Abstract
Obesity, type 2 diabetes mellitus and the metabolic syndrome are major risk factors for cardiovascular disease. Studies have demonstrated an association between low levels of testosterone and the above insulin-resistant states, with a prevalence of hypogonadism of up to 50% in men with type 2 diabetes mellitus. Low levels of testosterone are also associated with an increased risk of all-cause and cardiovascular mortality. Hypogonadism and obesity share a bidirectional relationship as a result of the complex interplay between adipocytokines, proinflammatory cytokines and hypothalamic hormones that control the pituitary–testicular axis. Interventional studies have shown beneficial effects of testosterone on components of the metabolic syndrome, type 2 diabetes mellitus and other cardiovascular risk factors, including insulin resistance and high levels of cholesterol. Biochemical evidence indicates that testosterone is involved in promoting glucose utilization by stimulating glucose uptake, glycolysis and mitochondrial oxidative phosphorylation. Testosterone is also involved in lipid homeostasis in major insulin-responsive target tissues, such as liver, adipose tissue and skeletal muscle.
Key Points
-
Testosterone deficiency is highly prevalent in men with the metabolic syndrome and type 2 diabetes mellitus
-
Low levels of testosterone are an independent risk factor that predicts subsequent development of the metabolic syndrome and type 2 diabetes mellitus
-
Population studies in community-dwelling men have shown that testosterone deficiency is associated with increased all-cause mortality and cardiovascular mortality
-
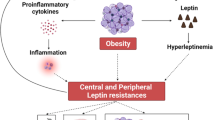
The hypogonadal–obesity–adipocytokine hypothesis summarises the complex interaction of the above components and their contribution to the vicious cycle of obesity causing hypogonadism and vice versa
-
Interventional studies of testosterone replacement therapy have shown improvements in insulin resistance, body composition, glycaemic control, lipid metabolism and other cardiovascular risk factors
-
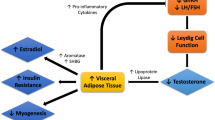
The benefit of testosterone on insulin sensitivity might be attributable to a complex regulatory influence on insulin signalling and glucose homeostasis in the major insulin-responsive target tissues
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
World Health Organization. Fact sheet No317, cardiovascular diseases [online], (2011).
British Heart Foundation. Coronary heart disease statistics in England 2012 [online], (2012).
American Heart Association. Men and cardiovascular diseases [online], (2012).
Stellato, R. K., Feldman, H. A., Hamdy, O., Horton, E. S. & McKinlay, J. B. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care 23, 490–494 (2000).
Haffner, S. M., Shaten, J., Stern, M. P., Smith, G. D. & Kuller, L. Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Am. J. Epidemiol. 143, 889–897 (1996).
Oh, J. Y., Barrett-Connor, E., Wedick, N. M., Wingard, D. L. & Rancho Bernardo, S. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care 25, 55–60 (2002).
Laaksonen, D. E. et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 27, 1036–1041 (2004).
Selvin, E. et al. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care 30, 234–238 (2007).
Kupelian, V. et al. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J. Clin. Endocrinol. Metab. 91, 843–850 (2006).
Jones, T. H. Testosterone deficiency: a risk factor for cardiovascular disease? Trends Endocrinol. Metab. 21, 496–503 (2010).
Kelly, D. M. & Jones, T. H. Testosterone: a vascular hormone in health and disease. J. Endocrinol. 217, R47–R71 (2013).
Jones, T. H. Effects of testosterone on type 2 diabetes and components of the metabolic syndrome. J. Diabetes 2, 146–156 (2010).
Kapoor, D., Aldred, H., Clark, S., Channer, K. S. & Jones, T. H. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 30, 911–917 (2007).
Ding, E. L., Song, Y., Malik, V. S. & Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 295, 1288–1299 (2006).
Hofstra, J. et al. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth. J. Med. 66, 103–109 (2008).
Dhindsa, S. et al. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J. Clin. Endocrinol. Metab. 89, 5462–5468 (2004).
Vermeulen, A., Verdonck, L. & Kaufman, J. M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab. 84, 3666–3672 (1999).
Couillard, C. et al. Contribution of body fatness and adipose tissue distribution to the age variation in plasma steroid hormone concentrations in men: the HERITAGE Family Study. J. Clin. Endocrinol. Metab. 85, 1026–1031 (2000).
Svartberg, J., von Mühlen, D., Sundsfjord, J. & Jorde, R. Waist circumference and testosterone levels in community dwelling men. The Tromsø study. Eur. J. Epidemiol. 19, 657–663 (2004).
Blouin, K. et al. Contribution of age and declining androgen levels to features of the metabolic syndrome in men. Metabolism 54, 1034–1040 (2005).
Singh, S. K., Goyal, R. & Pratyush, D. D. Is hypoandrogenemia a component of metabolic syndrome in males? Exp. Clin. Endocrinol. Diabetes 119, 30–35 (2011).
Grossmann, M. et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J. Clin. Endocrinol. Metab. 93, 1834–1840 (2008).
Biswas, M., Hampton, D., Newcombe, R. G. & Rees, D. A. Total and free testosterone concentrations are strongly influenced by age and central obesity in men with type 1 and type 2 diabetes but correlate weakly with symptoms of androgen deficiency and diabetes-related quality of life. Clin. Endocrinol. 76, 665–673 (2012).
Ogbera, O. A., Sonny, C., Olufemi, F. & Wale, A. Hypogonadism and subnormal total testosterone levels in men with type 2 diabetes mellitus. J. Coll. Physicians Surg. Pak. 21, 517–521 (2011).
Corona, G. et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int. J. Androl. 34, 528–540 (2011).
Wang, C. et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care 34, 1669–1675 (2011).
Laaksonen, D. E. et al. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. J. Clin. Endocrinol. Metab. 90, 712–719 (2005).
Bojesen, A., Host, C. & Gravholt, C. H. Klinefelter's syndrome, type 2 diabetes and the metabolic syndrome: the impact of body composition. Mol. Hum. Reprod. 16, 396–401 (2010).
Barrett-Connor, E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann. Intern. Med. 117, 807–811 (1992).
de Ronde, W. et al. Calculation of bioavailable and free testosterone in men: a comparison of 5 published algorithms. Clin. Chem. 52, 1777–1784 (2006).
Morris, P. D., Malkin, C. J., Channer, K. S. & Jones, T. H. A mathematical comparison of techniques to predict biologically available testosterone in a cohort of 1072 men. Eur. J. Endocrinol. 151, 241–249 (2004).
Kupelian, V. et al. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J. Clin. Endocrinol. Metab. 91, 843–850 (2006).
Peiris, A. N. et al. Relationship of insulin secretory pulses to sex hormone-binding globulin in normal men. J. Clin. Endocrinol. Metab. 76, 279–282 (1993).
Birkeland, K. I., Hanssen, K. F., Torjesen, P. A. & Vaaler, S. Level of sex hormone-binding globulin is positively correlated with insulin sensitivity in men with type 2 diabetes. J. Clin. Endocrinol. Metab. 76, 275–275 (1993).
Stanworth, R. D., Kapoor, D., Channer, K. S. & Jones, T. H. Statin therapy is associated with lower total but not bioavailable or free testosterone in men with type 2 diabetes. Diabetes Care 32, 541–546 (2009).
Kapoor, D., Channer, K. S. & Jones, T. H. Rosiglitazone increases bioactive testosterone and reduces waist circumference in hypogonadal men with type 2 diabetes. Diab. Vasc. Dis. Res. 5, 135–137 (2008).
Feldman, H. A. et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metab. 87, 589–598 (2002).
Vierhapper, H. & Nowotny, P. Reduced production rates of testosterone and dihydrotestosterone in healthy men treated with rosiglitazone. Metabolism 52, 230–232 (2003).
Ginsberg, H. N. Insulin resistance and cardiovascular disease. J. Clin. Invest. 106, 453–458 (2000).
Yeap, B. B. et al. Lower serum testosterone is independently associated with insulin resistance in non-diabetic older men: the Health In Men Study. Eur. J. Endocrinol. 161, 591–598 (2009).
Pitteloud, N. et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 28, 1636–1642 (2005).
Mårin, P. et al. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int. J. Obes. Relat. Metab. Disord. 16, 991 (1992).
Kapoor, D., Goodwin, E., Channer, K. S. & Jones, T. H. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur. J. Endocrinol. 154, 899–906 (2006).
Marin, P. Testosterone and regional fat distribution. Obes. Res. 3 (Suppl. 4), 609S–612S (1995).
Simon, D. et al. Androgen therapy improves insulin sensitivity and decreases leptin level in healthy adult men with low plasma total testosterone: a 3-month randomized placebo-controlled trial. Diabetes Care 24, 2149–2151 (2001).
Naharci, M. I., Pinar, M., Bolu, E. & Olgun, A. Effect of testosterone on insulin sensitivity in men with idiopathic hypogonadotropic hypogonadism. Endocr. Pract. 13, 629–635 (2007).
Jones, T. H. et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 34, 828–837 (2011).
Lebovitz, H. E. & Banerji, M. A. Insulin resistance and its treatment by thiazolidinediones. Recent Prog. Horm. Res. 56, 265–294 (2001).
Yialamas, M. A. et al. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 92, 4254–4259 (2007).
Boden, G. & Shulman, G. I. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Invest. 32 (Suppl. 3), 14–23 (2002).
McGarry, J. D. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51, 7–18 (2002).
Savage, D. B., Petersen, K. F. & Shulman, G. I. Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension 45, 828–833 (2005).
Jacob, S. et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 48, 1113–1119 (1999).
Petersen, K. F. et al. 13C/31P NMR studies on the mechanism of insulin resistance in obesity. Diabetes 47, 381–386 (1998).
Unger, R. H. & Orci, L. Lipotoxic diseases of nonadipose tissues in obesity. Int. J. Obes. Relat. Metab. Disord. 24 (Suppl. 4), S28–S32 (2000).
Hoyos, C. M. et al. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur. J. Endocrinol. 167, 531–541 (2012).
Marin, P., Oden, B. & Bjorntorp, P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J. Clin. Endocrinol. Metab. 80, 239–243 (1995).
Hotamisligil, G. S. Inflammatory pathways and insulin action. Int. J. Obes. Relat. Metab. Disord. 27 (Suppl. 3), S53–S55 (2003).
Malkin, C. J. et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J. Clin. Endocrinol. Metab. 89, 3313–3318 (2004).
Guay, A. T., Bansal, S. & Heatley, G. J. Effect of raising endogenous testosterone levels in impotent men with secondary hypogonadism: double blind placebo-controlled trial with clomiphene citrate. J. Clin. Endocrinol. Metab. 80, 3546–3552 (1995).
Guay, A. T., Jacobson, J., Perez, J. B., Hodge, M. B. & Velasquez, E. Clomiphene increases free testosterone levels in men with both secondary hypogonadism and erectile dysfunction: who does and does not benefit? Int. J. Impot. Res. 15, 156–165 (2003).
Jones, T. H. & Kennedy, R. L. Cytokines and hypothalamic-pituitary function. Cytokine 5, 531–538 (1993).
Mantzoros, C. S. The role of leptin in human obesity and disease: a review of current evidence. Ann. Int. Med. 130, 671 (1999).
Isidori, A. M. et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J. Clin. Endocrinol. Metab. 84, 3673–3680 (1999).
Herbison, A. E. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr. Rev. 19, 302–330 (1998).
Smith, J. T., Clifton, D. K. & Steiner, R. A. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction 131, 623–630 (2006).
George, J. T., Millar, R. P. & Anderson, R. A. Hypothesis: kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology 91, 302–307 (2010).
Iwasa, T. et al. Decreased expression of kisspeptin mediates acute immune/inflammatory stress-induced suppression of gonadotropin secretion in female rat. J. Endocrinol. Invest. 31, 656 (2008).
Singh, R., Artaza, J. N., Taylor, W. E., Gonzalez-Cadavid, N. F. & Bhasin, S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 144, 5081–5088 (2003).
Singh, R. et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology 147, 141–154 (2006).
Singh, A. B. et al. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J. Clin. Endocrinol. Metab. 87, 136–143 (2002).
Stanworth, R. D., Kapoor, D., Channer, K. S. & Jones, T. H. Androgen receptor CAG repeat polymorphism is associated with serum testosterone levels, obesity and serum leptin in men with type 2 diabetes. Eur. J. Endocrinol. 159, 739–746 (2008).
Jones, T. H. Testosterone associations with erectile dysfunction, diabetes, and the metabolic syndrome. Eur. Urol. Suppl. 6, 847–857 (2007).
Cohen, P. G. The hypogonadal–obesity cycle: role of aromatase in modulating the testosterone–estradiol shunt—a major factor in the genesis of morbid obesity. Med. Hypotheses 52, 49–51 (1999).
Barud, W., Palusinski, R., Beltowski, J. & Wojcicka, G. Inverse relationship between total testosterone and anti-oxidized low density lipoprotein antibody levels in ageing males. Atherosclerosis 164, 283–288 (2002).
Simon, D. et al. Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: The Telecom Study. J. Clin. Endocrinol. Metab. 82, 682–685 (1997).
Makinen, J. I. et al. Endogenous testosterone and serum lipids in middle-aged men. Atherosclerosis 197, 688–693 (2008).
Barrett-Connor, E. & Khaw, K. T. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation 78, 539–545 (1988).
Haffner, S. M., Mykkanen, L., Valdez, R. A. & Katz, M. S. Relationship of sex hormones to lipids and lipoproteins in nondiabetic men. J. Clin. Endocrinol. Metab. 77, 1610–1615 (1993).
Isidori, A. M. et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin. Endocrinol. 63, 381–394 (2005).
Permpongkosol, S., Tantirangsee, N. & Ratana-olarn, K. Treatment of 161 men with symptomatic late onset hypogonadism with long-acting parenteral testosterone undecanoate: effects on body composition, lipids, and psychosexual complaints. J. Sex. Med. 7, 3765–3774 (2010).
Kalinchenko, S. Y. et al. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin. Endocrinol. 73, 602–612 (2010).
Van Pottelbergh, I., Braeckman, L., De Bacquer, D., De Backer, G. & Kaufman, J. M. Differential contribution of testosterone and estradiol in the determination of cholesterol and lipoprotein profile in healthy middle-aged men. Atherosclerosis 166, 95–102 (2003).
Stanworth, R. D., Kapoor, D., Channer, K. S. & Jones, T. H. Dyslipidaemia is associated with testosterone, oestradiol and androgen receptor CAG repeat polymorphism in men with type 2 diabetes. Clin. Endocrinol. 74, 624–630 (2011).
Isidori, A. M. et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin. Endocrinol. 63, 280–293 (2005).
Kirkland, R. T. et al. Decrease in plasma high-density lipoprotein cholesterol levels at puberty in boys with delayed adolescence. Correlation with plasma testosterone levels. JAMA 257, 502–507 (1987).
Zmuda, J. M. et al. The effect of testosterone aromatization on high-density lipoprotein cholesterol level and postheparin lipolytic activity. Metabolism 42, 446–450 (1993).
Kekki, M. Lipoprotein-lipase action determining plasma high density lipoprotein cholesterol level in adult normolipaemics. Atherosclerosis 37, 143–150 (1980).
Langer, C. et al. Testosterone up-regulates scavenger receptor BI and stimulates cholesterol efflux from macrophages. Biochem. Biophys. Res. Commun. 296, 1051–1057 (2002).
Bagatell, C. J., Heiman, J. R., Matsumoto, A. M., Rivier, J. E. & Bremner, W. J. Metabolic and behavioral effects of high-dose, exogenous testosterone in healthy men. J. Clin. Endocrinol. Metab. 79, 561–567 (1994).
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002).
Zgliczynski, S. et al. Effect of testosterone replacement therapy on lipids and lipoproteins in hypogonadal and elderly men. Atherosclerosis 121, 35–43 (1996).
Uyanik, B. S., Ari, Z., Gumus, B., Yigitoglu, M. R. & Arslan, T. Beneficial effects of testosterone undecanoate on the lipoprotein profiles in healthy elderly men. A placebo controlled study. Jpn Heart J. 38, 73–82 (1997).
Nettleship, J. E., Jones, T. H., Channer, K. S. & Jones, R. D. Physiological testosterone replacement therapy attenuates fatty streak formation and improves high-density lipoprotein cholesterol in the Tfm mouse: an effect that is independent of the classic androgen receptor. Circulation 116, 2427–2434 (2007).
Scott, R. et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care 32, 493–498 (2009).
Jeppesen, J., Hein, H. O., Suadicani, P. & Gyntelberg, F. Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen Male Study. Circulation 97, 1029–1036 (1998).
Hokanson, J. E. & Austin, M. A. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a metaanalysis of population-based prospective studies. J. Cardiovasc. Risk 3, 213–219 (1996).
Alexandersen, P., Haarbo, J., Byrjalsen, I., Lawaetz, H. & Christiansen, C. Natural androgens inhibit male atherosclerosis: a study in castrated, cholesterol-fed rabbits. Circ. Res. 84, 813–819 (1999).
Zitzmann, M. et al. IPASS: a study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1,438 men. J. Sex. Med. 10, 579–588 (2013).
Heufelder, A. E., Saad, F., Bunck, M. C. & Gooren, L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J. Androl. 30, 726–733 (2009).
Agledahl, I., Hansen, J. B. & Svartberg, J. Impact of testosterone treatment on postprandial triglyceride metabolism in elderly men with subnormal testosterone levels. Scand. J. Clin. Lab. Invest. 68, 641–648 (2008).
Rosengren, A., Wilhelmsen, L., Eriksson, E., Risberg, B. & Wedel, H. Lipoprotein (a) and coronary heart disease: a prospective case-control study in a general population sample of middle aged men. BMJ 301, 1248–1251 (1990).
Zmuda, J. M., Thompson, P. D., Dickenson, R. & Bausserman, L. L. Testosterone decreases lipoprotein(a) in men. Am. J. Cardiol. 77, 1244–1247 (1996).
Ohlsson, C. et al. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men: The MrOS (Osteoporotic Fractures in Men) study in Sweden. J. Am. Coll. Cardiol. 58, 1674–1681 (2011).
Corona, G. et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur. J. Endocrinol. 165, 687–701 (2011).
Ruige, J. B., Mahmoud, A. M., De Bacquer, D. & Kaufman, J.-M. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart 97, 870–875 (2011).
Araujo, A. B. et al. Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 96, 3007–3019 (2011).
Jones, R. D., Nettleship, J. E., Kapoor, D., Jones, H. T. & Channer, K. S. Testosterone and atherosclerosis in aging men. Am. J. Cardiovasc. Drugs 5, 141–154 (2005).
Chew, K. K. et al. Erectile dysfunction as a predictor for subsequent atherosclerotic cardiovascular events: findings from a linked-data study. J. Sex. Med. 7, 192–202 (2010).
Jackson, G. et al. Cardiovascular aspects of sexual medicine. J. Sex. Med. 7, 1608–1626 (2010).
Araujo, A. B. et al. Erectile dysfunction and mortality. J. Sex. Med. 6, 2445–2454 (2009).
Shores, M. M. et al. Low testosterone is associated with decreased function and increased mortality risk: a preliminary study of men in a geriatric rehabilitation unit. J. Am. Geriatr. Soc. 52, 2077–2081 (2004).
Shores, M. M., Matsumoto, A. M., Sloan, K. L. & Kivlahan, D. R. Low serum testosterone and mortality in male veterans. Arch. Intern. Med. 166, 1660–1665 (2006).
Khaw, K. T. et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation 116, 2694–2701 (2007).
Malkin, C. J. et al. Low serum testosterone and increased mortality in men with coronary heart disease. Heart 96, 1821–1825 (2010).
Muraleedharan, V., Marsh, H. & Jones, H. in Society of Endocrinology, BES P163 (Birmingham, UK, 2011).
Hackett, G., Cole, N., Deshpande, A., Hall, A. & Wilkinson, P. The BLAST study: treating hypogonadism in type 2 diabetes with long acting testosterone undecanoate versus placebo significantly improves HbA1c, waist circumference, aging male symptom scores and all sexual function domains of the IIEF. Results continue to improve for 12 to 18 months. Presented at the European Society of Sexual Medicine meeting, Milan (2011).
Boyanov, M. A., Boneva, Z. & Christov, V. G. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male 6, 1–7 (2003).
Gopal, R. A. et al. Treatment of hypogonadism with testosterone in patients with type 2 diabetes mellitus. Endocr. Prac. 16, 570–576 (2010).
Bhattacharya, R. et al. Effect of 12 months of testosterone replacement therapy on metabolic syndrome components in hypogonadal men: data from the Testim Registry in the US (TRiUS). BMC Endoc. Disord. 11, 18 (2011).
Corona, G. et al. Testosterone and metabolic syndrome: a meta-analysis study. J. Sex. Med. 8, 272–283 (2011).
Grossmann, M. Low testosterone in men with type 2 diabetes: significance and treatment. J. Clin. Endocrinol. Metab. 96, 2341–2353 (2011).
Kaukua, J., Pekkarinen, T., Sane, T. & Mustajoki, P. Sex hormones and sexual function in obese men losing weight. Obes. Res. 11, 689–694 (2003).
Hammoud, A. et al. Effect of Roux-en-Y gastric bypass surgery on the sex steroids and quality of life in obese men. J. Clin. Endocrinol. Metab. 94, 1329–1332 (2009).
Reis, L. O. et al. Erectile dysfunction and hormonal imbalance in morbidly obese male is reversed after gastric bypass surgery: a prospective randomized controlled trial. Int. J. Androl. 33, 736–744 (2010).
Kelly, D. M. & Jones, T. H. Testosterone: a metabolic hormone in health and disease. J. Endocrinol. 217, R25–R45 (2013).
Yu, Y. H. & Ginsberg, H. N. Adipocyte signaling and lipid homeostasis sequelae of insulin-resistant adipose tissue. Circ. Res. 96, 1042–1052 (2005).
Lim, S. et al. Fat in liver/muscle correlates more strongly with insulin sensitivity in rats than abdominal fat. Obesity 17, 188–195 (2012).
Lam, T. K. T., Van de Werve, G. & Giacca, A. Free fatty acids increase basal hepatic glucose production and induce hepatic insulin resistance at different sites. Am. J. Physiol. Endocrinol. Metab. 284, E281–E290 (2003).
Kelly, D. M. et al. Testosterone increases hepatic liver X receptor and ApoE expression and improves lipid metabolism in the testicular feminized mouse: a potential protective mechanism against atherosclerosis and fatty liver disease. Endoc. Rev. 33, OR22–5 (2012).
Bryant, N. J., Govers, R. & James, D. E. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 3, 267–277 (2002).
Pessin, J. E. & Saltiel, A. R. Signaling pathways in insulin action: molecular targets of insulin resistance. J. Clin. Invest. 106, 165–170 (2000).
Chen, X., Li, X., Huang, H., Li, X. & Lin, J. F. Effects of testosterone on insulin receptor substrate-1 and glucose transporter 4 expression in cells sensitive to insulin [Chinese]. Zhonghua Yi Xue Za Zhi 86, 1474–1477 (2006).
Sato, K., Iemitsu, M., Aizawa, K. & Ajisaka, R. Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 294, E961–E968 (2008).
Muthusamy, T., Murugesan, P. & Balasubramanian, K. Sex steroids deficiency impairs glucose transporter 4 expression and its translocation through defective Akt phosphorylation in target tissues of adult male rat. Metabolism 58, 1581–1592 (2009).
Sesti, G. et al. Androgens increase insulin receptor mRNA levels, insulin binding, and insulin responsiveness in HEp-2 larynx carcinoma cells. Mol. Cell. Endocrinol. 86, 111–118 (1992).
Parthasarathy, C., Renuka, V. N. & Balasubramanian, K. Sex steroids enhance insulin receptors and glucose oxidation in Chang liver cells. Clin. Chim. Acta 399, 49–53 (2009).
Muthusamy, T., Murugesan, P., Srinivasan, C. & Balasubramanian, K. Sex steroids influence glucose oxidation through modulation of insulin receptor expression and IRS-1 serine phosphorylation in target tissues of adult male rat. Mol. Cell. Biochem. 352, 35–45 (2011).
Salehzadeh, F., Rune, A., Osler, M. & Al-Khalili, L. Testosterone or 17β-estradiol exposure reveals sex-specific effects on glucose and lipid metabolism in human myotubes. J. Endocrinol. 210, 219–229 (2011).
Bergamini, E., Bombara, G. & Pellegrino, C. The effect of testosterone on glycogen metabolism in rat levator ani muscle. Biochim. Biophys. Acta 177, 220–234 (1969).
McLaren, D., Kelly, D., Akhtar, S., Channer, K. & Jones, T. Low testosterone is associated with decreased expression of glut-4 and hexokinase 2 in muscle of the testicular feminised mouse. Endocrine Abstracts 29, P559 (2012).
Ramamani, A., Aruldhas, M. M. & Govindarajulu, P. Differential response of rat skeletal muscle glycogen metabolism to testosterone and estradiol. Can. J. Physiol. Pharmacol. 77, 300–304 (1999).
Leonard, S. L. The effect of castration and testosterone propionate injection on glycogen storage in skeletal muscle. Endocrinol. 51, 293–297 (1952).
Apostolakis, M., Matzelt, D. & Voigt, K. D. The effect of testosterone propionate on glycolytic and transamination enzyme activities in the liver, biceps muscle and levator ani muscle in rats [German]. Biochem. Z. 337, 414 (1963).
Max, S. R. Androgen-estrogen synergy in rat levator ani muscle: glucose-6-phosphate dehydrogenase. Mol. Cell. Endocrinol. 38, 103–107 (1984).
Mauvais-Jarvis, F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol. Metabol. 22, 24–33 (2011).
Traish, A. M., Abdallah, B. & Yu, G. Androgen deficiency and mitochondrial dysfunction: implications for fatigue, muscle dysfunction, insulin resistance, diabetes, and cardiovascular disease. Horm. Mol. Biol. Clin. Investig. 8, 431–444 (2011).
Ibebunjo, C., Eash, J. K., Li, C., Ma, Q. & Glass, D. J. Voluntary running, skeletal muscle gene expression, and signaling inversely regulated by orchidectomy and testosterone replacement. Am. J. Physiol. Endocrinol. Metab. 300, E327–E340 (2011).
Guo, W. et al. Testosterone plus low-intensity physical training in late life improves functional performance, skeletal muscle mitochondrial biogenesis, and mitochondrial quality control in male mice. PloS ONE 7, e51180 (2012).
van Breda, E. et al. Modulation of fatty-acid-binding protein content of rat heart and skeletal muscle by endurance training and testosterone treatment. Pflugers Arch. 421, 274–279 (1992).
Wu, S. Z. & Weng, X. Z. Therapeutic effects of an androgenic preparation on myocardial ischemia and cardiac function in 62 elderly male coronary heart disease patients. Chin. Med. J. (Engl.) 106, 415–418 (1993).
English, K. M., Steeds, R. P., Jones, T. H., Diver, M. J. & Channer, K. S. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: A randomized, double-blind, placebo-controlled study. Circulation 102, 1906–1911 (2000).
Malkin, C. J. et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart 90, 871–876 (2004).
English, K. M., Jones, R. D., Jones, T. H., Morice, A. H. & Channer, K. S. Testosterone acts as a coronary vasodilator by a calcium antagonistic action. J. Endocrinol. Invest. 25, 455–458 (2002).
Webb, C. M., McNeill, J. G., Hayward, C. S., de Zeigler, D. & Collins, P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation 100, 1690–1696 (1999).
Malkin, C. J. et al. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur. Heart J. 27, 57–64 (2006).
Pugh, P. J., Jones, R. D., West, J. N., Jones, T. H. & Channer, K. S. Testosterone treatment for men with chronic heart failure. Heart 90, 446–447 (2004).
Jain, P., Rademaker, A. W. & McVary, K. T. Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J. Urol. 164, 371–375 (2000).
Cherrier, M. M. et al. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology 57, 80–88 (2001).
Janowsky, J. S., Oviatt, S. K. & Orwoll, E. S. Testosterone influences spatial cognition in older men. Behav. Neurosci. 108, 325–332 (1994).
Cherrier, M. M. et al. The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology 64, 290–296 (2005).
Wang, C. et al. Testosterone replacement therapy improves mood in hypogonadal men—a clinical research center study. J. Clin. Endocrinol. Metab. 81, 3578–3583 (1996).
Wang, C. et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Int. J. Impot. Res. 21, 1–8 (2008).
Bhasin, S. et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 95, 2536–2559 (2010).
Fernandez-Balsells, M. M. et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 95, 2560–2575 (2010).
Fenely, M. R. & Carruthers, M. Is testosterone treatment good for the prostate? Study of safety during long-term treatment. J. Sex. Med. 9, 2138–2149 (2012).
Aversa, A. et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J. Sex. Med. 7, 3495–3503 (2010).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed to discussion of the content and wrote the article. P. M. Rao and T. H. Jones reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
T. H. Jones has received research grants and support and consultancy fees from Bayer Healthcare, been a consultant for ProStrakan in regard to the TIMES2 study, and has received honoraria for educational lectures and advisory boards from Bayer Healthcare, Clarus, Ferring, Lilly, Merck and Prostrakan. P. M. Rao and D. M. Kelly declare no competing interests.
Rights and permissions
About this article
Cite this article
Rao, P., Kelly, D. & Jones, T. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol 9, 479–493 (2013). https://doi.org/10.1038/nrendo.2013.122
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2013.122
This article is cited by
-
Association of the triglyceride glucose-body mass index with the extent of coronary artery disease in patients with acute coronary syndromes
Cardiovascular Diabetology (2024)
-
Predictive power of lipid-related indicators for testosterone deficiency: a comparative analysis, NHANES 2011–2016
International Urology and Nephrology (2024)
-
The effects of a low carbohydrate diet on erectile function and serum testosterone levels in hypogonadal men with metabolic syndrome: a randomized clinical trial
BMC Endocrine Disorders (2023)
-
Association between testosterone and serum soluble α-klotho in U.S. males: a cross-sectional study
BMC Geriatrics (2022)
-
The role of leptin and low testosterone in obesity
International Journal of Impotence Research (2022)