tschop, M. H. The role of ghrelin in the control of energy balance. Handb. Exp. Pharmacol. 209, 161–184 (2012).
Schwartz, G. J., McHugh, P. R. & Moran, T. H. Gastric loads and cholecystokinin synergistically stimulate rat gastric vagal afferents. Am. J. Physiol. 265, R872–R876 (1993).
Hamilton, R. B. & Norgren, R. Central projections of gustatory nerves in the rat. J. Comp. Neurol. 222, 560–577 (1984).
Berthoud, H. R., Carlson, N. R. & Powley, T. L. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am. J. Physiol. 260, R200–R207 (1991).
Berthoud, H. R., Jedrzejewska, A. & Powley, T. L. Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with dil injected into the dorsal vagal complex in the rat. J. Comp. Neurol. 301, 65–79 (1990).
Travagli, R. A., Hermann, G. E., Browning, K. N. & Rogers, R. C. Brainstem circuits regulating gastric function. Annu. Rev. Physiol. 68, 279–305 (2006).
Grill, H. J. & Hayes, M. R. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell. Metab. 16, 296–309 (2012).
Berthoud, H. R., Sutton, G. M., Townsend, R. L., Patterson, L. M. & Zheng, H. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol. Behav. 89, 517–524 (2006).
Rinaman, L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 1350, 18–34 (2010).
Schwartz, G. J. Brainstem integrative function in the central nervous system control of food intake. Forum Nutr. 63, 141–151 (2010).
Berthoud, H. R., Lenard, N. R. & Shin, A. C. Food reward, hyperphagia, and obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1266–R1277 (2011).
Kaplan, J. M., Seeley, R. J. & Grill, H. J. Daily caloric intake in intact and chronic decerebrate rats. Behav. Neurosci. 107, 876–881 (1993).
Seeley, R. J., Grill, H. J. & Kaplan, J. M. Neurological dissociation of gastrointestinal and metabolic contributions to meal size control. Behav. Neurosci. 108, 347–352 (1994).
Grill, H. J. & Smith, G. P. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. Am. J. Physiol. 254, R853–R856 (1988).
Hayes, M. R., Skibicka, K. P. & Grill, H. J. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149, 4059–4068 (2008).
Cota, D. et al. Hypothalamic mTOR signaling regulates food intake. Science 312, 927–930 (2006).
Levin, B. E., Magnan, C., Dunn-Meynell, A. & Le Foll, C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology 152, 2552–2557 (2011).
Guyenet, S. J. & Schwartz, M. W. Clinical review: Regulation of food intake, energy balance, and body fat mass: implications for the pathogenesis and treatment of obesity. J. Clin. Endocrinol. Metab. 97, 745–755 (2012).
Cone, R. D. et al. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 25 (Suppl. 5), S63–S67 (2001).
Rossi, M. et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology 139, 4428–4431 (1998).
Beck, B. Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1159–1185 (2006).
Belgardt, B. F. & Bruning, J. C. CNS leptin and insulin action in the control of energy homeostasis. Ann. NY Acad. Sci. 1212, 97–113 (2010).
Williams, K. W. & Elmquist, J. K. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat. Neurosci. 15, 1350–1355 (2012).
Mercer, J. G., Moar, K. M. & Hoggard, N. Localization of leptin receptor (Ob-R) messenger ribonucleic acid in the rodent hindbrain. Endocrinology 139, 29–34 (1998).
van Houten, M. & Posner, B. I. Specific binding and internalization of blood-borne [125I]-iodoinsulin by neurons of the rat area postrema. Endocrinology 109, 853–859 (1981).
Riedy, C. A., Chavez, M., Figlewicz, D. P. & Woods, S. C. Central insulin enhances sensitivity to cholecystokinin. Physiol. Behav. 58, 755–760 (1995).
Hallschmid, M., Benedict, C., Born, J., Fehm, H. L. & Kern, W. Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man. Physiol. Behav. 83, 55–64 (2004).
Sumithran, P. & Proietto, J. The defence of body weight: a physiological basis for weight regain after weight loss. Clin. Sci. (Lond.) 124, 231–241 (2013).
Edwards, K. L., Stapleton, M., Weis, J. & Irons, B. K. An update in incretin-based therapy: a focus on glucagon-like peptide-1 receptor agonists. Diabetes Technol. Ther. 14, 951–967 (2012).
Whitehouse, F. et al. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care 25, 724–730 (2002).
Hollander, P. A. et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care 26, 784–790 (2003).
Bradley, D. P., Kulstad, R. & Schoeller, D. A. Exenatide and weight loss. Nutrition 26, 243–249 (2010).
Younk, L. M., Mikeladze, M. & Davis, S. N. Pramlintide and the treatment of diabetes: a review of the data since its introduction. Expert Opin. Pharmacother. 12, 1439–1451 (2011).
Pal, R. & Sahu, A. Leptin signaling in the hypothalamus during chronic central leptin infusion. Endocrinology 144, 3789–3798 (2003).
Begg, D. P. et al. Reversal of diet-induced obesity increases insulin transport into cerebrospinal fluid and restores sensitivity to the anorexic action of central insulin in male rats. Endocrinology 154, 1047–1054 (2013).
Clegg, D. J. et al. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol. Behav. 103, 10–16 (2011).
El-Haschimi, K., Pierroz, D. D., Hileman, S. M., Bjorbaek, C. & Flier, J. S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Invest. 105, 1827–1832 (2000).
Born, J. et al. Sniffing neuropeptides: a transnasal approach to the human brain. Nat. Neurosci. 5, 514–516 (2002).
Hallschmid, M. et al. Intranasal Insulin reduces body fat in men but not in women. Diabetes 53, 3024–3029 (2004).
Proietto, J. & Thorburn, A. W. The therapeutic potential of leptin. Expert Opin. Investig. Drugs 12, 373–378 (2003).
Vanderweele, D. A., Haraczkiewicz, E. & Van Itallie, T. B. Elevated insulin and satiety in obese and normal weight rats. Appetite 3, 99–109 (1982).
Nicolaidis, S. & Rowland, N. Metering of intravenous versus oral nutrients and regulation of energy balance. Am. J. Physiol. 231, 661–668 (1976).
Swinnen, S. G., Simon, A. C., Holleman, F., Hoekstra, J. B. & Devries, J. H. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD006383. http://dx.doi.org/10.1002/14651858.CD006383.pub2 (2011).
Zachariah, S. et al. Insulin detemir reduces weight gain as a result of reduced food intake in patients with type 1 diabetes. Diabetes Care 34, 1487–1491 (2011).
Dornhorst, A. et al. Transferring to insulin detemir from NPH insulin or insulin glargine in type 2 diabetes patients on basal-only therapy with oral antidiabetic drugs improves glycaemic control and reduces weight gain and risk of hypoglycaemia: 14-week follow-up data from PREDICTIVE. Diabetes Obes. Metab. 10, 75–81 (2008).
Mayer, J. Glucostatic mechanism of regulation of food intake. N. Engl. J. Med. 249, 13–16 (1953).
Grossman, S. P. The role of glucose, insulin and glucagon in the regulation of food intake and body weight. Neurosci. Biobehav. Rev. 10, 295–315 (1986).
Langhans, W. Metabolic and glucostatic control of feeding. Proc. Nutr. Soc. 55, 497–515 (1996).
Faust, I. M. Johnson, P. R. & Hirsch, J. Surgical removal of adipose tissue alters feeding behavior and the development of obesity in rats. Science 197, 393–396 (1977).
Shi, H., Strader, A. D., Woods, S. C. & Seeley, R. J. Sexually dimorphic responses to fat loss after caloric restriction or surgical lipectomy. Am. J. Physiol. Endocrinol. Metab. 293, E316–E326 (2007).
Benatti, F. et al. Liposuction induces a compensatory increase of visceral fat which is effectively counteracted by physical activity: a randomized trial. J. Clin. Endocrinol. Metab. 97, 2388–2395 (2012).
Seeley, R. J., Matson, C. A., Chavez, M., Woods, S. C. & Schwartz, M. W. Behavioral, endocrine and hypothalamic responses to involuntary overfeeding. Am. J. Physiol. 271, R819–R823 (1996).
Hagan, M. et al. Role of the CNS melanocortin system in the response to overfeeding. J. Neurosci. 19, 2362–2367 (1999).
Sims, E. A. et al. Endocrine and metabolic effects of experimental obesity in man. Recent Prog. Horm. Res. 29, 457–496 (1973).
Kennedy, G. C. The hypothalamic control of food intake in rats. Proc. R. Soc. Lond. B Biol. Sci. 137, 535–549 (1950).
Sclafani, A., Lucas, F. & Ackroff, K. The importance of taste and palatability in carbohydrate-induced overeating in rats. Am. J. Physiol. 270, R1197–R1202 (1996).
Ballard, B. D., Gipson, M. T., Guttenberg, W. & Ramsey, K. Palatability of food as a factor influencing obese and normal-weight children's eating habits. Behav. Res. Ther. 18, 598–600 (1980).
Tamashiro, K. L. et al. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol. Behav. 80, 683–693 (2004).
de Wit, L. M. et al. Depressive and anxiety disorders and the association with obesity, physical, and social activities. Depress. Anxiety 27, 1057–1065 (2010).
Goularte, J. F., Ferreira, M. B. & Sanvitto, G. L. Effects of food pattern change and physical exercise on cafeteria diet-induced obesity in female rats. Br. J. Nutr. 108, 1511–1518 (2012).
Porikos, K. P. & Pi-Sunyer, F. X. Regulation of food intake in human obesity: studies with caloric dilution and exercise. Clin. Endocrinol. Metab. 13, 547–561 (1984).
Peterman, J. N. et al. Relationship between past food deprivation and current dietary practices and weight status among Cambodian refugee women in Lowell, MA. Am. J. Public Health 100, 1930–1937 (2010).
Swithers, S. E. & Davidson, T. L. Influence of early dietary experience on energy regulation in rats. Physiol. Behav. 86, 669–680 (2005).
de Castro, J. M. Prior day's intake has macronutrient-specific delayed negative feedback effects on the spontaneous food intake of free-living humans. J. Nutr. 128, 61–67 (1998).
Birch, L. L., Johnson, S. L., Andresen, G., Peters, J. C. & Schulte, M. C. The variability of young children's energy intake. N. Engl. J. Med. 324, 232–235 (1991).
Vallerand, A. L., Lupien, J. & Bukowiecki, L. J. Cold exposure reverses the diabetogenic effects of high-fat feeding. Diabetes 35, 329–334 (1986).
Dunlap, S. & Heinrichs, S. C. Neuronal depletion of omega-3 fatty acids induces flax seed dietary self-selection in the rat. Brain Res. 1250, 113–119 (2009).
Rozin, P. Are carbohydrate and protein intakes separately regulated. J. Comp. Physiol. Psychol. 65, 23–29 (1968).
Berthoud, H. R. The neurobiology of food intake in an obesogenic environment. Proc. Nutr. Soc. 71, 478–487 (2012).
Berthoud, H. R. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr. Opin. Neurobiol. 21, 888–896 (2011).
Chrousos, G. P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 5, 374–381 (2009).
Ulrich-Lai, Y. M. & Herman, J. P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409 (2009).
Maniam, J. & Morris, M. J. The link between stress and feeding behaviour. Neuropharmacology 63, 97–110 (2012).
Dallman, M. F. Stress-induced obesity and the emotional nervous system. Trends Endocrinol. Metab. 21, 159–165 (2010).
Adam, T. C. & Epel, E. S. Stress, eating and the reward system. Physiol. Behav. 91, 449–458 (2007).
Schwartz, M. W., Dallman, M. F. & Woods, S. C. The hypothalamic response to starvation: implications for the study of wasting disorders. Am. J. Physiol. 269, R949–R957 (1995).
Seeley, R. J. et al. Behavioral, endocrine, and hypothalamic responses to involuntary overfeeding. Am. J. Physiol. 271, R819–R823 (1996).
Hanson, E. S. & Dallman, M. F. Neuropeptide Y (NPY) may integrate responses of hypothalamic feeding systems and the hypothalamo-pituitary-adrenal axis. J. Neuroendocrinol. 7, 273–279 (1995).
Strack, A. M., Sebastian, R. J., Schwartz, M. W. & Dallman, M. F. Glucocorticoids and insulin: reciprocal signals for energy balance. Am. J. Physiol. 268, 142–149 (1995).
Glowa, J. & Gold, P. Corticotropin releasing hormone produces profound anorexigenic effects in the rhesus monkey. Neuropeptides 18, 55–61 (1991).
Richard, D., Huang, Q. & Timofeeva, E. The corticotropin-releasing hormone system in the regulation of energy balance in obesity. Int. J. Obes. Relat. Metab. Disord. 24, S36–S39 (2000).
Green, P. K., Wilkinson, C. W. & Woods, S. C. Intraventricular corticosterone increases the rate of body weight gain in underweight adrenalectomized rats. Endocrinology 130, 269–275 (1992).
Zakrzewska, K. E. et al. Induction of obesity and hyperleptinemia by central glucocorticoid infusion in the rat. Diabetes 48, 365–370 (1999).
White, B. D., Dean, R. G. & Martin, R. J. Adrenalectomy decreases neuropeptide Y mRNA levels in the arcuate nucleus. Brain Res. Bull. 25, 711–715 (1990).
Dallman, M. F., Akana, S. F., Strack, A. M., Hanson, E. S. & Sebastian, R. J. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Ann. NY Acad. Sci. 771, 730–742 (1995).
Zakrzewska, K. E. et al. Selective dependence of intracerebroventricular neuropeptide Y-elicited effects on central glucocorticoids. Endocrinology 140, 3183–3187 (1999).
Jahng, J. W. et al. Dexamethasone reduces food intake, weight gain and the hypothalamic 5-HT concentration and increases plasma leptin in rats. Eur. J. Pharmacol. 581, 64–70 (2008).
Miell, J. P., Englaro, P. & Blum, W. F. Dexamethasone induces an acute and sustained rise in circulating leptin levels in normal human subjects. Horm. Metab. Res. 28, 704–707 (1996).
Mostyn, A., Keisler, D. H., Webb, R., Stephenson, T. & Symonds, M. E. The role of leptin in the transition from fetus to neonate. Proc. Nutr. Soc. 60, 187–194 (2001).
Fried, S. K., Russell, C. D., Grauso, N. L. & Brolin, R. E. Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J. Clin. Invest. 92, 2191–2198 (1993).
Nieuwenhuizen, A. G. & Rutters, F. The hypothalamic–pituitary–adrenal–axis in the regulation of energy balance. Physiol. Behav. 94, 169–177 (2008).
Chuang, J. C. et al. Ghrelin mediates stress-induced food-reward behavior in mice. J. Clin. Invest. 121, 2684–2692 (2011).
Disse, E. et al. Peripheral ghrelin enhances sweet taste food consumption and preference, regardless of its caloric content. Physiol. Behav. 101, 277–281 (2010).
Richardson, R. D., Omachi, K., Kermani, R. & Woods, S. C. Intraventricular insulin potentiates the anorexic effect of corticotropin releasing hormone in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R1321–R1326 (2002).
Bello, N. T. & Hajnal, A. Dopamine and binge eating behaviors. Pharmacol. Biochem. Behav. 97, 25–33 (2010).
Foster, M. T. et al. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology 150, 2325–2333 (2009).
Dallman, M. F. et al. Chronic stress and obesity: a new view of “comfort food”. Proc. Natl Acad. Sci. USA 100, 11696–11701 (2003).
Ulrich-Lai, Y. M. et al. Pleasurable behaviors reduce stress via brain reward pathways. Proc. Natl Acad. Sci. USA 107, 20529–20534 (2010).
Bhatnagar, S. et al. Corticosterone facilitates saccharin intake in adrenalectomized rats: does corticosterone increase stimulus salience? J. Neuroendocrinol. 12, 453–460 (2000).
Clegg, D. J. Minireview: the year in review of estrogen regulation of metabolism. Mol. Endocrinol. 26, 1957–1960 (2012).
Brown, L. M., Gent, L., Davis, K. & Clegg, D. J. Metabolic impact of sex hormones on obesity. Brain Res. 1350, 77–85 (2010).
Asarian, L. & Geary, N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 148, 5656–5666 (2007).
Thammacharoen, S., Lutz, T. A., Geary, N. & Asarian, L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 149, 1609–1617 (2008).
Buffenstein, R., Poppitt, S. D., McDevitt, R. M. & Prentice, A. M. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol. Behav. 58, 1067–1077 (1995).
Asarian, L. & Geary, N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1251–1263 (2006).
Xu, Y. et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell. Metab. 14, 453–465 (2011).
Chai, J. K. et al. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am. J. Physiol. 276, R1366–R1373 (1999).
Putnam, K., Shoemaker, R., Yiannikouris, F. & Cassis, L. A. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 302, H1219–H1230 (2012).
Epstein, A. N., Fitzsimons, J. T. & Simons, B. J. Drinking caused by the intracranial injection of angiotensin into the rat. J. Physiol. 200, 98P–100P (1969).
Abraham, S. F., Denton, D. A. & Weisinger, R. S. The specificity of the dipsogenic effect of angiotensin II. Pharmacol. Biochem. Behav. 4, 363–368 (1976).
Giacchetti, G. et al. Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am. J. Hypertens. 15, 381–388 (2002).
Harte, A. L. et al. Insulin increases angiotensinogen expression in human abdominal subcutaneous adipocytes. Diabetes Obes. Metab. 5, 462–467 (2003).
Iwashita, M. et al. Valsartan, independently of AT1 receptor or PPARγ, suppresses LPS-induced macrophage activation and improves insulin resistance in cocultured adipocytes. Am. J. Physiol. Endocrinol. Metab. 302, E286–E296 (2012).
Massiera, F. et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 15, 2727–2729 (2001).
Weisinger, R. S., Begg, D. P. & Jois, M. Antagonists of the renin-angiotensin system and the prevention of obesity. Curr. Opin. Investig. Drugs 10, 1069–1077 (2009).
Jayasooriya, A. P. et al. Mice lacking angiotensin-converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance. Proc. Natl Acad. Sci. USA 105, 6531–6536 (2008).
Massiera, F. et al. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology 142, 5220–5225 (2001).
Fitzsimons, J. T. Angiotensin, thirst, and sodium appetite. Physiol. Rev. 78, 583–686 (1998).
Miselis, R. R. The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res. 230, 1–23 (1981).
de Kloet, A. D. et al. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J. Neurosci. 33, 4825–4833 (2013).
Yamamoto, R. et al. Angiotensin II type 1 receptor signaling regulates feeding behavior through anorexigenic corticotropin-releasing hormone in hypothalamus. J. Biol. Chem. 286, 21458–21465 (2011).
Enalapril in essential hypertension: a comparative study with propranolol. Enalapril in Hypertension Study Group (UK). Br. J. Clin. Pharmacol. 18, 51–56 (1984).
de Kloet, A. D., Krause, E. G. & Woods, S. C. The renin angiotensin system and the metabolic syndrome. Physiol. Behav. 100, 525–534 (2010).
Minamino, N., Makino, Y., Tateyama, H., Kangawa, K. & Matsuo, H. Characterization of immunoreactive human C-type natriuretic peptide in brain and heart. Biochem. Biophys. Res. Commun. 179, 535–542 (1991).
Bartels, E. D., Nielsen, J. M., Bisgaard, L. S., Goetze, J. P. & Nielsen, L. B. Decreased expression of natriuretic peptides associated with lipid accumulation in cardiac ventricle of obese mice. Endocrinology 151, 5218–5225 (2010).
Inuzuka, M. et al. C-type natriuretic peptide as a new regulator of food intake and energy expenditure. Endocrinology 151, 3633–3642 (2010).
Gray, J. M. et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430, 317–322 (2004).
Valentino, M. A. et al. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J. Clin. Invest. 121, 3578–3588 (2011).
Berkseth, K. E., Schur, E. & Schwartz, M. W. A role for natriuretic peptides in the central control of energy balance? Diabetes 62, 1379–1381 (2013).
Ehrlich, K. J. & Fitts, D. A. Atrial natriuretic peptide in the subfornical organ reduces drinking induced by angiotensin or in response to water deprivation. Behav. Neurosci. 104, 365–372 (1990).
Villarreal, D., Freeman, R. H., Davis, J. O., Verburg, K. M. & Vari, R. C. Renal mechanisms for suppression of renin secretion by atrial natriuretic factor. Hypertension 8, II28–II35 (1986).
Brolin, R. E., Robertson, L. B., Kenler, H. A. & Cody, R. P. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann. Surg. 220, 782–790 (1994).
Kenler, H. A., Brolin, R. E. & Cody, R. P. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am. J. Clin. Nutr. 52, 87–92 (1990).
Ullrich, J., Ernst, B., Wilms, B., Thurnheer, M. & Schultes, B. Roux-en-Y gastric bypass surgery reduces hedonic hunger and improves dietary habits in severely obese subjects. Obes. Surg. 23, 50–55 (2013).
Chambers, A. P. et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141, 950–958 (2011).
Liou, A. P. et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl Med. 5, 178ra141 (2013).
Shin, A. C., Zheng, H., Townsend, R. L., Sigalet, D. L. & Berthoud, H. R. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 151, 1588–1597 (2010).
Stefater, M. A. et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 138, 2426–2436 (2010).
Wilson-Perez, H. E. et al. The effect of vertical sleeve gastrectomy on food choice in rats. Int. J. Obes. (Lond.) 37, 288–295 (2013).
Lingvay, I., Guth, E., Islam, A. & Livingston, E. Rapid improvement of diabetes after gastric bypass surgery: is it the diet or surgery? Diabetes Care http://dx.doi.org/10.2337/dc12-2316.
Stefater, M. A., Wilson-Perez, H. E., Chambers, A. P., Sandoval, D. A. & Seeley, R. J. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr. Rev. 33, 595–622 (2012).
Korner, J. et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J. Clin. Endocrinol. Metab. 90, 359–365 (2005).
le Roux, C. W. et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann. Surg. 243, 108–114 (2006).
Ramon, J. M. et al. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J. Gastrointest. Surg. 16, 1116–1122 (2012).
Warde-Kamar, J., Rogers, M., Flancbaum, L. & Laferrere, B. Calorie intake and meal patterns up to 4 years after Roux-en-Y gastric bypass surgery. Obes. Surg. 14, 1070–1079 (2004).
Stemmer, K. et al. Roux-en-Y gastric bypass surgery but not vertical sleeve gastrectomy decreases bone mass in male rats. Endocrinology 154, 2015–2024 (2013).
Pournaras, D. J. & le Roux, C. W. The effect of bariatric surgery on gut hormones that alter appetite. Diabetes Metab. 35, 508–512 (2009).
Chambers, A. P. et al. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology 144, 50–52 e55 (2013).
Wilson-Perez, H. E. et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide-1 receptor deficiency. Diabetes 62, 2380–2385 (2013).
Cano, V., Merino, B., Ezquerra, L., Somoza, B. & Ruiz-Gayo, M. A cholecystokinin-1 receptor agonist (CCK-8) mediates increased permeability of brain barriers to leptin. Br. J. Pharmacol. 154, 1009–1015 (2008).
Kohli, R. et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G652–G660 (2010).
Haluzikova, D. et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity (Silver Spring) http://dx.doi.org/10.1002/oby.20208.
Ryan, K. K. et al. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology 154, 9–15 (2013).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Shoelson, S. E., Lee, J. & Goldfine, A. B. Inflammation and insulin resistance. J. Clin. Invest. 116, 1793–1801 (2006).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Premaratna, S. D. et al. Angiotensin-converting enzyme inhibition reverses diet-induced obesity, insulin resistance and inflammation in C57BL/6J mice. Int. J. Obes (Lond.) 36, 233–243 (2012).
Hotamisligil, G. S. Inflammatory pathways and insulin action. Int. J. Obes. Relat. Metab. Disord. 27 (Suppl. 3), S53–S55 (2003).
Fantino, M. & Wieteska, L. Evidence for a direct central anorectic effect of tumor-necrosis-factor-α in the rat. Physiol. Behav. 53, 477–483 (1993).
Bernstein, I. L., Taylor, E. M. & Bentson, K. L. TNF-induced anorexia and learned food aversions are attenuated by area postrema lesions. Am. J. Physiol. 260, R906–R910 (1991).
Plata-Salaman, C. R., Oomura, Y. & Kai, Y. Tumor necrosis factor and interleukin-1B: suppression of food intake by direct action in the central nervous system. Brain Res. 448, 106–114 (1988).
Smith, B. K. & Kluger, M. J. Anti-TNF-α antibodies normalized body temperature and enhanced food intake in tumor-bearing rats. Am. J. Physiol. 265, R615–R619 (1993).
Bluthe, R. M. et al. Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrinology 19, 197–207 (1994).
De Souza, C. T. et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146, 4192–4199 (2005).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 (2012).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Begg, D., Woods, S. The endocrinology of food intake. Nat Rev Endocrinol 9, 584–597 (2013). https://doi.org/10.1038/nrendo.2013.136
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2013.136
This article is cited by
-
Blood–brain borders: a proposal to address limitations of historical blood–brain barrier terminology
Fluids and Barriers of the CNS (2024)
-
Position statement on nutrition therapy for overweight and obesity: nutrition department of the Brazilian association for the study of obesity and metabolic syndrome (ABESO—2022)
Diabetology & Metabolic Syndrome (2023)
-
Circulating metabolite homeostasis achieved through mass action
Nature Metabolism (2022)
-
An Exploration of the Role of Sugar-Sweetened Beverage in Promoting Obesity and Health Disparities
Current Obesity Reports (2021)
-
Treatment studies with cannabinoids in anorexia nervosa: a systematic review
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2021)