Abstract
Medullary thyroid carcinoma (MTC) and the multiple endocrine neoplasia (MEN) type 2 syndromes are rare but important endocrine diseases that are increasingly managed by pediatric providers. MTC is generally associated with a favorable prognosis when diagnosed during childhood, where it frequently occurs secondary to activating mutations in the RET proto-oncogene and arises from pre-existing C-cell hyperplasia. MEN2A accounts for 90–95% of childhood MTC cases and is most commonly due to mutations in codon 634 of RET. MEN2B is associated with the most aggressive clinical presentation of MTC and is almost always due to the Met918Thr mutation of RET. Surgery is the primary treatment and only chance of cure, although the advent of targeted therapies seems to be improving progression-free survival in advanced cases. Since the discovery of the role of RET in MEN2A, considerable advances in the management of this syndrome have occurred, and most of the children with MEN2A who have undergone early thyroidectomy will now lead full, productive lives. Strong genotype–phenotype correlations have facilitated the development of guidelines for interventions. Contemporary approaches for deciding the appropriate age at which surgery should take place incorporate data from ultrasonography and calcitonin measurements in addition to the results of genotyping. To optimize care and to facilitate ongoing research, children with MTC and the MEN2 syndromes are optimally treated at tertiary centers with multidisciplinary expertise.
Key Points
-
Medullary thyroid carcinoma (MTC) during childhood is rare and almost always results from a dominantly inherited or de novo activating mutation in the RET proto-oncogene
-
C-cell hyperplasia is the initial stage of tumorigenesis that leads to the development of microscopic noninvasive MTC and ultimately to locoregional and distant metastases due to frankly invasive carcinoma
-
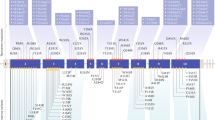
Multiple endocrine neoplasia (MEN) type 2A, which accounts for 90–95% of childhood MEN2 cases, is most frequently caused by mutations in RET exons 10 (codons 609, 611, 618 and 620) and 11 (codon 634)
-
MEN2B is almost always due to the Met918Thr mutation (exon 16) in the RET proto-oncogene and is associated with the most aggressive clinical presentation of MTC during childhood
-
MTC is primarily a surgical disease; the best hope of cure is for an experienced thyroid surgeon to remove the thyroid in at-risk patients before metastasis occurs
-
Strong genotype–phenotype correlations facilitate guidelines for treating children with MEN2; current approaches for determining the optimal timing for thyroidectomy incorporate ultrasonography and calcitonin data in addition to the genotype
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hazard, J. B., Hawk, W. A. & Crile, G. Jr. Medullary (solid) carcinoma of the thyroid; a clinicopathologic entity. J. Clin. Endocrinol. Metab. 19, 152–161 (1959).
Wolfe, H. J. et al. C-cell hyperplasia preceding medullary thyroid carcinoma. N. Engl. J. Med. 289, 437–441 (1973).
Hogan, A. R. et al. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J. Surg. Res. 156, 167–172 (2009).
Donis-Keller, H. et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum. Mol. Genet. 2, 851–856 (1993).
Mulligan, L. M. et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 363, 458–460 (1993).
Hofstra, R. M. et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 367, 375–376 (1994).
Carlson, K. M. et al. Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc. Natl Acad. Sci. USA 91, 1579–1583 (1994).
Kloos, R. T. et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19, 565–612 (2009).
Jiménez, C., Hu, M. I. & Gagel, R. F. Management of medullary thyroid carcinoma. Endocrinol. Metab. Clin. North Am. 37, 481–496 (2008).
Sipple, J. H. The association of pheochromocytoma with carcinoma of the thyroid gland. Am. J. Med. 31, 163–166 (1961).
Cushman, P. Jr. Familial endocrine tumors; report of two unrelated kindred affected with pheochromocytomas, one also with multiple thyroid carcinomas. Am. J. Med. 32, 352–360 (1962).
Steiner, A. L., Goodman, A. D. & Powers, S. R. Study of a kindred with pheochromocytoma, medullary thyroid carcinoma, hyperparathyroidism and Cushing's disease: multiple endocrine neoplasia, type 2. Medicine (Baltimore) 47, 371–409 (1968).
Simpson, N. E. & Kidd, K. K. Where is the locus for multiple endocrine neoplasia type 2A? Henry Ford Hosp. Med. J. 35, 168–171 (1987).
Mathew, C. G. et al. A linked genetic marker for multiple endocrine neoplasia type 2A on chromosome 10. Nature 328, 527–528 (1987).
Takahashi, M., Ritz, J. & Cooper, G. M. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 42, 581–588 (1985).
Williams, E. D. & Pollock, D. J. Multiple mucosal neuromata with endocrine tumours: a syndrome allied to von Recklinghausen's disease. J. Pathol. Bacteriol. 91, 71–80 (1966).
Chong, G. C., Beahrs, O. H., Sizemore, G. W. & Woolner, L. H. Medullary carcinoma of the thyroid gland. Cancer 35, 695–704 (1975).
Hansford, J. R. & Mulligan, L. M. Multiple endocrine neoplasia type 2 and RET: from neoplasia to neurogenesis. J. Med. Genet. 37, 817–827 (2000).
Marx, S. J. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat. Rev. Cancer 5, 367–375 (2005).
Arighi, E., Borrello, M. G. & Sariola, H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 16, 441–467 (2005).
Drosten, M. & Pützer, B. M. Mechanisms of Disease: cancer targeting and the impact of oncogenic RET for medullary thyroid carcinoma therapy. Nat. Clin. Pract. Oncol. 3, 564–574 (2006).
Wells, S. A. Jr & Santoro, M. Targeting the RET pathway in thyroid cancer. Clin. Cancer Res. 15, 7119–7123 (2009).
Traugott, A. L. & Moley, J. F. The RET protooncogene. Cancer Treat. Res. 153, 303–319 (2010).
Phay, J. E. & Shah, M. H. Targeting RET receptor tyrosine kinase activation in cancer. Clin. Cancer Res. 16, 5936–5941 (2010).
Torino, F., Paragliola, R. M., Barnabei, A. & Corsello, S. M. Medullary thyroid cancer: a promising model for targeted therapy. Curr. Mol. Med. 10, 608–625 (2010).
Eng, C. et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA 276, 1575–1579 (1996).
Wells, S. A. Jr et al. Predictive DNA testing and prophylactic thyroidectomy in patients at risk for multiple endocrine neoplasia type 2A. Ann. Surg. 220, 237–247 (1994).
Szinnai, G., Sarnacki, S. & Polak, M. Hereditary medullary thyroid carcinoma: how molecular genetics made multiple endocrine neoplasia type 2 a paediatric disease. Endocr. Dev. 10, 173–187 (2007).
Waguespack, S. G. A perspective from pediatric endocrinology on the hereditary medullary thyroid carcinoma syndromes. Thyroid 19, 543–546 (2009).
Frohnauer, M. K. & Decker, R. A. Update on the MEN 2A c804 RET mutation: is prophylactic thyroidectomy indicated? Surgery 128, 1052–1057 (2000).
Erlic, Z. et al. Pathogenicity of DNA variants and double mutations in multiple endocrine neoplasia type 2 and von Hippel-Lindau syndrome. J. Clin. Endocrinol. Metab. 95, 308–313 (2010).
Crockett, D. K. et al. Predicting phenotypic severity of uncertain gene variants in the RET proto-oncogene. PLoS ONE 6, e18380 (2011).
Margraf, R. L. et al. Multiple endocrine neoplasia type 2 RET protooncogene database: repository of MEN2-associated RET sequence variation and reference for genotype/phenotype correlations. Hum. Mutat. 30, 548–556 (2009).
Brandi, M. L. et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J. Clin. Endocrinol. Metab. 86, 5658–5671 (2001).
Guillem, J. G. et al. ASCO/SSO review of current role of risk-reducing surgery in common hereditary cancer syndromes. J. Clin. Oncol. 24, 4642–4660 (2006).
Tuttle, R. M. et al. Medullary carcinoma. J. Natl Compr. Canc. Netw. 8, 512–530 (2010).
Chen, H. et al. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas 39, 775–783 (2010).
Waguespack, S., Wells, S., Ross, J. & Bleyer, A. in Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival 1975–2000 (eds Bleyer, A., O'Leary, M., Barr, R. & Ries, L.) 143–154 (National Cancer Institute, Bethesda, 2006).
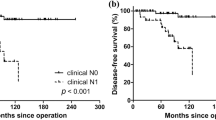
Raval, M. V. et al. Influence of lymph node metastases on survival in pediatric medullary thyroid cancer. J. Pediatr. Surg. 45, 1947–1954 (2010).
Elisei, R. et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J. Clin. Endocrinol. Metab. 93, 682–687 (2008).
Gagel, R. F. et al. Natural history of the familial medullary thyroid carcinoma-pheochromocytoma syndrome and the identification of preneoplastic stages by screening studies: a five-year report. Trans. Assoc. Am. Physicians 88, 177–191 (1975).
Machens, A. Early malignant progression of hereditary medullary thyroid cancer. N. Engl. J. Med. 350, 943 (2004).
Skinner, M. A. et al. Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N. Engl. J. Med. 353, 1105–1113 (2005).
Rohmer, V. et al. Prognostic factors of disease-free survival after thyroidectomy in 170 young patients with a RET germline mutation: a multicenter study of the Groupe Francais d'Etude des Tumeurs Endocrines. J. Clin. Endocrinol. Metab. 96, E509–E518 (2011).
de Groot, J. W. et al. Determinants of life expectancy in medullary thyroid cancer: age does not matter. Clin. Endocrinol. (Oxf.) 65, 729–736 (2006).
Wray, C. J. et al. Failure to recognize multiple endocrine neoplasia 2B: more common than we think? Ann. Surg. Oncol. 15, 293–301 (2008).
Cupisti, K. et al. Long-term clinical and biochemical follow-up in medullary thyroid carcinoma: a single institution's experience over 20 years. Ann. Surg. 246, 815–821 (2007).
Boostrom, S. Y. et al. Need for a revised staging consensus in medullary thyroid carcinoma. Arch. Surg. 144, 663–669 (2009).
Machens, A. et al. Early malignant progression of hereditary medullary thyroid cancer. N. Engl. J. Med. 349, 1517–1525 (2003).
Etit, D. et al. Histopathologic and clinical features of medullary microcarcinoma and C-cell hyperplasia in prophylactic thyroidectomies for medullary carcinoma: a study of 42 cases. Arch. Pathol. Lab. Med. 132, 1767–1773 (2008).
Dralle, H. et al. Prophylactic thyroidectomy in 75 children and adolescents with hereditary medullary thyroid carcinoma: German and Austrian experience. World J. Surg. 22, 744–750 (1998).
Zenaty, D. et al. Medullary thyroid carcinoma identified within the first year of life in children with hereditary multiple endocrine neoplasia type 2A (codon 634) and 2B. Eur. J. Endocrinol. 160, 807–813 (2009).
Meijer, J. A. et al. Calcitonin and carcinoembryonic antigen doubling times as prognostic factors in medullary thyroid carcinoma: a structured meta-analysis. Clin. Endocrinol. (Oxf.) 72, 534–542 (2010).
Costante, G., Durante, C., Francis, Z., Schlumberger, M. & Filetti, S. Determination of calcitonin levels in C-cell disease: clinical interest and potential pitfalls. Nat. Clin. Pract. Endocrinol. Metab. 5, 35–44 (2009).
Machens, A., Lorenz, K. & Dralle, H. Individualization of lymph node dissection in RET (rearranged during transfection) carriers at risk for medullary thyroid cancer: value of pretherapeutic calcitonin levels. Ann. Surg. 250, 305–310 (2009).
Chougnet, C., Brassard, M., Leboulleux, S., Baudin, E. & Schlumberger, M. Molecular targeted therapies for patients with refractory thyroid cancer. Clin. Oncol. (R. Coll. Radiol.) 22, 448–455 (2010).
Sherman, S. I. Targeted therapies for thyroid tumors. Mod. Pathol. 24 (Suppl. 2), S44–S52 (2011).
Caprelsa® (vandetanib) package insert (http://www1.astrazeneca-us.com/pi/vandetanib.pdf). (AstraZeneca, 2011).
Wells, S. A. Jr et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J. Clin. Oncol. 28, 767–772 (2010).
Robinson, B. G., Paz-Ares, L., Krebs, A., Vasselli, J. & Haddad, R. Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancer. J. Clin. Endocrinol. Metab. 95, 2664–2671 (2010).
Fox, E. et al. Phase I/II trial of vandetanib in children and adolescents with hereditary medullary thyroid carcinoma [abstract]. J. Clin. Oncol. 27 (Suppl.), a10014 (2009).
Frank-Raue, K., Rondot, S. & Raue, F. Molecular genetics and phenomics of RET mutations: Impact on prognosis of MTC. Mol. Cell. Endocrinol. 322, 2–7 (2010).
Wohllk, N. et al. Relevance of RET proto-oncogene mutations in sporadic medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 81, 3740–3745 (1996).
Schuffenecker, I. et al. Prevalence and parental origin of de novo RET mutations in multiple endocrine neoplasia type 2A and familial medullary thyroid carcinoma. Le Groupe d'Etude des Tumeurs a Calcitonine. Am. J. Hum. Genet. 60, 233–237 (1997).
Elisei, R. et al. RET genetic screening in patients with medullary thyroid cancer and their relatives: experience with 807 individuals at one center. J. Clin. Endocrinol. Metab. 92, 4725–4729 (2007).
Romei, C. et al. Multiple endocrine neoplasia type 2 syndromes (MEN 2): results from the ItaMEN network analysis on the prevalence of different genotypes and phenotypes. Eur. J. Endocrinol. 163, 301–308 (2010).
Machens, A., Lorenz, K. & Dralle, H. Constitutive RET tyrosine kinase activation in hereditary medullary thyroid cancer: clinical opportunities. J. Intern. Med. 266, 114–125 (2009).
Frank-Raue, K., Rondot, S., Schulze, E. & Raue, F. Change in the spectrum of RET mutations diagnosed between 1994 and 2006. Clin. Lab. 53, 273–282 (2007).
Machens, A. et al. Genotype-phenotype correlations in hereditary medullary thyroid carcinoma: oncological features and biochemical properties. J. Clin. Endocrinol. Metab. 86, 1104–1109 (2001).
Kouvaraki, M. A. et al. Genotype–phenotype analysis in multiple endocrine neoplasia type 1. Arch. Surg. 137, 641–647 (2002).
Decker, R. A., Peacock, M. L. & Watson, P. Hirschsprung disease in MEN 2A: increased spectrum of RET exon 10 genotypes and strong genotype–phenotype correlation. Hum. Mol. Genet. 7, 129–134 (1998).
Moore, S. W. & Zaahl, M. G. Multiple endocrine neoplasia syndromes, children, Hirschsprung's disease and RET. Pediatr. Surg. Int. 24, 521–530 (2008).
Moore, S. W. & Zaahl, M. Familial associations in medullary thyroid carcinoma with Hirschsprung disease: the role of the RET-C620 “Janus” genetic variation. J. Pediatr. Surg. 45, 393–396 (2010).
Gagel, R. F. When “The 7-Year Itch” is indicative of an endocrine malignant condition. Endocr. Pract. 8, 72–74 (2002).
Verga, U. et al. Frequent association between MEN 2A and cutaneous lichen amyloidosis. Clin. Endocrinol. (Oxf.) 59, 156–161 (2003).
Rothberg, A. E., Raymond, V. M., Gruber, S. B. & Sisson, J. Familial medullary thyroid carcinoma associated with cutaneous lichen amyloidosis. Thyroid 19, 651–655 (2009).
Bergholm, U., Bergström, R. & Ekbom, A. Long-term follow-up of patients with medullary carcinoma of the thyroid. Cancer 79, 132–138 (1997).
Raue, F. German medullary thyroid carcinoma/multiple endocrine neoplasia registry. German MTC/MEN Study Group. Medullary Thyroid Carcinoma/Multiple Endocrine Neoplasia Type 2. Langenbecks Arch. Surg. 383, 334–336 (1998).
O'Riordain, D. S. et al. Medullary thyroid carcinoma in multiple endocrine neoplasia types 2A and 2B. Surgery 116, 1017–1023 (1994).
Schuffenecker, I. et al. Risk and penetrance of primary hyperparathyroidism in multiple endocrine neoplasia type 2A families with mutations at codon 634 of the RET proto-oncogene. Groupe D'etude des Tumeurs à Calcitonine. J. Clin. Endocrinol. Metab. 83, 487–491 (1998).
Carling, T. & Udelsman, R. Parathyroid surgery in familial hyperparathyroid disorders. J. Intern. Med. 257, 27–37 (2005).
Magalhães, P. K., Antonini, S. R., de Paula, F. J., de Freitas, L. C. & Maciel, L. M. Primary hyperparathyroidism as the first clinical manifestation of multiple endocrine neoplasia type 2A in a 5-year-old child. Thyroid 21, 547–550 (2011).
Scholten, A. et al. Evolution of surgical treatment of primary hyperparathyroidism in patients with multiple endocrine neoplasia type 2A. Endocr. Pract. 17, 7–15 (2011).
Cranston, A. N. et al. RET is constitutively activated by novel tandem mutations that alter the active site resulting in multiple endocrine neoplasia type 2B. Cancer Res. 66, 10179–10187 (2006).
Jasim, S. et al. Multiple endocrine neoplasia type 2B with a RET proto-oncogene A883F mutation displays a more indolent form of medullary thyroid carcinoma compared with a RET M918T mutation. Thyroid 21, 189–192 (2011).
Carlson, K. M. et al. Parent-of-origin effects in multiple endocrine neoplasia type 2B. Am. J. Hum. Genet. 55, 1076–1082 (1994).
Jones, B. A. & Sisson, J. C. Early diagnosis and thyroidectomy in multiple endocrine neoplasia, type 2b. J. Pediatr. 102, 219–223 (1983).
Kaufman, F. R., Roe, T. F., Isaacs, H. Jr & Weitzman, J. J. Metastatic medullary thyroid carcinoma in young children with mucosal neuroma syndrome. Pediatrics 70, 263–267 (1982).
Saltzman, C. L., Herzenberg, J. E., Phillips, W. A., Hensinger, R. N. & Hopwood, N. J. Thick lips, bumpy tongue, and slipped capital femoral epiphysis—a deadly combination. J. Pediatr. Orthop. 8, 219–222 (1988).
O'Riordain, D. S. et al. Multiple endocrine neoplasia type 2B: more than an endocrine disorder. Surgery 118, 936–942 (1995).
Brauckhoff, M. et al. Premonitory symptoms preceding metastatic medullary thyroid cancer in MEN 2B: An exploratory analysis. Surgery 144, 1044–1050 (2008).
Brauckhoff, M. et al. Multiple endocrine neoplasia 2B syndrome due to codon 918 mutation: clinical manifestation and course in early and late onset disease. World J. Surg. 28, 1305–1311 (2004).
Camacho, C. P. et al. Early diagnosis of multiple endocrine neoplasia type 2B: a challenge for physicians. Arq. Bras. Endocrinol. Metabol. 52, 1393–1398 (2008).
Parker, D. G., Robinson, B. G. & O'Donnell, B. A. External ophthalmic findings in multiple endocrine neoplasia type 2B. Clin. Experiment. Ophthalmol. 32, 420–423 (2004).
Vasen, H. F. et al. The natural course of multiple endocrine neoplasia type IIb. A study of 18 cases. Arch. Intern. Med. 152, 1250–1252 (1992).
Leboulleux, S. et al. Medullary thyroid carcinoma as part of a multiple endocrine neoplasia type 2B syndrome: influence of the stage on the clinical course. Cancer 94, 44–50 (2002).
Evans, C. A., Nesbitt, I. M., Walker, J. & Cohen, M. C. MEN 2B syndrome should be part of the working diagnosis of constipation of the newborn. Histopathology 52, 646–648 (2008).
Waguespack, S. G. & Rich, T. A. Multiple endocrine neoplasia [corrected] syndrome type 2B in early childhood: long-term benefit of prophylactic thyroidectomy. Cancer 116, 2284 (2010).
Yip, L. et al. Multiple endocrine neoplasia type 2: evaluation of the genotype-phenotype relationship. Arch. Surg. 138, 409–416 (2003).
Quayle, F. J., Fialkowski, E. A., Benveniste, R. & Moley, J. F. Pheochromocytoma penetrance varies by RET mutation in MEN 2A. Surgery 142, 800–805 (2007).
Iihara, M. et al. A nationwide clinical survey of patients with multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma in Japan. Jpn J. Clin. Oncol. 27, 128–134 (1997).
Machens, A. et al. Codon-specific development of pheochromocytoma in multiple endocrine neoplasia type 2. J. Clin. Endocrinol. Metab. 90, 3999–4003 (2005).
Nguyen, L. et al. Pheochromocytoma in multiple endocrine neoplasia type 2: a prospective study. Eur. J. Endocrinol. 144, 37–44 (2001).
Frank-Raue, K. et al. Risk profiles and penetrance estimations in multiple endocrine neoplasia type 2A caused by germline RET mutations located in exon 10. Hum. Mutat. 32, 51–58 (2011).
Nilsson, O. et al. Adrenal and extra-adrenal pheochromocytomas in a family with germline RET V804L mutation. JAMA 281, 1587–1588 (1999).
Nomura, T. et al. Laparoscopic resection of periadrenal paraganglioma in a patient with multiple endocrine neoplasia type 2A. Surg. Laparosc. Endosc. Percutan. Tech. 21, e31–e33 (2011).
Modigliani, E. et al. Pheochromocytoma in multiple endocrine neoplasia type 2: European study. The Euromen Study Group. J. Intern. Med. 238, 363–367 (1995).
Carney, J. A., Sizemore, G. W. & Tyce, G. M. Bilateral adrenal medullary hyperplasia in multiple endocrine neoplasia, type 2: the precursor of bilateral pheochromocytoma. Mayo Clin. Proc. 50, 3–10 (1975).
Lairmore, T. C., Ball, D. W., Baylin, S. B. & Wells, S. A. Jr. Management of pheochromocytomas in patients with multiple endocrine neoplasia type 2 syndromes. Ann. Surg. 217, 595–601 (1993).
Gagel, R. F. et al. The clinical outcome of prospective screening for multiple endocrine neoplasia type 2a. An 18-year experience. N. Engl. J. Med. 318, 478–484 (1988).
Calmettes, C., Rosenberg-Gourgin, M., Caron, J. & Feingold, N. Pheochromocytoma: a frequent indicator for MEN 2. Henry Ford Hosp. Med. J. 40, 276–277 (1992).
Bryant, J., Farmer, J., Kessler, L. J., Townsend, R. R. & Nathanson, K. L. Pheochromocytoma: the expanding genetic differential diagnosis. J. Natl Cancer Inst. 95, 1196–1204 (2003).
Jiménez, C., Cote, G., Arnold, A. & Gagel, R. F. Review: Should patients with apparently sporadic pheochromocytomas or paragangliomas be screened for hereditary syndromes? J. Clin. Endocrinol. Metab. 91, 2851–2858 (2006).
Waguespack, S. G. et al. A current review of the etiology, diagnosis, and treatment of pediatric pheochromocytoma and paraganglioma. J. Clin. Endocrinol. Metab. 95, 2023–2037 (2010).
Eisenhofer, G. et al. Age at diagnosis of pheochromocytoma differs according to catecholamine phenotype and tumor location. J. Clin. Endocrinol. Metab. 96, 375–384 (2011).
Chevinsky, A. H., Minton, J. P. & Falko, J. M. Metastatic pheochromocytoma associated with multiple endocrine neoplasia syndrome type II. Arch. Surg. 125, 935–938 (1990).
Ayala-Ramirez, M. et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J. Clin. Endocrinol. Metab. 96, 717–725 (2011).
Eisenhofer, G. et al. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr. Relat. Cancer 18, 97–111 (2010).
Eisenhofer, G. et al. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin. Chem. 57, 411–420 (2011).
van Duinen, N. et al. Pheochromocytomas detected by biochemical screening in predisposed subjects are associated with lower prevalence of clinical and biochemical manifestations and smaller tumors than pheochromocytomas detected by signs and symptoms. Eur. J. Endocrinol. 163, 121–127 (2010).
Havekes, B., Romijn, J. A., Eisenhofer, G., Adams, K. & Pacak, K. Update on pediatric pheochromocytoma. Pediatr. Nephrol. 24, 943–950 (2009).
Resta, R. et al. A new definition of genetic counseling: National Society of Genetic Counselors' Task Force report. J. Genet. Couns. 15, 77–83 (2006).
National Cancer Institute Cancer Genetics Risk Assessment and Counseling (PDQ®) [online], (2011).
Lips, C. J., Höppener, J. W., Van Nesselrooij, B. P. & Van der Luijt, R. B. Counselling in multiple endocrine neoplasia syndromes: from individual experience to general guidelines. J. Intern. Med. 257, 69–77 (2005).
Grosfeld, F. J., Beemer, F. A., Lips, C. J., Hendriks, K. S. & ten Kroode, H. F. Parents' responses to disclosure of genetic test results of their children. Am. J. Med. Genet. 94, 316–323 (2000).
Grosfeld, F. J. et al. Distress in MEN 2 family members and partners prior to DNA test disclosure. Multiple endocrine neoplasia type 2. Am. J. Med. Genet. 91, 1–7 (2000).
Vernon, S. W. et al. Correlates of psychologic distress in colorectal cancer patients undergoing genetic testing for hereditary colon cancer. Health Psychol. 16, 73–86 (1997).
Croyle, R. T., Smith, K. R., Botkin, J. R., Baty, B. & Nash, J. Psychological responses to BRCA1 mutation testing: preliminary findings. Health Psychol. 16, 63–72 (1997).
Audrain, J. et al. Genetic counseling and testing for breast-ovarian cancer susceptibility: what do women want? J. Clin. Oncol. 16, 133–138 (1998).
Grubbs, E. G. et al. Do the recent American Thyroid Association (ATA) Guidelines accurately guide the timing of prophylactic thyroidectomy in MEN2A? Surgery 148, 1302–1309 (2010).
Frank-Raue, K. et al. Long-term outcome in 46 gene carriers of hereditary medullary thyroid carcinoma after prophylactic thyroidectomy: impact of individual RET genotype. Eur. J. Endocrinol. 155, 229–236 (2006).
Rodríguez, G. J. et al. Prophylactic thyroidectomy in MEN 2A syndrome: experience in a single center. J. Am. Coll. Surg. 195, 159–166 (2002).
Samaan, N. A., Yang, K. P., Schultz, P. & Hickey, R. C. Diagnosis, management, and pathogenetic studies in medullary thyroid carcinoma syndrome. Henry Ford Hosp. Med. J. 37, 132–137 (1989).
Basuyau, J. P., Mallet, E., Leroy, M. & Brunelle, P. Reference intervals for serum calcitonin in men, women, and children. Clin. Chem. 50, 1828–1830 (2004).
Wang, T. S., Roman, S. A. & Sosa, J. A. Predictors of outcomes following pediatric thyroid and parathyroid surgery. Curr. Opin. Oncol. 21, 23–28 (2009).
Sosa, J. A. et al. Clinical and economic outcomes of thyroid and parathyroid surgery in children. J. Clin. Endocrinol. Metab. 93, 3058–3065 (2008).
Engiz, O. et al. Early prophylactic thyroidectomy for RET mutation-positive MEN 2B. Pediatr. Int. 51, 590–593 (2009).
Tuggle, C. T. et al. Pediatric endocrine surgery: who is operating on our children? Surgery 144, 869–877 (2008).
Carty, S. E. et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid 19, 1153–1158 (2009).
Spinelli, C., Di Giacomo, M., Costanzo, S., Elisei, R. & Miccoli, P. Role of RET codonic mutations in the surgical management of medullary thyroid carcinoma in pediatric age multiple endocrine neoplasm type 2 syndromes. J. Pediatr. Surg. 45, 1610–1616 (2010).
Acknowledgements
The authors acknowledge all of the children and families with MEN2 who have taught them so much about this disease. The authors thank Michelle D. Williams and Douglas B. Evans for contributing to Figure 2, and also acknowledge Lucy Faith's support of their surgical endocrinology education and research programs. Written consent for publication was obtained from a patient and a responsible relative of a patient for the inclusion of Figure 4. C. P. Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape, LLC-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Contributions
S. G. Waguespack wrote the article, and all authors researched the data for the article, provided substantial contributions to discussion of the content and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Waguespack, S., Rich, T., Perrier, N. et al. Management of medullary thyroid carcinoma and MEN2 syndromes in childhood. Nat Rev Endocrinol 7, 596–607 (2011). https://doi.org/10.1038/nrendo.2011.139
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2011.139
This article is cited by
-
Risk factor analysis of distant metastases in patients with primary medullary thyroid cancer: a population-based study
European Archives of Oto-Rhino-Laryngology (2024)
-
Predictive factors of malignancy in pediatric patients with thyroid nodules and performance of the Italian classification (SIAPEC 2014) in the outcome of the cytological FNA categories
Endocrine (2021)
-
Comparison of pediatric and adult medullary thyroid carcinoma based on SEER program
Scientific Reports (2020)