Abstract
The development of new methods for noninvasive imaging is an area of biotechnology that is of great relevance for the diagnosis and characterization of diabetes mellitus. Noninvasive imaging can be used to study the dynamics of β-cell mass and function; β-cell death; vascularity, innervation and autoimmune attack of pancreatic islets; and the efficacy of islet transplantation to remedy β-cell loss in patients with diabetes mellitus. In this Review, we focus on the application of MRI for monitoring islet transplantation and on the potential causes of islet graft failure, which are still poorly understood. Questions that have been addressed by MRI studies encompass graft longevity, and the effects of immune rejection, glucose toxic effects, and the transplanted islets' purity on graft fate. We also highlight novel technologies for simultaneous imaging and delivery of experimental therapies that aim to extend the lifespan and functionality of islet grafts. On the basis of this evidence, MRI represents a valuable platform for a thorough investigation of β-cell function in the context of islet transplantation. State-of-the-art multimodality approaches, such as PET–MRI, can extend our current capabilities and help answer the critical questions that currently inhibit the prevention and cure of diabetes mellitus.
Key Points
-
The development of new methods for noninvasive imaging can improve the diagnosis and characterization of diabetes mellitus
-
Noninvasive imaging can help to monitor the dynamics of the β-cell mass, function and death of β cells, vascularity, innervation, autoimmune activation and infiltration of pancreatic islets
-
Although islet transplantation is a promising approach, substantial graft loss can occur owing to allorejection, autoimmune attack, glucose toxic effects, mechanical stress and microvascular disruption of the islets during transplantation
-
The use of MRI to assess transplanted islets can help to optimize transplantation protocols, explore the anatomy and physiology of transplanted islets and study the immunology of islet engraftment
-
MRI has improved our understanding of immune rejection, toxic effects of glucose, the influence of transplanted islets' purity on graft fate and facilitated delivery of experimental therapies to extend grafts' lifespan
-
State-of-the-art multimodality approaches, such as PET–MRI, can extend our current capabilities and help tackle the obstacles that currently inhibit the prevention and cure of diabetes mellitus
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
U. S. Department of Health and Human Services, Centers for Disease Control and Prevention CDC National Diabetes Fact Sheet, 2007: General Information[online] (2008).
Moore, A., Bonner-Weir, S. & Weissleder, R. Non-invasive in vivo measurement of β-cell mass in mouse model of diabetes. Diabetes 50, 2231–2236 (2001).
Ladriere, L., Malaisse-Lagae, F., Alejandro, R. & Malaisse, W. J. Pancreatic fate of a 125I-labelled mouse monoclonal antibody directed against pancreatic B-cell surface ganglioside(s) in control and diabetic rats. Cell Biochem. Funct. 19, 107–115 (2001).
Ladriere, L., Malaisse-Lagae, F. & Malaisse, W. J. Uptake of tritiated glibenclamide by endocrine and exocrine pancreas. Endocrine 13, 133–136 (2000).
Hohmeier, H. E. et al. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 (2000).
Samli, K. N., McGuire, M. J., Newgard, C. B., Johnston, S. A. & Brown, K. C. Peptide-mediated targeting of the islets of Langerhans. Diabetes 54, 2103–2108 (2005).
Medarova, Z., Bonner-Weir, S., Lipes, M. & Moore, A. Imaging β-cell death with a near-infrared probe. Diabetes 54, 1780–1788 (2005).
Medarova, Z. et al. Noninvasive magnetic resonance imaging of microvascular changes in type 1 diabetes. Diabetes 56, 2677–2682 (2007).
Simpson, N. R. et al. Visualizing pancreatic β-cell mass with 11C-DTBZ. Nucl. Med. Biol. 33, 855–864 (2006).
Souza, F. et al. Longitudinal noninvasive PET-based β cell mass estimates in a spontaneous diabetes rat model. J. Clin. Invest. 116, 1506–1513 (2006).
Kung, M. P. et al. In vivo imaging of β-cell mass in rats using 18F-FP-(+)-DTBZ: a potential PET ligand for studying diabetes mellitus. J. Nucl. Med. 49, 1171–1176 (2008).
Medarova, Z., Tsai, S., Evgenov, N., Santamaria, P. & Moore, A. In vivo imaging of a diabetogenic CD8+ T cell response during type 1 diabetes progression. Magn. Reson. Med. 59, 712–720 (2008).
Moore, A., Grimm, J., Han, B. & Santamaria, P. Tracking the recruitment of diabetogenic CD8+ T-cells to the pancreas in real time. Diabetes 53, 1459–1466 (2004).
Kaufman, D., Baker, M., Nelson, J., Zhang, X. & Chen, X. In vivo, real-time, non-invasive bioluminescent imaging of transplanted islets in a functional murine model. Presented at Imaging the Pancreatic β Cell, April 21–22, Bethesda, MD (2003).
Powers, A. et al. Using bioluminescence to non-invasively image and assess transplanted islet mass. Presented at Imaging the Pancreatic β Cell, April 21–22, Bethesda, MD (2003).
Lu, Y. et al. Repetitive microPET imaging of implanted human islets in mice. Presented at Imaging the Pancreatic β Cell, April 21–22, Bethesda, MD (2003).
Lu, Y. et al. Bioluminescent monitoring of islet graft survival after transplantation. Mol. Ther. 9, 428–435 (2004).
Lu, Y. et al. Noninvasive imaging of islet grafts using positron-emission tomography. Proc. Natl Acad. Sci. USA 103, 11294–11299 (2006).
Fowler, M. et al. Assessment of pancreatic islet mass after islet transplantation using in vivo bioluminescence imaging. Transplantation 79, 768–776 (2005).
Toso, C. et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am. J. Transplant. 8, 701–706 (2008).
Toso, C. et al. Positron-emission tomography imaging of early events after transplantation of islets of Langerhans. Transplantation 79, 353–355 (2005).
Eich, T., Eriksson, O., Lundgren, T. & Nordic Network for Clinical Islet Transplantation. Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. N. Engl. J. Med. 356, 2754–2755 (2007).
Eich, T. et al. Positron emission tomography: a real-time tool to quantify early islet engraftment in a preclinical large animal model. Transplantation 84, 893–898 (2007).
Evgenov, N. V., Medarova, Z., Dai, G., Bonner-Weir, S. & Moore, A. In vivo imaging of islet transplantation. Nat. Med. 12, 144–148 (2006).
Evgenov, N. V. et al. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes 55, 2419–2428 (2006).
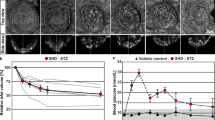
Evgenov, N. V., Pratt, J., Pantazopoulos, P. & Moore, A. Effects of glucose toxicity and islet purity on in vivo magnetic resonance imaging of transplanted pancreatic islets. Transplantation 85, 1091–1098 (2008).
Biancone, L. et al. Magnetic resonance imaging of gadolinium-labeled pancreatic islets for experimental transplantation. NMR Biomed. 20, 40–48 (2007).
Koblas, T. et al. Magnetic resonance imaging of intrahepatically transplanted islets using paramagnetic beads. Transplant. Proc. 37, 3493–3495 (2005).
Tai, J. H. et al. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes 55, 2931–2938 (2006).
Butler, A. E. et al. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 (2003).
Shapiro, A. M. et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343, 230–238 (2000).
Sosnovik, D. E., Nahrendorf, M. & Weissleder, R. Magnetic nanoparticles for MR imaging: agents, techniques and cardiovascular applications. Basic Res. Cardiol. 103, 122–130 (2008).
Ryan, E. A. et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes 50, 710–719 (2001).
Shapiro, A. M. et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 355, 1318–1330 (2006).
Davalli, A. M. et al. A selective decrease in the β cell mass of human islets transplanted into diabetic nude mice. Transplantation 59, 817–820 (1995).
Davalli, A. M. et al. Function, mass, and replication of porcine and rat islets transplanted into diabetic nude mice. Diabetes 44, 104–111 (1995).
Rossetti, L., Giaccari, A. & DeFronzo, R. Glucose toxicity. Diabetes Care 13, 610–630 (1990).
Moore, W., Bieser, K., Geng, Z., Tong, P. & Kover, K. Decreased survival of islet allografts in rats with advanced chronic complications of diabetes. Cell Transplant. 11, 707–713 (2002).
Inverardi, L., Kenyon, N. S. & Ricordi, C. Islet transplantation: immunological perspectives. Curr. Opin. Immunol. 15, 507–511 (2003).
Pileggi, A., Ricordi, C., Alessiani, M. & Inverardi, L. Factors influencing islet of Langerhans graft function and monitoring. Clin. Chim. Acta 310, 3–16 (2001).
Castano, L. & Eisenbarth, G. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu. Rev. Immunol. 8, 647–679 (1990).
Atkinson, M. & Maclaren, N. The pathogenesis of insulin-dependent diabetes mellitus. N. Engl. J. Med. 331, 1428–1436 (1994).
Virostko, J. et al. Factors influencing quantification of in vivo bioluminescence imaging: application to assessment of pancreatic islet transplants. Mol. Imaging 3, 333–342 (2004).
Roth, D. J., Jansen, E. D., Powers, A. C. & Wang, T. G. Bioluminescent imaging of an NF-κB transgenic mouse model. Transplantation 81, 1185–1190 (2006).
Chen, X., Zhang, X., Larson, C. S., Baker, M. S. & Kaufman, D. B. In vivo bioluminescence imaging of transplanted islets and early detection of graft rejection. Transplantation 81, 1421–1427 (2006).
Bertera, S. et al. Body window-enabled in vivo multicolour imaging of transplanted mouse islets expressing as insulin–Timer fusion protein. Biotechniques 35, 718–722 (2003).
Kim, S. et al. Quantitative micro positron emission tomography (PET) imaging for the in vivo determination of pancreatic islet graft survival. Nat. Med. 12, 1423–1428 (2006).
Medarova, Z. & Moore, A. Non-invasive detection of transplanted pancreatic islets. Diabetes Obes. Metab. 10 (Suppl. 4), 88–97 (2008).
Medarova, Z. & Moore, A. Cellular and molecular imaging of the diabetic pancreas. In Molecular and Cellular MR Imaging Part III Ch 19 (eds Modo, M. M. J. & Bulte, J. W. M.) 343–369 (CRC, 2007).
Jirák, D. et al. MRI of transplanted pancreatic islets. Magn. Reson. Med. 52, 1228–1233 (2004).
Medarova, Z., Evgenov, N. V., Dai, G., Bonner-Weir, S. & Moore, A. In vivo multimodal imaging of transplanted pancreatic islets. Nat. Protoc. 1, 429–435 (2006).
Barshes, N. R. et al. Achievement of insulin independence via pancreatic islet transplantation using a remote isolation center: a first-year review. Transplant. Proc. 36, 1127–1129 (2004).
Kriz, J. et al. Magnetic resonance imaging of pancreatic islets in tolerance and rejection. Transplantation 80, 1596–1603 (2005).
Arnush, M. et al. IL-1 produced and released endogenously within human islets inhibits β cell function. J. Clin. Invest. 102, 516–526 (1998).
Barnett, B. P. et al. Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat. Med. 13, 986–991 (2007).
Hathout, E. et al. In vivo imaging demonstrates a time-line for new vessel formation in islet transplantation. Pediatr. Transplant. doi:10.1111/j.1399–30462008.01088.x
Hathout, E. et al. In vivo magnetic resonance imaging of vascularization in islet transplantation. Transpl. Int. 20, 1059–1065 (2007).
Gimi, B. et al. Functional MR microimaging of pancreatic β-cell activation. Cell Transplant. 15, 195–203 (2006).
Antkowiak, P. F. et al. Non-invasive assessment of pancreatic β cell function in vivo using manganese-enhanced magnetic resonance imaging. Am. J. Physiol. Endocrinol. Metab. 296, E573–E578 (2009).
Medarova, Z. et al. Multifunctional magnetic nanocarriers for image-tagged siRNA delivery to intact pancreatic Islets. Transplantation 86, 1170–1177 (2008).
Claiborn, K. C. & Stoffers, D. A. Toward a cell-based cure for diabetes: advances in production and transplant of β cells. Mt Sinai J. Med. 75, 362–371 (2008).
Caravan, P. Strategies for increasing the sensitivity of gadolinium-based MRI contrast agents. Chem. Soc. Rev. 35, 512–23 (2006).
Heyn, C. et al. In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn. Reson. Med. 55, 23–29 (2006).
Moore, A., Medarova, Z., Potthast, A. & Dai, G. In vivo targeting of underglycosylated MUC-1 tumour antigen using a multimodal imaging probe. Cancer Res. 64, 1821–1827 (2004).
Wagner, S., Schnorr, J., Pilgrimm, H., Hamm, B. & Taupitz, M. Monomer-coated very small superparamagnetic iron oxide particles as contrast medium for magnetic resonance imaging: preclinical in vivo characterization. Invest. Radiol. 37, 167–177 (2002).
Medarova, Z., Pham, W., Farrar, C., Petkova, V. & Moore, A. In vivo imaging of siRNA delivery and silencing in tumours. Nat. Med. 13, 372–377 (2007).
Arbab, A. S. & Frank, J. A. Cellular MRI and its role in stem cell therapy. Regen. Med. 3, 199–215 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Medarova, Z., Moore, A. MRI as a tool to monitor islet transplantation. Nat Rev Endocrinol 5, 444–452 (2009). https://doi.org/10.1038/nrendo.2009.130
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2009.130
This article is cited by
-
PET probes for imaging pancreatic islet cells
Clinical and Translational Imaging (2017)
-
Surgical approaches to chronic pancreatitis: indications and imaging findings
Abdominal Radiology (2016)
-
Managing diabetes with nanomedicine: challenges and opportunities
Nature Reviews Drug Discovery (2015)
-
Total pancreatectomy and islet autotransplantation for chronic pancreatitis: spectrum of postoperative CT findings
Abdominal Imaging (2015)
-
Inside the pancreas: progress and challenges of human beta cell mass quantification
Diabetologia (2014)