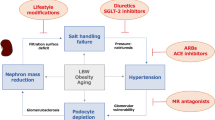
Abstract
Chronic kidney disease (CKD) is defined by persistent urine abnormalities, structural abnormalities or impaired excretory renal function suggestive of a loss of functional nephrons. The majority of patients with CKD are at risk of accelerated cardiovascular disease and death. For those who progress to end-stage renal disease, the limited accessibility to renal replacement therapy is a problem in many parts of the world. Risk factors for the development and progression of CKD include low nephron number at birth, nephron loss due to increasing age and acute or chronic kidney injuries caused by toxic exposures or diseases (for example, obesity and type 2 diabetes mellitus). The management of patients with CKD is focused on early detection or prevention, treatment of the underlying cause (if possible) to curb progression and attention to secondary processes that contribute to ongoing nephron loss. Blood pressure control, inhibition of the renin–angiotensin system and disease-specific interventions are the cornerstones of therapy. CKD complications such as anaemia, metabolic acidosis and secondary hyperparathyroidism affect cardiovascular health and quality of life, and require diagnosis and treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
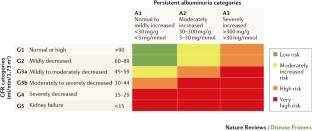
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3, 1–150 (2013). This study is the latest classification of CKD, now implementing albuminuria in a 2D matrix for stratification of the risk of CKD progression and complications.
Zoccali, C. et al. The systemic nature of CKD. Nat. Rev. Nephrol. 13, 344–358 (2017).
Hill, N. R. et al. Global prevalence of chronic kidney disease — a systematic review and meta-analysis. PLoS ONE 11, e0158765 (2016).
Zhang, L. et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379, 815–822 (2012).
Arora, P. et al. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ 185, E417–E423 (2013).
White, S. L., Polkinghorne, K. R., Atkins, R. C. & Chadban, S. J. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am. J. Kidney Dis. 55, 660–670 (2010).
Levey, A. S. & Coresh, J. Chronic kidney disease. Lancet 379, 165–180 (2012).
Girndt, M., Trocchi, P., Scheidt-Nave, C., Markau, S. & Stang, A. The prevalence of renal failure. Results from the German Health Interview and Examination Survey for Adults, 2008–2011 (DEGS1). Dtsch. Arztebl. Int. 113, 85–91 (2016).
Bruck, K. et al. CKD prevalence varies across the European general population. J. Am. Soc. Nephrol. 27, 2135–2147 (2016).
Fraser, S. D. et al. Exploration of chronic kidney disease prevalence estimates using new measures of kidney function in the health survey for England. PLoS ONE 10, e0118676 (2015).
Glassock, R. J., Warnock, D. G. & Delanaye, P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat. Rev. Nephrol. 13, 104–114 (2017).
Stanifer, J. W., Muiru, A., Jafar, T. H. & Patel, U. D. Chronic kidney disease in low- and middle-income countries. Nephrol. Dial. Transplant. 31, 868–874 (2016).
Stanifer, J. W. et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob. Health 2, e174–e181 (2014).
Ene-Iordache, B. et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob. Health 4, e307–e319 (2016).
ESPN/ERA-EDTA Registry. Annual Report. ESPN/ERA-EDTA Registrywww.espn-reg.org/index.jsp (2014).
Chesnaye, N. et al. Demographics of paediatric renal replacement therapy in Europe: a report of the ESPN/ERA-EDTA registry. Pediatr. Nephrol. 29, 2403–2410 (2014).
Saran, R. et al. US Renal Data System 2016 Annual Data Report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 69 (Suppl. 1), A7–A8 (2017).
Harambat, J., van Stralen, K. J., Kim, J. J. & Tizard, E. J. Epidemiology of chronic kidney disease in children. Pediatr. Nephrol. 27, 363–373 (2012).
Jha, V. et al. Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272 (2013).
Stanifer, J. W. et al. Traditional medicines and kidney disease in low- and middle-income countries: opportunities and challenges. Semin. Nephrol. 37, 245–259 (2017).
Charlton, J. R., Springsteen, C. H. & Carmody, J. B. Nephron number and its determinants in early life: a primer. Pediatr. Nephrol. 29, 2299–2308 (2014).
Khalsa, D. D., Beydoun, H. A. & Carmody, J. B. Prevalence of chronic kidney disease risk factors among low birth weight adolescents. Pediatr. Nephrol. 31, 1509–1516 (2016).
Komenda, P. et al. The prevalence of CKD in rural Canadian indigenous peoples: results from the First Nations Community Based Screening to Improve Kidney Health and Prevent Dialysis (FINISHED) screen, triage, and treat program. Am. J. Kidney Dis. 68, 582–590 (2016).
Gifford, F. J., Gifford, R. M., Eddleston, M. & Dhaun, N. Endemic nephropathy around the world. Kidney Int. Rep. 2, 282–292 (2017).
Glaser, J. et al. Climate change and the emergent epidemic of CKD from heat stress in rural communities: the case for heat stress nephropathy. Clin. J. Am. Soc. Nephrol. 11, 1472–1483 (2016).
Jayasumana, C. et al. Chronic interstitial nephritis in agricultural communities: a worldwide epidemic with social, occupational and environmental determinants. Nephrol. Dial. Transplant. 32, 234–241 (2017).
Levin, A. et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 390, 1888–1917 (2017). This study presents a roadmap on how to close gaps in global kidney health.
ERA-EDTA Registry. ERA-EDTA Registry Annual Report 2014 (Department of Medical Informatics, Amsterdam, The Netherlands, 2016).
Li, P. K. et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 13, 90–103 (2017).
Bello, A. K. et al. Assessment of global kidney health care status. JAMA 317, 1864–1881 (2017). This study provides the latest overview about kidney health care in all regions of the world, revealing wide variation of access to nephrology specialists, quality of diagnostic workup and preferences for kidney replacement therapy.
Liyanage, T. et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 385, 1975–1982 (2015).
D. A. L. Ys, G. B. D. & Collaborators, H. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1603–1658 (2016).
Jager, K. J. & Fraser, S. D. S. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol. Dial. Transplant. 32 (Suppl. 2), ii121–ii128 (2017).
Thomas, B. et al. Global cardiovascular and renal outcomes of reduced GFR. J. Am. Soc. Nephrol. 28, 2167–2179 (2017).
GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544 (2016).
Nitsch, D. et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ 346, f324 (2013). This is a meta-analysis that provides the rationale for all-cause mortality risk prediction using eGFR and albuminuria levels as implemented in the KDIGO CKD stages.
van de Luijtgaarden, M. W. et al. Trends in dialysis modality choice and related patient survival in the ERA-EDTA Registry over a 20-year period. Nephrol. Dial. Transplant. 31, 120–128 (2016).
Bertram, J. F., Douglas-Denton, R. N., Diouf, B., Hughson, M. D. & Hoy, W. E. Human nephron number: implications for health and disease. Pediatr. Nephrol. 26, 1529–1533 (2011).
Brenner, B. M., Meyer, T. W. & Hostetter, T. H. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N. Engl. J. Med. 307, 652–659 (1982).
Hostetter, T. H., Olson, J. L., Rennke, H. G., Venkatachalam, M. A. & Brenner, B. M. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am. J. Physiol. 241, F85–F93 (1981).
Benghanem Gharbi, M. et al. Chronic kidney disease, hypertension, diabetes, and obesity in the adult population of Morocco: how to avoid “over”- and “under”-diagnosis of CKD. Kidney Int. 89, 1363–1371 (2016). This is a CKD population study performed in Morocco presenting percentiles for repeated estimates of GFR, which are extremely useful for patient care.
Ruggenenti, P., Cravedi, P. & Remuzzi, G. Mechanisms and treatment of CKD. J. Am. Soc. Nephrol. 23, 1917–1928 (2012).
Laouari, D. et al. TGF-α mediates genetic susceptibility to chronic kidney disease. J. Am. Soc. Nephrol. 22, 327–335 (2011). This is the first description of the transforming growth factor-α–epithelial growth factor receptor axis as a driver of compensatory growth of remnant nephrons. Targeting this pathway can limit the adaptive response from turning into a maladaptive mechanism of CKD progression.
Helal, I., Fick-Brosnahan, G. M., Reed-Gitomer, B. & Schrier, R. W. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 8, 293–300 (2012).
Grams, M. E. et al. Kidney-failure risk projection for the living kidney-donor candidate. N. Engl. J. Med. 374, 411–421 (2016).
Mueller, T. F. & Luyckx, V. A. The natural history of residual renal function in transplant donors. J. Am. Soc. Nephrol. 23, 1462–1466 (2012).
D'Agati, V. D. et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 12, 453–471 (2016).
Tonneijck, L. et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J. Am. Soc. Nephrol. 28, 1023–1039 (2017).
Denic, A. et al. The substantial loss of nephrons in healthy human kidneys with aging. J. Am. Soc. Nephrol. 28, 313–320 (2017).
Hodgin, J. B. et al. Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J. Am. Soc. Nephrol. 26, 3162–3178 (2015).
Kriz, W. & Lemley, K. V. The role of the podocyte in glomerulosclerosis. Curr. Opin. Nephrol. Hypertens. 8, 489–497 (1999).
Kriz, W. & Lemley, K. V. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J. Am. Soc. Nephrol. 26, 258–269 (2015).
Benigni, A., Gagliardini, E. & Remuzzi, G. Changes in glomerular perm-selectivity induced by angiotensin II imply podocyte dysfunction and slit diaphragm protein rearrangement. Semin. Nephrol. 24, 131–140 (2004).
Rizzo, P. et al. Nature and mediators of parietal epithelial cell activation in glomerulonephritides of human and rat. Am. J. Pathol. 183, 1769–1778 (2013).
Clark, W. F. et al. Dipstick proteinuria as a screening strategy to identify rapid renal decline. J. Am. Soc. Nephrol. 22, 1729–1736 (2011).
Abbate, M., Zoja, C. & Remuzzi, G. How does proteinuria cause progressive renal damage? J. Am. Soc. Nephrol. 17, 2974–2984 (2006).
Schnaper, H. W. The tubulointerstitial pathophysiology of progressive kidney disease. Adv. Chron. Kidney Dis. 24, 107–116 (2017).
Kaissling, B., Lehir, M. & Kriz, W. Renal epithelial injury and fibrosis. Biochim. Biophys. Acta 1832, 931–939 (2013).
Peired, A. et al. Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J. Am. Soc. Nephrol. 24, 1756–1768 (2013). This study provides a description of how proteinuria suppresses podocyte regeneration from local podocyte precursors inside the glomerulus.
Brenner, B. M., Garcia, D. L. & Anderson, S. Glomeruli and blood pressure. Less of one, more the other? Am. J. Hypertens. 1, 335–347 (1988).
Low Birth, W. & Nephron Number Working, G. The impact of kidney development on the life course: a consensus document for action. Nephron 136, 3–49 (2017).
Hirano, D. et al. Association between low birth weight and childhood-onset chronic kidney disease in Japan: a combined analysis of a nationwide survey for paediatric chronic kidney disease and the National Vital Statistics Report. Nephrol. Dial. Transplant. 31, 1895–1900 (2016).
Ruggajo, P. et al. Low birth weight and risk of progression to end stage renal disease in IgA nephropathy — a retrospective registry-based cohort study. PLoS ONE 11, e0153819 (2016).
Becherucci, F., Roperto, R. M., Materassi, M. & Romagnani, P. Chronic kidney disease in children. Clin. Kidney J. 9, 583–591 (2016).
Luyckx, V. A. et al. A developmental approach to the prevention of hypertension and kidney disease: a report from the Low Birth Weight and Nephron Number Working Group. Lancet 390, 424–428 (2017).
Oliveira, B., Kleta, R., Bockenhauer, D. & Walsh, S. B. Genetic, pathophysiological, and clinical aspects of nephrocalcinosis. Am. J. Physiol. Renal Physiol. 311, F1243–F1252 (2016).
Trautmann, A. et al. Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin. J. Am. Soc. Nephrol. 10, 592–600 (2015).
Eckardt, K. U. et al. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management — A KDIGO consensus report. Kidney Int. 88, 676–683 (2015).
Nicolaou, N., Renkema, K. Y., Bongers, E. M., Giles, R. H. & Knoers, N. V. Genetic, environmental, and epigenetic factors involved in CAKUT. Nat. Rev. Nephrol. 11, 720–731 (2015).
Cain, J. E., Di Giovanni, V., Smeeton, J. & Rosenblum, N. D. Genetics of renal hypoplasia: insights into the mechanisms controlling nephron endowment. Pediatr. Res. 68, 91–98 (2010).
Uy, N. & Reidy, K. Developmental genetics and congenital anomalies of the kidney and urinary tract. J. Pediatr. Genet. 5, 51–60 (2016).
Vivante, A. & Hildebrandt, F. Exploring the genetic basis of early-onset chronic kidney disease. Nat. Rev. Nephrol. 12, 133–146 (2016).
Kottgen, A. et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat. Genet. 41, 712–717 (2009).
Dummer, P. D. et al. APOL1 kidney disease risk variants: an evolving landscape. Semin. Nephrol. 35, 222–236 (2015).
Kruzel-Davila, E. et al. APOL1-mediated cell injury involves disruption of conserved trafficking processes. J. Am. Soc. Nephrol. 28, 1117–1130 (2017).
Denic, A. et al. Single-nephron glomerular filtration rate in healthy adults. N. Engl. J. Med. 376, 2349–2357 (2017).
Lu, J. L. et al. Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol. 3, 704–714 (2015).
Kramer, H. et al. Waist circumference, body mass index, and ESRD in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am. J. Kidney Dis. 67, 62–69 (2016).
Chang, A. et al. Lifestyle-related factors, obesity, and incident microalbuminuria: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am. J. Kidney Dis. 62, 267–275 (2013).
Foster, M. C. et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am. J. Kidney Dis. 52, 39–48 (2008).
Vivante, A. et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch. Intern. Med. 172, 1644–1650 (2012).
Dunlop, W. Serial changes in renal haemodynamics during normal human pregnancy. Br. J. Obstet. Gynaecol. 88, 1–9 (1981).
Nevis, I. F. et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin. J. Am. Soc. Nephrol. 6, 2587–2598 (2011).
Anders, H. J., Davis, J. M. & Thurau, K. Nephron protection in diabetic kidney disease. N. Engl. J. Med. 375, 2096–2098 (2016).
Vallon, V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu. Rev. Med. 66, 255–270 (2015). A comprehensive overview on the mechanism of action of SGLT2 inhibitors in diabetic kidney disease.
van Bommel, E. J. et al. SGLT2 inhibition in the diabetic kidney-from mechanisms to clinical outcome. Clin. J. Am. Soc. Nephrol. 12, 700–710 (2017).
Anguiano Gomez, L., Lei, Y., Devarapu, S. K. & Anders, H. J. The diabetes pandemic suggests unmet needs for ‘CKD with diabetes’ in addition to ‘diabetic nephropathy’. Implications for pre-clinical research and drug testing. Nephrol. Dial. Transplant.http://dx.doi.org/10.1093/ndt/gfx219 (2017).
Wanner, C. et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 375, 323–334 (2016). This is the first study to show profound nephroprotective effects of an SGLT2 inhibitor in patients with CKD and diabetes.
Bellomo, R., Kellum, J. A. & Ronco, C. Acute kidney injury. Lancet 380, 756–766 (2012).
Venkatachalam, M. A., Weinberg, J. M., Kriz, W. & Bidani, A. K. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J. Am. Soc. Nephrol. 26, 1765–1776 (2015).
Barton, A. L., Mallard, A. S. & Parry, R. G. One year's observational study of acute kidney injury incidence in primary care; frequency of follow-up serum creatinine and mortality risk. Nephron 130, 175–181 (2015).
Eckardt, K. U. et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 382, 158–169 (2013).
Portale, A. A. et al. Disordered FGF23 and mineral metabolism in children with CKD. Clin. J. Am. Soc. Nephrol. 9, 344–353 (2014).
Denburg, M. R. et al. Fracture burden and risk factors in childhood CKD: results from the CKiD cohort study. J. Am. Soc. Nephrol. 27, 543–550 (2016).
Eriksen, B. O. et al. Elevated blood pressure is not associated with accelerated glomerular filtration rate decline in the general non-diabetic middle-aged population. Kidney Int. 90, 404–410 (2016).
Freedman, B. I. & Cohen, A. H. Hypertension-attributed nephropathy: what's in a name? Nat. Rev. Nephrol. 12, 27–36 (2016).
Flynn, J. T. et al. Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension 52, 631–637 (2008).
Vaziri, N. D. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am. J. Physiol. Renal Physiol. 290, F262–F272 (2006).
Speer, T. et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38, 754–768 (2013).
Foley, R. N., Parfrey, P. S. & Sarnak, M. J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 32, S112–119 (1998).
Raschenberger, J. et al. Association of relative telomere length with cardiovascular disease in a large chronic kidney disease cohort: the GCKD study. Atherosclerosis 242, 529–534 (2015).
Grabner, A. et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 22, 1020–1032 (2015).
de Jager, D. J. et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302, 1782–1789 (2009).
De Cosmo, S., Menzaghi, C., Prudente, S. & Trischitta, V. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol. Dial. Transplant. 28, 29–36 (2013).
Cozzolino, M., Ketteler, M. & Zehnder, D. The vitamin D system: a crosstalk between the heart and kidney. Eur. J. Heart Fail. 12, 1031–1041 (2010).
Vervloet, M. & Cozzolino, M. Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int. 91, 808–817 (2017).
Carrero, J. J. et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 23, 77–90 (2013).
Buchanan, C. et al. Intradialytic cardiac magnetic resonance imaging to assess cardiovascular responses in a short-term trial of hemodiafiltration and hemodialysis. J. Am. Soc. Nephrol. 28, 1269–1277 (2017).
van der Heijden, B. J., van Dijk, P. C., Verrier-Jones, K., Jager, K. J. & Briggs, J. D. Renal replacement therapy in children: data from 12 registries in Europe. Pediatr. Nephrol. 19, 213–221 (2004).
Tonshoff, B., Kiepe, D. & Ciarmatori, S. Growth hormone/insulin-like growth factor system in children with chronic renal failure. Pediatr. Nephrol. 20, 279–289 (2005).
Rhee, C. M. et al. Thyroid functional disease: an under-recognized cardiovascular risk factor in kidney disease patients. Nephrol. Dial. Transplant. 30, 724–737 (2015).
Andersen, K. et al. Intestinal dysbiosis, barrier dysfunction, and bacterial translocation account for CKD-related systemic inflammation. J. Am. Soc. Nephrol. 28, 76–83 (2017).
Lau, W. L., Kalantar-Zadeh, K. & Vaziri, N. D. The gut as a source of inflammation in chronic kidney disease. Nephron 130, 92–98 (2015).
Lu, R., Kiernan, M. C., Murray, A., Rosner, M. H. & Ronco, C. Kidney-brain crosstalk in the acute and chronic setting. Nat. Rev. Nephrol. 11, 707–719 (2015).
Roumelioti, M. E. et al. Sleep and fatigue symptoms in children and adolescents with CKD: a cross-sectional analysis from the chronic kidney disease in children (CKiD) study. Am. J. Kidney Dis. 55, 269–280 (2010).
De Broe, M. E., Gharbi, M. B., Zamd, M. & Elseviers, M. Why overestimate or underestimate chronic kidney disease when correct estimation is possible? Nephrol. Dial. Transplant. 32 (Suppl. 2), ii136–ii141 (2017).
Rule, A. D. et al. For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int. 75, 1071–1078 (2009).
Rule, A. D. & Glassock, R. J. GFR estimating equations: getting closer to the truth? Clin. J. Am. Soc. Nephrol. 8, 1414–1420 (2013).
Stevens, L. A. et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 75, 652–660 (2009).
Inker, L. A. et al. Performance of glomerular filtration rate estimating equations in a community-based sample of Blacks and Whites: the multiethnic study of atherosclerosis. Nephrol. Dial. Transplant. gfx042 (2017).
Praditpornsilpa, K. et al. The need for robust validation for MDRD-based glomerular filtration rate estimation in various CKD populations. Nephrol. Dial. Transplant. 26, 2780–2785 (2011).
Inker, L. A. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 367, 20–29 (2012).
Pottel, H. et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol. Dial. Transplant. 31, 798–806 (2016).
Delanaye, P. et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: How to measure glomerular filtration rate with iohexol? Clin. Kidney J. 9, 682–699 (2016).
Delanaye, P. et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: Why to measure glomerular filtration rate with iohexol? Clin. Kidney J. 9, 700–704 (2016).
Matsushita, K. et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 3, 514–525 (2015).
Fotheringham, J., Campbell, M. J., Fogarty, D. G., El Nahas, M. & Ellam, T. Estimated albumin excretion rate versus urine albumin-creatinine ratio for the estimation of measured albumin excretion rate: derivation and validation of an estimated albumin excretion rate equation. Am. J. Kidney Dis. 63, 405–414 (2014).
Glassock, R. J. Evaluation of proteinuria redux. Kidney Int. 90, 938–940 (2016).
Azurmendi, P. J. et al. Early renal and vascular changes in ADPKD patients with low-grade albumin excretion and normal renal function. Nephrol. Dial. Transplant. 24, 2458–2463 (2009).
Glassock, R. J., Fervenza, F. C., Hebert, L. & Cameron, J. S. Nephrotic syndrome redux. Nephrol. Dial. Transplant. 30, 12–17 (2015).
Atwell, T. D. et al. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR Am. J. Roentgenol. 194, 784–789 (2010).
Lees, J. S. et al. Risk factors for bleeding complications after nephrologist-performed native renal biopsy. Clin. Kidney J. 10, 573–577 (2017).
Xu, D. M., Chen, M., Zhou, F. D. & Zhao, M. H. Risk factors for severe bleeding complications in percutaneous renal biopsy. Am. J. Med. Sci. 353, 230–235 (2017).
Glassock, R. J. Con: kidney biopsy: an irreplaceable tool for patient management in nephrology. Nephrol. Dial. Transplant. 30, 528–531 (2015).
Li, J., An, C., Kang, L., Mitch, W. E. & Wang, Y. Recent advances in magnetic resonance imaging assessment of renal fibrosis. Adv. Chron. Kidney Dis. 24, 150–153 (2017).
Becherucci, F. et al. Lessons from genetics: is it time to revise the therapeutic approach to children with steroid-resistant nephrotic syndrome? J. Nephrol. 29, 543–550 (2016).
Siwy, J. et al. Noninvasive diagnosis of chronic kidney diseases using urinary proteome analysis. Nephrol. Dial. Transplant. gfw337 (2016).
Peralta, C. A. & Estrella, M. M. Preventive nephrology in the era of “I” evidence: should we screen for chronic kidney disease? Kidney Int. 92, 19–21 (2017).
Taal, M. W. Screening for chronic kidney disease: preventing harm or harming the healthy? PLoS Med. 9, e1001345 (2012).
Moyer, V. A. & Force, U. S. P. S. T. Screening for chronic kidney disease: U. S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 157, 567–570 (2012).
Shardlow, A., McIntyre, N. J., Fluck, R. J., McIntyre, C. W. & Taal, M. W. Chronic kidney disease in primary care: outcomes after five years in a prospective cohort study. PLoS Med. 13, e1002128 (2016).
Perkovic, V. et al. Management of patients with diabetes and CKD: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 90, 1175–1183 (2016).
Hoerger, T. J. et al. A health policy model of CKD: 2. The cost-effectiveness of microalbuminuria screening. Am. J. Kidney Dis. 55, 463–473 (2010).
Ozyilmaz, A. et al. Screening for albuminuria with subsequent screening for hypertension and hypercholesterolaemia identifies subjects in whom treatment is warranted to prevent cardiovascular events. Nephrol. Dial. Transplant. 28, 2805–2815 (2013).
Tanaka, F. et al. Low-grade albuminuria and incidence of cardiovascular disease and all-cause mortality in nondiabetic and normotensive individuals. J. Hypertens. 34, 506–512 (2016).
Grams, M. E. et al. Race, APOL1 risk, and eGFR decline in the general population. J. Am. Soc. Nephrol. 27, 2842–2850 (2016).
Fink, H. A. et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the U. S. Preventive Services Task Force American College of Physicians Clinical Practice Guideline. Ann. Intern. Med. 156, 570–581 (2012). This study presents a critical discussion of the benefits and risks of CKD screening.
Imai, E. et al. Kidney disease screening program in Japan: history, outcome, and perspectives. Clin. J. Am. Soc. Nephrol. 2, 1360–1366 (2007).
Caley, M., Chohan, P., Hooper, J. & Wright, N. The impact of NHS Health Checks on the prevalence of disease in general practices: a controlled study. Br. J. Gen. Pract. 64, e516–e521 (2014).
Khwaja, A. & Throssell, D. A critique of the UK NICE guidance for the detection and management of individuals with chronic kidney disease. Nephron Clin. Pract. 113, c207–c213 (2009).
Smith, J. M., Mott, S. A., Hoy, W. E. & International Federation of Kidney Foundations. Status of chronic kidney disease prevention programs: International Federation of Kidney Foundation Members 2005/2007. Kidney Int. 74, 1516–1525 (2008).
Tonelli, M. et al. How to advocate for the inclusion of chronic kidney disease in a national noncommunicable chronic disease program. Kidney Int. 85, 1269–1274 (2014).
Boulware, L. E., Jaar, B. G., Tarver-Carr, M. E., Brancati, F. L. & Powe, N. R. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA 290, 3101–3114 (2003).
Komenda, P. et al. Cost-effectiveness of primary screening for CKD: a systematic review. Am. J. Kidney Dis. 63, 789–797 (2014).
Kovesdy, C. P., Furth, S. L., Zoccali, C. & World Kidney Day Steering Committee. Obesity and kidney disease: hidden consequences of the epidemic. Can. J. Kidney Health Dis. 4, 2054358117698669 (2017).
Oellgaard, J. et al. Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long-term renal benefits. Kidney Int. 91, 982–988 (2017).
Rebholz, C. M. et al. Dietary acid load and incident chronic kidney disease: results from the ARIC study. Am. J. Nephrol. 42, 427–435 (2015).
Asghari, G. et al. Adherence to the Mediterranean diet is associated with reduced risk of incident chronic kidney diseases among Tehranian adults. Hypertens. Res. 40, 96–102 (2017).
Dunkler, D. et al. Dietary risk factors for incidence or progression of chronic kidney disease in individuals with type 2 diabetes in the European Union. Nephrol. Dial. Transplant. 30 (Suppl. 4), iv76–iv85 (2015).
Liu, Y. et al. Dietary habits and risk of kidney function decline in an urban population. J. Ren. Nutr. 27, 16–25 (2017).
Snelson, M., Clarke, R. E. & Coughlan, M. T. Stirring the pot: can dietary modification alleviate the burden of CKD? Nutrients 9, 265 (2017).
Smyth, A. et al. Diet and major renal outcomes: a prospective cohort study. The NIH-AARP Diet and Health Study. J. Ren. Nutr. 26, 288–298 (2016).
Rebholz, C. M. et al. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am. J. Kidney Dis. 68, 853–861 (2016).
Dobre, M., Rahman, M. & Hostetter, T. H. Current status of bicarbonate in CKD. J. Am. Soc. Nephrol. 26, 515–523 (2015).
Banerjee, T. et al. High dietary acid load predicts ESRD among adults with CKD. J. Am. Soc. Nephrol. 26, 1693–1700 (2015).
Scialla, J. J. et al. Higher net acid excretion is associated with a lower risk of kidney disease progression in patients with diabetes. Kidney Int. 91, 204–215 (2017).
Gross, O. et al. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int. 81, 494–501 (2012).
Schievink, B. et al. Early renin-angiotensin system intervention is more beneficial than late intervention in delaying end-stage renal disease in patients with type 2 diabetes. Diabetes Obes. Metab. 18, 64–71 (2016).
Noris, M. & Remuzzi, G. Glomerular diseases dependent on complement activation, including atypical hemolytic uremic syndrome, membranoproliferative glomerulonephritis, and C3 glomerulopathy: core curriculum 2015. Am. J. Kidney Dis. 66, 359–375 (2015).
Hildebrand, A. M., Huang, S. H. & Clark, W. F. Plasma exchange for kidney disease: what is the best evidence? Adv. Chron. Kidney Dis. 21, 217–227 (2014).
Rauen, T. et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N. Engl. J. Med. 373, 2225–2236 (2015). This study shows that if conservative treatment is done well, it can be very potent in preventing CKD progression in IgA nephropathy.
Staplin, N. et al. Smoking and adverse outcomes in patients with CKD: the Study of Heart and Renal Protection (SHARP). Am. J. Kidney Dis. 68, 371–380 (2016).
Cravedi, P., Ruggenenti, P. & Remuzzi, G. Intensified inhibition of renin-angiotensin system: a way to improve renal protection? Curr. Hypertens. Rep. 9, 430–436 (2007).
Schrier, R. W. et al. Blood pressure in early autosomal dominant polycystic kidney disease. N. Engl. J. Med. 371, 2255–2266 (2014).
Weir, M. R. et al. Effectiveness of patiromer in the treatment of hyperkalemia in chronic kidney disease patients with hypertension on diuretics. J. Hypertens. 35 (Suppl. 1), S57–S63 (2017).
Holtkamp, F. A. et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 80, 282–287 (2011).
Ruggenenti, P. et al. Role of remission clinics in the longitudinal treatment of CKD. J. Am. Soc. Nephrol. 19, 1213–1224 (2008). This study shows that if conservative treatment is done well, it can be very potent in preventing CKD progression in many forms of kidney disease.
Daina, E. et al. A multidrug, antiproteinuric approach to alport syndrome: a ten-year cohort study. Nephron 130, 13–20 (2015).
Cheung, A. K. et al. Effects of intensive BP control in CKD. J. Am. Soc. Nephrol. 28, 2812–2823 (2017).
Li, K. et al. Effects of bariatric surgery on renal function in obese patients: a systematic review and meta analysis. PLoS ONE 11, e0163907 (2016).
Guideline development group. Clinical Practice Guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR <45 mL/min). Nephrol. Dial. Transplant. 30 (Suppl. 2), ii1–ii142 (2015).
Neal, B. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377, 644–657 (2017).
Zinman, B. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 (2015).
Wanner, C., Amann, K. & Shoji, T. The heart and vascular system in dialysis. Lancet 388, 276–284 (2016).
Rossignol, P. et al. Cardiovascular outcome trials in patients with chronic kidney disease: challenges associated with selection of patients and endpoints. Eur. Heart J. ehx209 (2017).
Xu, X. et al. Efficacy of folic acid therapy on the progression of chronic kidney disease: the renal substudy of the China stroke primary prevention trial. JAMA Intern. Med. 176, 1443–1450 (2016).
Baigent, C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377, 2181–2192 (2011).
Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2, 279–335 (2012).
Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. Suppl. 2, 337–414 (2012).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int. Suppl. 7, 1–59 (2017).
Sumida, K. & Kovesdy, C. P. Disease trajectories before ESRD: implications for clinical management. Semin. Nephrol. 37, 132–143 (2017).
Tangri, N. et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA 315, 164–174 (2016).
Ricardo, A. C. et al. Influence of nephrologist care on management and outcomes in adults with chronic kidney disease. J. Gen. Intern. Med. 31, 22–29 (2016).
Tordoir, J. et al. EBPG on vascular access. Nephrol. Dial. Transplant. 22 (Suppl. 2), ii88–ii117(2007).
Ravani, P. et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J. Am. Soc. Nephrol. 24, 465–473 (2013).
Xue, H. et al. Hemodialysis access usage patterns in the incident dialysis year and associated catheter-related complications. Am. J. Kidney Dis. 61, 123–130 (2013).
Alencar de Pinho, N. et al. Vascular access conversion and patient outcome after hemodialysis initiation with a nonfunctional arteriovenous access: a prospective registry-based study. BMC Nephrol. 18, 74 (2017).
Wallace, E. L. et al. Catheter insertion and perioperative practices within the ISPD North American Research Consortium. Perit. Dial. Int. 36, 382–386 (2016).
Leurs, P., Machowska, A. & Lindholm, B. Timing of dialysis initiation: when to start? Which treatment? J. Ren. Nutr. 25, 238–241 (2015).
Abramowicz, D. et al. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrol. Dial. Transplant. 30, 1790–1797 (2015).
Sebille, V. et al. Prospective, multicenter, controlled study of quality of life, psychological adjustment process and medical outcomes of patients receiving a preemptive kidney transplant compared to a similar population of recipients after a dialysis period of less than three years — the PreKit-QoL study protocol. BMC Nephrol. 17, 11 (2016).
Chang, P. et al. Living donor age and kidney allograft half-life: implications for living donor paired exchange programs. Clin. J. Am. Soc. Nephrol. 7, 835–841 (2012).
Allen, P. J. et al. Recurrent glomerulonephritis after kidney transplantation: risk factors and allograft outcomes. Kidney Int. 92, 461–469 (2017).
Carson, R. C., Juszczak, M., Davenport, A. & Burns, A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin. J. Am. Soc. Nephrol. 4, 1611–1619 (2009).
Morton, R. L. et al. Conservative management and end-of-life care in an Australian cohort with ESRD. Clin. J. Am. Soc. Nephrol. 11, 2195–2203 (2016).
Verberne, W. R. et al. Comparative survival among older adults with advanced kidney disease managed conservatively versus with dialysis. Clin. J. Am. Soc. Nephrol. 11, 633–640 (2016).
Crail, S. Walker, R., Brown, M. & Renal Supportive Care Working Group. Renal supportive and palliative care: position statement. Nephrology 18, 393–400 (2013).
Birmele, B. et al. Death after withdrawal from dialysis: the most common cause of death in a French dialysis population. Nephrol. Dial. Transplant. 19, 686–691 (2004).
Cox, K. J., Parshall, M. B., Hernandez, S. H., Parvez, S. Z. & Unruh, M. L. Symptoms among patients receiving in-center hemodialysis: a qualitative study. Hemodial. Int. 21, 524–533 (2017).
Jesky, M. D. et al. Health-related quality of life impacts mortality but not progression to end-stage renal disease in pre-dialysis chronic kidney disease: a prospective observational study. PLoS ONE 11, e0165675 (2016).
Rebollo Rubio, A. & Morales Asencio, J. M. & Eugenia Pons Raventos, M. Depression anxiety and health-related quality of life amongst patients who are starting dialysis treatment. J. Ren. Care 43, 73–82 (2017). This is a study showing that improving quality of life starts with its proper assessment.
Davison, S. N., Jhangri, G. S. & Johnson, J. A. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int. 69, 1621–1625 (2006).
Davison, S. N. Pain in hemodialysis patients: prevalence, cause, severity, and management. Am. J. Kidney Dis. 42, 1239–1247 (2003).
Pereira, B. D. S. et al. Beyond quality of life: a cross sectional study on the mental health of patients with chronic kidney disease undergoing dialysis and their caregivers. Health Qual. Life Outcomes 15, 74 (2017).
Tonelli, M. The roads less traveled? Diverging research and clinical priorities for dialysis patients and those with less severe CKD. J. Kidney Dis. 63, 124–132 (2014).
Tinetti, M. E., Fried, T. R. & Boyd, C. M. Designing health care for the most common chronic condition — multimorbidity. JAMA 307, 2493–2494 (2012).
Cabrera, V. J., Hansson, J., Kliger, A. S. & Finkelstein, F. O. Symptom management of the patient with CKD: the role of dialysis. Clin. J. Am. Soc. Nephrol. 12, 687–693 (2017).
Manfredini, F. et al. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J. Am. Soc. Nephrol. 28, 1259–1268 (2017).
Cameron, J. I., Whiteside, C., Katz, J. & Devins, G. M. Differences in quality of life across renal replacement therapies: a meta-analytic comparison. Am. J. Kidney Dis. 35, 629–637 (2000).
Iyasere, O. U. et al. Quality of life and physical function in older patients on dialysis: a comparison of assisted peritoneal dialysis with hemodialysis. Clin. J. Am. Soc. Nephrol. 11, 423–430 (2016). This is a paper that evaluates alternative options to haemodialysis for older patients with ESRD.
Vanholder, R. et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat. Rev. Nephrol. 13, 393–409 (2017).
Dew, M. A. et al. Does transplantation produce quality of life benefits? A quantitative analysis of the literature. Transplantation 64, 1261–1273 (1997).
Rhee, C. M., Brunelli, S. M., Subramanian, L. & Tentori, F. Measuring patient experience in dialysis: a new paradigm of quality assessment. J. Nephrol. http://dx.doi.org/10.1007/s40620-017-0401-2 (2017).
Wuttke, M. & Kottgen, A. Insights into kidney diseases from genome-wide association studies. Nat. Rev. Nephrol. 12, 549–562 (2016).
Beeman, S. C. et al. MRI-based glomerular morphology and pathology in whole human kidneys. Am. J. Physiol. Renal Physiol. 306, F1381–F1390 (2014).
Boor, P., Ostendorf, T. & Floege, J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat. Rev. Nephrol. 6, 643–656 (2010).
Tampe, D. & Zeisberg, M. Potential approaches to reverse or repair renal fibrosis. Nat. Rev. Nephrol. 10, 226–237 (2014).
Goicoechea, M. et al. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am. J. Kidney Dis. 65, 543–549 (2015).
de Zeeuw, D. et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 369, 2492–2503 (2013).
Pergola, P. E. et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 365, 327–336 (2011).
Lazzeri, E., Romagnani, P. & Lasagni, L. Stem cell therapy for kidney disease. Expert Opin. Biol. Ther. 15, 1455–1468 (2015).
Lasagni, L. et al. Podocyte regeneration driven by renal progenitors determines glomerular disease remission and can be pharmacologically enhanced. Stem Cell Rep. 5, 248–263 (2015).
Mazzinghi, B., Romagnani, P. & Lazzeri, E. Biologic modulation in renal regeneration. Expert Opin. Biol. Ther. 16, 1403–1415 (2016).
Pichaiwong, W. et al. Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J. Am. Soc. Nephrol. 24, 1088–1102 (2013).
Cianciolo Cosentino, C. et al. Histone deacetylase inhibitor enhances recovery after AKI. J. Am. Soc. Nephrol. 24, 943–953 (2013).
Klinkhammer, B. M., Goldschmeding, R., Floege, J. & Boor, P. Treatment of renal fibrosis-turning challenges into opportunities. Adv. Chron. Kidney Dis. 24, 117–129 (2017).
Kramann, R. et al. Pharmacological GLI2 inhibition prevents myofibroblast cell-cycle progression and reduces kidney fibrosis. J. Clin. Invest. 125, 2935–2951 (2015).
Peired, A. J., Sisti, A. & Romagnani, P. Mesenchymal stem cell-based therapy for kidney disease: a review of clinical evidence. Stem Cells Int. 2016, 4798639 (2016).
Ninichuk, V. et al. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 70, 121–129 (2006).
Xinaris, C. et al. Functional human podocytes generated in organoids from amniotic fluid stem cells. J. Am. Soc. Nephrol. 27, 1400–1411 (2016).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 536, 238 (2016).
Takasato, M. & Little, M. H. Making a kidney organoid using the directed differentiation of human pluripotent stem cells. Methods Mol. Biol. 1597, 195–206 (2017).
Xinaris, C., Brizi, V. & Remuzzi, G. Organoid models and applications in biomedical research. Nephron 130, 191–199 (2015).
Anders, H. J., Jayne, D. R. & Rovin, B. H. Hurdles to the introduction of new therapies for immune-mediated kidney diseases. Nat. Rev. Nephrol. 12, 205–216 (2016).
Holderied, A. & Anders, H. J. Animal models of kidney inflammation in translational medicine. Drug Discov. Today Dis. Models 11, 19–27 (2014).
Levin, A., Lancashire, W. & Fassett, R. G. Targets, trends, excesses, and deficiencies: refocusing clinical investigation to improve patient outcomes. Kidney Int. 83, 1001–1009 (2013).
Jayne, D. R. et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J. Am. Soc. Nephrol. (2017).
Glassock, R., Delanaye, P. & El Nahas, M. An age-calibrated classification of chronic kidney disease. JAMA 314, 559–560 (2015).
Levey, A. S., Inker, L. A. & Coresh, J. Chronic kidney disease in older people. JAMA 314, 557–558 (2015).
Poggio, E. D. et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int. 75, 1079–1087 (2009).
Pottel, H., Hoste, L., Yayo, E. & Delanaye, P. Glomerular filtration rate in healthy living potential kidney donors: a meta-analysis supporting the construction of the full age spectrum equation. Nephron 135, 105–119 (2017).
Glassock, R. J. & Rule, A. D. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int. 82, 270–277 (2012).
Hallan, S. I. et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA 308, 2349–2360 (2012).
Warnock, D. G., Delanaye, P. & Glassock, R. J. Risks for all-cause mortality: stratified by age, estimated glomerular filtration rate and albuminuria. Nephron 136, 292–297 (2017).
Denic, A., Glassock, R. J. & Rule, A. D. Structural and functional changes with the aging kidney. Adv. Chron. Kidney Dis. 23, 19–28 (2016).
Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group. KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int. Suppl. 3, 259–305 (2013).
Klessens, C. Q. et al. An autopsy study suggests that diabetic nephropathy is underdiagnosed. Kidney Int. 90, 149–156 (2016).
Acknowledgements
P.R. is supported by the European Research Council under the Consolidator Grant RENOIR (ERC-2014-CoG), grant number 648274. G.R. and H.-J.A. have received support from the European Union's research and innovation programme (under grant agreement Horizon 2020, NEPHSTROM No. 634086). Z.M. has received research grants from the French government (the Investisssement d'Avenir programme). H.-J.A. has received support from the Deutsche Forschungsgemeinschaft (AN372/16-2, 23–1 and 24–1). The views expressed here are the responsibility of the authors only. The EU Commission takes no responsibility for any use made of the information set out.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all sections of the Primer, with H.-J.A. coordinating the project.
Corresponding author
Ethics declarations
Competing interests
R.G. has received speaker honoraria from Genentech and consultancy honoraria from Bristol Myers Squibb; he has conducted compensated editorial tasks for the American Society of Nephrology and Karger and Wolters-Kluwer; and he owns stock in Reata. Z.M. has received grants for research from Amgen, Baxter, Dohme-Chibret, Fresenius Medical Care, GlaxoSmithKline, Lilly, Merck Sharp, Otsuka, and Sanofi-Genzyme; and has received personal fees and grants to charities from Amgen, Bayer and Sanofi-Genzyme. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Romagnani, P., Remuzzi, G., Glassock, R. et al. Chronic kidney disease. Nat Rev Dis Primers 3, 17088 (2017). https://doi.org/10.1038/nrdp.2017.88
Published:
DOI: https://doi.org/10.1038/nrdp.2017.88
This article is cited by
-
Objectively measured daily steps as an outcome in a clinical trial of chronic kidney disease: a systematic review
BMC Nephrology (2024)
-
Diffusion tensor imaging of brain changes in patients with chronic kidney disease before cognitive impairment with 3 T MRI device
Egyptian Journal of Radiology and Nuclear Medicine (2024)
-
Uremic toxins mediate kidney diseases: the role of aryl hydrocarbon receptor
Cellular & Molecular Biology Letters (2024)
-
Realtime monitoring of thrombus formation in vivo using a self-reporting vascular access graft
Communications Medicine (2024)
-
Duration of detrusor overactivity as an independent predictive factor of upper urinary tract deterioration in patients with traumatic spinal cord injury: results of a retrospective cohort study
Spinal Cord (2024)