Abstract
The long-term survival of patients with thyroid cancer and the possibility of tumour recurrence up to 30–40 years after the achievement of a disease-free status illustrate the importance of lifelong follow-up in these individuals. This Review discusses the most innovative aspects of follow-up protocols for patients with differentiated thyroid cancer, that is, of papillary or follicular hystotype, and those with medullary thyroid cancer. Particular focus is placed on the relevance of new ultrasensitive assays for thyroglobulin measurement and the option of using recombinant human TSH to stimulate thyroglobulin secretion. Methods to compensate for the loss of diagnostic significance of serum thyroglobulin levels in patients with differentiated thyroid cancer and circulating anti-thyroglobulin antibodies are highlighted, as well as the role of the postoperative calcitonin stimulation test and the clinical relevance of determining the doubling time of calcitonin and carcinoembryonic antigen in patients with medullary thyroid cancer. Moreover, this Review gives some insights into the role of molecular thyroid cancer testing, both for prognostic and for therapeutic purposes. Finally, a general overview of traditional imaging procedures, such as neck ultrasonography, CT, MRI and bone scintigraphy, is provided alongside a comparison with new nuclear imaging tests such as PET.
Key Points
-
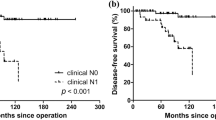
95% of patients with differentiated papillary or follicular thyroid cancer (DTC) and 45–50% of those with medullary thyroid cancer (MTC) survive in the long term
-
However, the probability that these patients will experience tumour recurrence, even after several years in clinical remission, is not negligible
-
Detectable levels of serum thyroglobulin are indicative of persistent or recurrent DTC
-
Thyroglobulin stimulation with exogenous recombinant human TSH is preferable to withdrawal of suppressive levothyroxine therapy, as the latter approach can cause hypothyroidism, which severely affects patients' quality of life
-
The same laboratory, and possibly the same thyroglobulin assay, should be used for the follow-up of a patient with DTC, to account for variations in assay specificity and sensitivity
-
Calcitonin is the most reliable tumour marker for diagnosis and follow-up of MTC; however, carcinoembryonic antigen is a valid marker to determine disease progression
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schlumberger, M. J. Papillary and follicular thyroid carcinoma. N. Engl. J. Med. 338, 297–306 (1998).
Siironen, P. et al. Anaplastic and poorly differentiated thyroid carcinoma: therapeutic strategies and treatment outcome of 52 consecutive patients. Oncology 79, 400–408 (2010).
Gharib, H. et al. Medullary thyroid carcinoma: clinicopathologic features and long-term follow-up of 65 patients treated during 1946 through 1970. Mayo Clin. Proc. 67, 934–940 (1992).
Leung, A. M. et al. Factors determining the persistence or recurrence of well-differentiated thyroid cancer treated by thyroidectomy and/or radioiodine in the Boston, Massachusetts area: a retrospective chart review. Thyroid Res. 4, 9 (2011).
Franc, S. et al. Complete surgical lymph node resection does not prevent authentic recurrences of medullary thyroid carcinoma. Clin. Endocrinol. (Oxf.) 55, 403–409 (2001).
Cooper, D. S. et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19, 1167–1214 (2009).
Pacini, F. et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 154, 787–803 (2006).
Kloos, R. T. et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19, 565–612 (2009).
Watkinson, J. C. The British Thyroid Association guidelines for the management of thyroid cancer in adults. Nucl. Med. Commun. 25, 897–900 (2004).
Paschke, R. et al. Thyroid nodule guidelines: agreement, disagreement and need for future research. Nat. Rev. Endocrinol. 7, 354–361 (2011).
Mazzaferri, E. L. et al. A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 88, 1433–1441 (2003).
Cailleux, A. F., Baudin, E., Travagli, J. P., Ricard, M. & Schlumberger, M. Is diagnostic iodine-131 scanning useful after total thyroid ablation for differentiated thyroid cancer? J. Clin. Endocrinol. Metab. 85, 175–178 (2000).
Pacini, F. et al. Diagnostic 131-iodine whole-body scan may be avoided in thyroid cancer patients who have undetectable stimulated serum Tg levels after initial treatment. J. Clin. Endocrinol. Metab. 87, 1499–1501 (2002).
Incerti, C. Recombinant human thyroid-stimulating hormone (rhTSH): clinical development. J. Endocrinol. Invest. 22 (Suppl.), 8–16 (1999).
Pacini, F. et al. Prediction of disease status by recombinant human TSH-stimulated serum Tg in the postsurgical follow-up of differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 86, 5686–5690 (2001).
Kloos, R. T. & Mazzaferri, E. L. A single recombinant human thyrotropin-stimulated serum thyroglobulin measurement predicts differentiated thyroid carcinoma metastases three to five years later. J. Clin. Endocrinol. Metab. 90, 5047–5057 (2005).
Castagna, M. G. et al. The use of ultrasensitive thyroglobulin assays reduces but does not abolish the need for TSH stimulation in patients with differentiated thyroid carcinoma. J. Endocrinol. Invest. 34, e219–e223 (2011).
Brassard, M. et al. Long-term follow-up of patients with papillary and follicular thyroid cancer: a prospective study on 715 patients. J. Clin. Endocrinol. Metab. 96, 1352–1359 (2011).
Malandrino, P. et al. Risk-adapted management of differentiated thyroid cancer assessed by a sensitive measurement of basal serum thyroglobulin. J. Clin. Endocrinol. Metab. 96, 1703–1709 (2011).
Schlumberger, M. et al. Comparison of seven serum thyroglobulin assays in the follow-up of papillary and follicular thyroid cancer patients. J. Clin. Endocrinol. Metab. 92, 2487–2495 (2007).
Castagna, M. G. et al. Limited value of repeat recombinant human thyrotropin (rhTSH)-stimulated thyroglobulin testing in differentiated thyroid carcinoma patients with previous negative rhTSH-stimulated thyroglobulin and undetectable basal serum thyroglobulin levels. J. Clin. Endocrinol. Metab. 93, 76–81 (2008).
Persoon, A. C. et al. A sensitive Tg assay or rhTSH stimulated Tg: what's the best in the long-term follow-up of patients with differentiated thyroid carcinoma? PLoS ONE 2, e816 (2007).
Miyauchi, A. et al. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid 21, 707–716 (2011).
Pacini, F., Sabra, M. M. & Tuttle, R. M. Clinical relevance of thyroglobulin doubling time in the management of patients with differentiated thyroid cancer. Thyroid 21, 691–692 (2011).
Spencer, C. A. & Lopresti, J. S. Measuring thyroglobulin and thyroglobulin autoantibody in patients with differentiated thyroid cancer. Nat. Clin. Pract. Endocrinol. Metab. 4, 223–233 (2008).
Chen, L. et al. Pulmonary fibrosis following radioiodine therapy of pulmonary metastases from differentiated thyroid carcinoma. Thyroid 20, 337–340 (2010).
Sherman, S. I. Targeted therapies for thyroid tumors. Mod. Pathol. 24 (Suppl. 2), S44–S52 (2011).
Emmertsen, K. K., Nielsen, H. E., Mosekilde, L. & Hansen, H. H. Pentagastrin, calcium and whisky stimulated serum calcitonin in medullary carcinoma of the thyroid. Acta Radiol. Oncol. 19, 85–89 (1980).
Melvin, K. E. & Tashjian, A. H. Jr. The syndrome of excessive thyrocalcitonin produced by medullary carcinoma of the thyroid. Proc. Natl Acad. Sci. USA 59, 1216–1222 (1968).
Fugazzola, L. et al. Disappearance rate of serum calcitonin after total thyroidectomy for medullary thyroid carcinoma. Int. J. Biol. Markers 9, 21–24 (1994).
Pellegriti, G. et al. Long-term outcome of medullary thyroid carcinoma in patients with normal postoperative medical imaging. Br. J. Cancer 88, 1537–1542 (2003).
Doyle, P. et al. Potency and tolerance of calcitonin stimulation with high-dose calcium versus pentagastrin in normal adults. J. Clin. Endocrinol. Metab. 94, 2970–2974 (2009).
Colombo, C. et al. Comparison of calcium and pentagastrin tests for the diagnosis and follow-up of medullary thyroid cancer. J. Clin. Endocrinol. Metab. 97, 905–913 (2012).
Dalouzy, J. C. et al. Discovery of a new broad resonance in 19Ne: implications for the destruction of the cosmic γ-ray emitter 18F. Phys. Rev. Lett. 102, 162503 (2009).
Pacini, F. et al. Medullary thyroid cancer. An immunohistochemical and humoral study using six separate antigens. Am. J. Clin. Pathol. 95, 300–308 (1991).
Kratzsch, J. et al. Basal and stimulated calcitonin and procalcitonin by various assays in patients with and without medullary thyroid cancer. Clin. Chem. 57, 467–474 (2011).
Meijer, J. A. et al. Calcitonin and carcinoembryonic antigen doubling times as prognostic factors in medullary thyroid carcinoma: a structured meta-analysis. Clin. Endocrinol. (Oxf.) 72, 534–542 (2010).
Barbet, J., Campion, L., Kraeber-Bodéré, F. & Chatal, J. F. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 90, 6077–6084 (2005).
Nikiforova, M. N. & Nikiforov, Y. E. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev. Mol. Diagn. 8, 83–95 (2008).
Nikiforova, M. N. et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J. Clin. Endocrinol. Metab. 88, 5399–5404 (2003).
Xing, M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr. Rev. 28, 742–762 (2007).
Elisei, R. et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J. Clin. Endocrinol. Metab. 93, 3943–3949 (2008).
Elisei, R. et al. RET genetic screening in patients with medullary thyroid cancer and their relatives: experience with 807 individuals at one center. J. Clin. Endocrinol. Metab. 92, 4725–4729 (2007).
Elisei, R. et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J. Clin. Endocrinol. Metab. 93, 682–687 (2008).
Moura, M. M. et al. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br. J. Cancer 100, 1777–1783 (2009).
Moura, M. M., Cavaco, B. M., Pinto, A. E. & Leite, V. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J. Clin. Endocrinol. Metab. 96, E863–E868 (2011).
Tang, W. et al. DNA extraction from formalin-fixed, paraffin-embedded tissue. Cold Spring Harb. Protoc. 2009, pdb.prot5138 (2009).
Leboulleux, S. et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 92, 3590–3594 (2007).
Pacini, F. et al. Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 74, 1401–1404 (1992).
Torlontano, M. et al. Follow-up of low risk patients with papillary thyroid cancer: role of neck ultrasonography in detecting lymph node metastases. J. Clin. Endocrinol. Metab. 89, 3402–3407 (2004).
Frasoldati, A. et al. Diagnosis of neck recurrences in patients with differentiated thyroid carcinoma. Cancer 97, 90–96 (2003).
Pacini, F., Castagna, M. G., Cipri, C. & Schlumberger, M. Medullary thyroid carcinoma. Clin. Oncol. (R. Coll. Radiol.) 22, 475–485 (2010).
Giraudet, A. L. et al. Imaging medullary thyroid carcinoma with persistent elevated calcitonin levels. J. Clin. Endocrinol. Metab. 92, 4185–4190 (2007).
Urhan, M. et al. Iodine-123 as a diagnostic imaging agent in differentiated thyroid carcinoma: a comparison with iodine-131 post-treatment scanning and serum thyroglobulin measurement. Eur. J. Nucl. Med. Mol. Imaging 34, 1012–1017 (2007).
Blum, M., Tiu, S., Chu, M., Goel, S. & Friedman, K. I-131 SPECT/CT elucidates cryptic findings on planar whole-body scans and can reduce needless therapy with I-131 in post-thyroidectomy thyroid cancer patients. Thyroid 21, 1235–1247 (2011).
Pineda, J. D., Lee, T., Ain, K., Reynolds, J. C. & Robbins, J. Iodine-131 therapy for thyroid cancer patients with elevated thyroglobulin and negative diagnostic scan. J. Clin. Endocrinol. Metab. 80, 1488–1492 (1995).
Pacini, F. et al. Therapeutic doses of iodine-131 reveal undiagnosed metastases in thyroid cancer patients with detectable serum thyroglobulin levels. J. Nucl. Med. 28, 1888–1891 (1987).
Pacini, F. et al. Outcome of differentiated thyroid cancer with detectable serum Tg and negative diagnostic 131I whole body scan: comparison of patients treated with high 131I activities versus untreated patients. J. Clin. Endocrinol. Metab. 86, 4092–4097 (2001).
van Tol, K. M. et al. Outcome in patients with differentiated thyroid cancer with negative diagnostic whole-body scanning and detectable stimulated thyroglobulin. Eur. J. Endocrinol. 148, 589–596 (2003).
Robbins, R. J. et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-18F-fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J. Clin. Endocrinol. Metab. 91, 498–505 (2006).
Sisson, J. C., Ackermann, R. J., Meyer, M. A., Wahl, R. L. Uptake of 18-fluoro-2-deoxy-D-glucose by thyroid cancer: implications for diagnosis and therapy. J. Clin. Endocrinol. Metab. 77, 1090–1094 (1993).
Faggiano, A. et al. Secretive and proliferative tumor profile helps to select the best imaging technique to identify postoperative persistent or relapsing medullary thyroid cancer. Endocr. Relat. Cancer 16, 225–231 (2009).
Ong, S. C. et al. Diagnostic accuracy of 18F-FDG PET in restaging patients with medullary thyroid carcinoma and elevated calcitonin levels. J. Nucl. Med. 48, 501–507 (2007).
Giraudet, A. L. et al. Imaging medullary thyroid carcinoma with persistent elevated calcitonin levels. J. Clin. Endocrinol. Metab. 92, 4185–4190 (2007).
Treglia, G. et al. Comparison of 18F-DOPA, 18F-FDG and 68Ga-somatostatin analogue PET/CT in patients with recurrent medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging http://dx.doi.org/10.1007/s00259-011-2031-6.
Kauhanen, S. et al. Complementary roles of 18F-DOPA PET/CT and 18F-FDG PET/CT in medullary thyroid cancer. J. Nucl. Med. 52, 1855–1863 (2011).
Beheshti, M. et al. The value of 18F-DOPA PET–CT in patients with medullary thyroid carcinoma: comparison with 18F-FDG PET–CT. Eur. Radiol. 19, 1425–1434 (2009).
Palyga, I. et al. The role of PET–CT scan with somatostatin analogue labelled with gallium 68 (68Ga-DOTA-TATE PET–CT) in diagnosing patients with disseminated medullary thyroid carcinoma (MTC). Endokrynol. Pol. 61, 507–511 (2010).
Bodei, L. et al. Receptor radionuclide therapy with 90Y-DOTATOC in patients with medullary thyroid carcinomas. Cancer Biother. Radiopharm. 19, 65–71 (2004).
Papotti, M., Kumar, U., Volante, M., Pecchioni, C. & Patel, Y. C. Immunohistochemical detection of somatostatin receptor types 1–5 in medullary carcinoma of the thyroid. Clin. Endocrinol. (Oxf.) 54, 641–649 (2001).
Castellani, M. R. et al. MIBG for diagnosis and therapy of medullary thyroid carcinoma: is there still a role? Q. J. Nucl. Med. Mol. Imaging 52, 430–440 (2008).
Pacini, F. et al. Thyroid autoantibodies in thyroid cancer: incidence and relationship with tumour outcome. Acta Endocrinol. (Copenh.) 119, 373–380 (1988).
Chiovato, L. et al. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann. Intern. Med. 139, 346–351 (2003).
Kim, W. G. et al. Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulin-negative patients with differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 93, 4683–4689 (2008).
Elisei, R. et al. Low specificity of blood thyroglobulin messenger ribonucleic acid assay prevents its use in the follow-up of differentiated thyroid cancer patients. J. Clin. Endocrinol. Metab. 89, 33–39 (2004).
Biscolla, R. P., Cerutti, J. M. & Maciel, R. M. Detection of recurrent thyroid cancer by sensitive nested reverse transcription-polymerase chain reaction of thyroglobulin and sodium/iodide symporter messenger ribonucleic acid transcripts in peripheral blood. J. Clin. Endocrinol. Metab. 85, 3623–3627 (2000).
Chia, S. Y. et al. Thyroid-stimulating hormone receptor messenger ribonucleic acid measurement in blood as a marker for circulating thyroid cancer cells and its role in the preoperative diagnosis of thyroid cancer. J. Clin. Endocrinol. Metab. 92, 468–475 (2007).
Boldarine, V. T. et al. Development of a sensitive and specific quantitative reverse transcription-polymerase chain reaction assay for blood thyroglobulin messenger ribonucleic acid in the follow-up of patients with differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 95, 1726–1733 (2010).
Torosian, L. et al. Blood thyroglobulin and TSH receptor mRNA detection by RT-PCR in the follow-up of differentiated thyroid cancer patients. Rev. Esp. Med. Nucl. 29, 109–113 (2010).
Acknowledgements
Work described in this Review was supported in part by grants from the Ministero della Istruzione Universitaria e Ricerca Scientifica (MIUR), the Associazione Italiana per la Ricerca sul Cancro (AIRC), the Istituto Toscano Tumori (ITT) and the Ministero della Salute, Progetto Ricerca Oncologica RF-CAM 2006353005.
Author information
Authors and Affiliations
Contributions
R. Elisei researched the data and wrote the article. Both authors provided a substantial contribution to discussions of the content. A. Pinchera reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Elisei, R., Pinchera, A. Advances in the follow-up of differentiated or medullary thyroid cancer. Nat Rev Endocrinol 8, 466–475 (2012). https://doi.org/10.1038/nrendo.2012.38
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2012.38
This article is cited by
-
Immunotherapy for advanced thyroid cancers — rationale, current advances and future strategies
Nature Reviews Endocrinology (2020)
-
Medullary thyroid carcinoma treated with percutaneous ultrasound-guided radiofrequency ablation
Endocrine (2019)
-
Novel Graphene Biosensor Based on the Functionalization of Multifunctional Nano-bovine Serum Albumin for the Highly Sensitive Detection of Cancer Biomarkers
Nano-Micro Letters (2019)
-
Surgical management of medullary thyroid carcinoma
Updates in Surgery (2017)