Abstract
Neurofibromatosis type 1 is a complex autosomal dominant disorder caused by germline mutations in the NF1 tumour suppressor gene. Nearly all individuals with neurofibromatosis type 1 develop pigmentary lesions (café-au-lait macules, skinfold freckling and Lisch nodules) and dermal neurofibromas. Some individuals develop skeletal abnormalities (scoliosis, tibial pseudarthrosis and orbital dysplasia), brain tumours (optic pathway gliomas and glioblastoma), peripheral nerve tumours (spinal neurofibromas, plexiform neurofibromas and malignant peripheral nerve sheath tumours), learning disabilities, attention deficits, and social and behavioural problems, which can negatively affect quality of life. With the identification of NF1 and the generation of accurate preclinical mouse strains that model some of these clinical features, therapies that target the underlying molecular and cellular pathophysiology for neurofibromatosis type 1 are becoming available. Although no single treatment exists, current clinical management strategies include early detection of disease phenotypes (risk assessment) and biologically targeted therapies. Similarly, new medical and behavioural interventions are emerging to improve the quality of life of patients. Although considerable progress has been made in understanding this condition, numerous challenges remain; a collaborative and interdisciplinary approach is required to manage individuals with neurofibromatosis type1 and to develop effective treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Gutmann, D. H., Wood, D. L. & Collins, F. S. Identification of the neurofibromatosis type 1 gene product. Proc. Natl Acad. Sci. USA 88, 9658–9662 (1991).
Uusitalo, E. et al. Incidence and mortality of neurofibromatosis: a total population study in Finland. J. Invest. Dermatol. 135, 904–906 (2015).
Risch, N., Reich, E. W., Wishnick, M. M. & McCarthy, J. G. Spontaneous mutation and parental age in humans. Am. J. Hum. Genet. 41, 218–248 (1987).
Liu, Q., Zoellner, N., Gutmann, D. H. & Johnson, K. J. Parental age and neurofibromatosis type 1: a report from the NF1 Patient Registry Initiative. Fam. Cancer 14, 317–324 (2014). This study highlights the value of patient-reported registries for epidemiological research.
Bunin, G. R., Needle, M. & Riccardi, V. M. Paternal age and sporadic neurofibromatosis 1: a case–control study and consideration of the methodologic issues. Genet. Epidemiol. 14, 507–516 (1997).
Snajderova, M. et al. The importance of advanced parental age in the origin of neurofibromatosis type 1. Am. J. Med. Genet. A 158A, 519–523 (2012).
Poyhonen, M., Kytola, S. & Leisti, J. Epidemiology of neurofibromatosis type 1 (NF1) in northern Finland. J. Med. Genet. 37, 632–636 (2000).
Sergeyev, A. S. On the mutation rate of neurofibromatosis. Humangenetik 28, 129–138 (1975).
Samuelsson, B. & Axelsson, R. Neurofibromatosis. A clinical and genetic study of 96 cases in Gothenburg, Sweden. Acta Derm. Venereol. Suppl. (Stockh.) 95, 67–71 (1981).
McKeever, K., Shepherd, C. W., Crawford, H. & Morrison, P. J. An epidemiological, clinical and genetic survey of neurofibromatosis type 1 in children under sixteen years of age. Ulster Med. J. 77, 160–163 (2008).
Sorensen, S. A., Mulvihill, J. J. & Nielsen, A. On the natural history of von Recklinghausen neurofibromatosis. Ann. NY Acad. Sci. 486, 30–37 (1986).
Evans, D. G. et al. Mortality in neurofibromatosis 1: in North West England: an assessment of actuarial survival in a region of the UK since 1989. Eur. J. Hum. Genet. 19, 1187–1191 (2011). This report describes a detailed analysis of patient survival in neurofibromatosis type 1.
Rasmussen, S. A., Yang, Q. & Friedman, J. M. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am. J. Hum. Genet. 68, 1110–1118 (2001).
Masocco, M. et al. Mortality associated with neurofibromatosis type 1: a study based on Italian death certificates. Orphanet J. Rare Dis. 6, 11 (2011).
Zoller, M., Rembeck, B., Akesson, H. O. & Angervall, L. Life expectancy, mortality and prognostic factors in neurofibromatosis type 1. A twelve-year follow-up of an epidemiological study in Goteborg, Sweden. Acta Derm. Venereol. 75, 136–140 (1995).
Duong, T. A. et al. Mortality associated with neurofibromatosis 1: a cohort study of 1895 patients in 1980–2006 in France. Orphanet J. Rare Dis. 6, 18 (2011).
Messiaen, L. M. et al. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum. Mutat. 15, 541–555 (2000). This important study highlights how improvements in genetic testing facilitated the development of NF1 genetic testing.
Pasmant, E. et al. NF1 microdeletions in neurofibromatosis type 1: from genotype to phenotype. Hum. Mutat. 31, E1506–E1518 (2010).
Rojnueangnit, K. et al. High incidence of Noonan syndrome features including short stature and pulmonic stenosis in patients carrying NF1 missense mutations affecting p. Arg1809: genotype-phenotype correlation. Hum. Mutat. 36, 1052–1063 (2015).
Upadhyaya, M. et al. An absence of cutaneous neurofibromas associated with a 3-bp inframe deletion in exon 17 of the NF1 gene (c.2970–2972 delAAT): evidence of a clinically significant NF1 genotype–phenotype correlation. Am. J. Hum. Genet. 80, 140–151 (2007).
Viskochil, D. et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell 62, 187–192 (1990).
Wallace, M. R. et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science 249, 181–186 (1990).
DeClue, J. E., Cohen, B. D. & Lowy, D. R. Identification and characterization of the neurofibromatosis type 1 protein product. Proc. Natl Acad. Sci. USA 88, 9914–9918 (1991).
Basu, T. N. et al. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 356, 713–715 (1992).
Dasgupta, B., Yi, Y., Chen, D. Y., Weber, J. D. & Gutmann, D. H. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 65, 2755–2760 (2005). This is the first report to demonstrate that neurofibromin negatively regulates mTOR signalling, which is relevant to neurofibromatosis type 1-associated tumour growth.
Johannessen, C. M. et al. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc. Natl Acad. Sci. USA 102, 8573–8578 (2005).
Lin, A. L. & Gutmann, D. H. Advances in the treatment of neurofibromatosis-associated tumours. Nat. Rev. Clin. Oncol. 10, 616–624 (2013).
De Schepper, S. et al. Somatic mutation analysis in NF1 cafe au lait spots reveals two NF1 hits in the melanocytes. J. Invest. Dermatol. 128, 1050–1053 (2008).
Diwakar, G., Zhang, D., Jiang, S. & Hornyak, T. J. Neurofibromin as a regulator of melanocyte development and differentiation. J. Cell Sci. 121, 167–177 (2008).
Stevenson, D. A. et al. Double inactivation of NF1 in tibial pseudarthrosis. Am. J. Hum. Genet. 79, 143–148 (2006). This is the first report to demonstrate that biallelic NF1 loss occurs in non-tumour features of neurofibromatosis type 1.
Wang, W. et al. Mice lacking Nf1 in osteochondroprogenitor cells display skeletal dysplasia similar to patients with neurofibromatosis type I. Hum. Mol. Genet. 20, 3910–3924 (2011).
Sharma, R. et al. Hyperactive Ras/MAPK signaling is critical for tibial nonunion fracture in neurofibromin-deficient mice. Hum. Mol. Genet. 22, 4818–4828 (2013). This study demonstrates that hyperactive RAS underlies the skeletal defects that are observed in tibial fracture associated with neurofibromatosis type 1.
Yang, F. C. et al. Hyperactivation of p21ras and PI3K cooperate to alter murine and human neurofibromatosis type 1-haploinsufficient osteoclast functions. J. Clin. Invest. 116, 2880–2891 (2006).
He, Y. et al. c-Fms signaling mediates neurofibromatosis type-1 osteoclast gain-in-functions. PLoS ONE 7, e46900 (2012).
de la Croix Ndong, J. et al. Asfotase-alpha improves bone growth, mineralization and strength in mouse models of neurofibromatosis type-1. Nat. Med. 20, 904–910 (2014).
Elefteriou, F. et al. ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab. 4, 441–451 (2006).
Costa, R. M. et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature 415, 526–530 (2002). This report reveals that neurofibromin controls mouse learning through RAS regulation of GABA signalling.
Li, W. et al. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr. Biol. 15, 1961–1967 (2005).
Oyibo, H. K., Znamenskiy, P., Oviedo, H. V., Enquist, L. W. & Zador, A. M. Long-term Cre-mediated retrograde tagging of neurons using a novel recombinant pseudorabies virus. Front. Neuroanat. 8, 86 (2014).
Cui, Y. et al. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell 135, 549–560 (2008).
Molosh, A. I. et al. Social learning and amygdala disruptions in Nf1 mice are rescued by blocking p21-activated kinase. Nat. Neurosci. 17, 1583–1590 (2014).
Chen, Y. H. et al. Mouse low-grade gliomas contain cancer stem cells with unique molecular and functional properties. Cell Rep. 10, 1899–1912 (2015).
Diggs-Andrews, K. A. et al. Dopamine deficiency underlies learning deficits in neurofibromatosis-1 mice. Ann. Neurol. 73, 309–315 (2013). This study demonstrates that neurofibromin controls mouse learning by increasing the levels of dopamine in the brain.
Brown, J. A. et al. Reduced striatal dopamine underlies the attention system dysfunction in neurofibromatosis-1 mutant mice. Hum. Mol. Genet. 19, 4515–4528 (2010).
Scheithauer, B. W., Erlandson, R. A. & Woodruff, J. M. Tumors of the Peripheral Nervous System (American Registry of Pathology, 1999).
Le, L. Q., Shipman, T., Burns, D. K. & Parada, L. F. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell Stem Cell 4, 453–463 (2009).
Li, H. et al. Analysis of steroid hormone effects on xenografted human NF1 tumor Schwann cells. Cancer Biol. Ther. 10, 758–764 (2010).
Jouhilahti, E. M. et al. The development of cutaneous neurofibromas. Am. J. Pathol. 178, 500–505 (2011).
Wu, J. et al. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in Desert Hedgehog-expressing cells. Cancer Cell 13, 105–116 (2008).
Le, L. Q. et al. Susceptible stages in Schwann cells for NF1-associated plexiform neurofibroma development. Cancer Res. 71, 4686–4695 (2011).
Mayes, D. A. et al. Perinatal or adult Nf1 inactivation using tamoxifen-inducible PlpCre each cause neurofibroma formation. Cancer Res. 71, 4675–4685 (2011).
Chen, Z. et al. Cells of origin in the embryonic nerve roots for NF1-associated plexiform neurofibroma. Cancer Cell 26, 695–706 (2014). This report reveals that Schwann cell precursors represent the likely cell of origin for plexiform neurofibromas in mice.
Joseph, N. M. et al. The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells. Cancer Cell 13, 129–140 (2008).
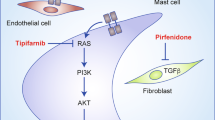
Yang, F. C. et al. Nf1-dependent tumors require a microenvironment containing Nf1+/− and c-kit-dependent bone marrow. Cell 135, 437–448 (2008). This landmark study demonstrates that mast cells increase neoplastic Schwann cell growth through KIT ligand, which opens the door to imatinib as a potential therapy for plexiform neurofibromas.
Yang, F. C. et al. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for Nf1+/− mast cells. J. Clin. Invest. 112, 1851–1861 (2003).
Yang, F. C. et al. Nf1+/− mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Hum. Mol. Genet. 15, 2421–2437 (2006).
Robertson, K. A. et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial. Lancet Oncol. 13, 1218–1224 (2012).
Prada, C. E. et al. Neurofibroma-associated macrophages play roles in tumor growth and response to pharmacological inhibition. Acta Neuropathol. 125, 159–168 (2013). This report suggests that macrophages also participate in the growth of plexiform neurofibromas in mice.
Wu, J. et al. Preclincial testing of sorafenib and RAD001 in the Nfflox/flox;DhhCre mouse model of plexiform neurofibroma using magnetic resonance imaging. Pediatr. Blood Cancer 58, 173–180 (2012).
Jessen, W. J. et al. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J. Clin. Invest. 123, 340–347 (2013).
Gregorian, C. et al. PTEN dosage is essential for neurofibroma development and malignant transformation. Proc. Natl Acad. Sci. USA 106, 19479–19484 (2009).
Keng, V. W. et al. PTEN and NF1 inactivation in Schwann cells produces a severe phenotype in the peripheral nervous system that promotes the development and malignant progression of peripheral nerve sheath tumors. Cancer Res. 72, 3405–3413 (2012).
Cichowski, K. et al. Mouse models of tumor development in neurofibromatosis type 1. Science 286, 2172–2176 (1999). This important study is the first to report the generation of mouse MPNSTs.
Lothe, R. A. et al. Biallelic inactivation of TP53 rarely contributes to the development of malignant peripheral nerve sheath tumors. Genes Chromosomes Cancer 30, 202–206 (2001).
Hirbe, A. C. et al. Spatially- and temporally-controlled postnatal p53 knockdown cooperates with embryonic Schwann cell precursor Nf1 gene loss to promote malignant peripheral nerve sheath tumor formation. Oncotarget 7, 7403–7414 (2016). This report describes the first mouse model of MPNST in which the timing and location of tumour development could be controlled.
DeClue, J. E. et al. Epidermal growth factor receptor expression in neurofibromatosis type 1-related tumors and NF1 animal models. J. Clin. Invest. 105, 1233–1241 (2000).
Mo, W. et al. CXCR4/CXCL12 mediate autocrine cell- cycle progression in NF1-associated malignant peripheral nerve sheath tumors. Cell 152, 1077–1090 (2013).
Lee, W. et al. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat. Genet. 46, 1227–1232 (2014). This study reveals the importance of SUZ12 and PRC2 in the pathogenesis of MPNSTs.
De Raedt, T. et al. PRC2 loss amplifies Ras-driven transcription and confers sensitivity to BRD4-based therapies. Nature 514, 247–251 (2014).
Bajenaru, M. L. et al. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 63, 8573–8577 (2003). This is the first report of a mouse model for neurofibromatosis type 1-associated optic pathway glioma.
Gutmann, D. H. et al. Somatic neurofibromatosis type 1 (NF1) inactivation characterizes NF1-associated pilocytic astrocytoma. Genome Res. 23, 431–439 (2013).
Lee, D. Y., Yeh, T. H., Emnett, R. J., White, C. R. & Gutmann, D. H. Neurofibromatosis-1 regulates neuroglial progenitor proliferation and glial differentiation in a brain region-specific manner. Genes Dev. 24, 2317–2329 (2010).
Lee, D. Y., Gianino, S. M. & Gutmann, D. H. Innate neural stem cell heterogeneity determines the patterning of glioma formation in children. Cancer Cell 22, 131–138 (2012).
Hegedus, B. et al. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell 1, 443–457 (2007).
Pong, W. W., Higer, S. B., Gianino, S. M., Emnett, R. J. & Gutmann, D. H. Reduced microglial CX3CR1 expression delays neurofibromatosis-1 glioma formation. Ann. Neurol. 73, 303–308 (2013).
Daginakatte, G. C. & Gutmann, D. H. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum. Mol. Genet. 16, 1098–1112 (2007). This is the first study to demonstrate that microglia in the tumour microenvironment are important drivers of optic pathway glioma growth in mice.
Daginakatte, G. C., Gianino, S. M., Zhao, N. W., Parsadanian, A. S. & Gutmann, D. H. Increased c-Jun-NH2-kinase signaling in neurofibromatosis-1 heterozygous microglia drives microglia activation and promotes optic glioma proliferation. Cancer Res. 68, 10358–10366 (2008).
Simmons, G. W. et al. Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J. Neuropathol. Exp. Neurol. 70, 51–62 (2011).
Solga, A. C. et al. RNA sequencing of tumor-associated microglia reveals CCL5 as a stromal chemokine critical for neurofibromatosis-1 glioma growth. Neoplasia 17, 776–788 (2015).
Dasgupta, B., Li, W., Perry, A. & Gutmann, D. H. Glioma formation in neurofibromatosis 1 reflects preferential activation of K-RAS in astrocytes. Cancer Res. 65, 236–245 (2005).
Banerjee, S., Crouse, N. R., Emnett, R. J., Gianino, S. M. & Gutmann, D. H. Neurofibromatosis-1 regulates mTOR-mediated astrocyte growth and glioma formation in a TSC/Rheb-independent manner. Proc. Natl Acad. Sci. USA 108, 15996–16001 (2011).
Kaul, A., Toonen, J. A., Cimino, P. J., Gianino, S. M. & Gutmann, D. H. Akt- or MEK-mediated mTOR inhibition suppresses Nf1 optic glioma growth. Neuro Oncol. 17, 843–853 (2015).
Kaul, A., Toonen, J. A., Gianino, S. M. & Gutmann, D. H. The impact of coexisting genetic mutations on murine optic glioma biology. Neuro Oncol. 17, 670–677 (2015).
Hegedus, B. et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 68, 1520–1528 (2008).
Hegedus, B. et al. Optic nerve dysfunction in a mouse model of neurofibromatosis-1 optic glioma. J. Neuropathol. Exp. Neurol. 68, 542–551 (2009).
Brown, J. A., Gianino, S. M. & Gutmann, D. H. Defective cAMP generation underlies the sensitivity of CNS neurons to neurofibromatosis-1 heterozygosity. J. Neurosci. 30, 5579–5589 (2010).
Diggs-Andrews, K. A. et al. Sex is a major determinant of neuronal dysfunction in neurofibromatosis type 1. Ann. Neurol. 75, 309–316 (2014). This report is the first to demonstrate that sex is a major determinant of vision loss in children and mice with neurofibromatosis type 1-associated optic pathway glioma.
Diggs-Andrews, K. A. et al. Reply: to PMID 24375753. Ann. Neurol. 75, 800–801 (2014).
Toonen, J. A., Solga, A. C., Ma, Y. & Gutmann, D. H. Estrogen activation of microglia underlies the sexually dimorphic differences in Nf1 optic glioma-induced retinal pathology. J. Exp. Med. 214, 17–25 (2017).
Toonen, J. A. et al. NF1 germline mutation differentially dictates optic glioma formation and growth in neurofibromatosis-1. Hum. Mol. Genet. 25, 1703–1713 (2016). This is the first study to show that the germline NF1 mutation could differentially dictate the formation of optic pathway gliomas in mice.
Anastasaki, C. & Gutmann, D. H. Neuronal NF1/RAS regulation of cyclic AMP requires atypical PKC activation. Hum. Mol. Genet. 23, 6712–6721 (2014).
Toonen, J. A., Ma, Y. & Gutmann, D. H. Defining the temporal course of murine neurofibromatosis-1 optic gliomagenesis reveals a therapeutic window to attenuate retinal dysfunction. Neuro Oncol. http://dx.doi.org/10.1093/neuonc/now267 (2016).
[No authors listed.] Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch. Neurol. 45, 575–578 (1988).
Evans, D. G. et al. Comprehensive RNA analysis of the NF1 gene in classically affected NF1 affected individuals meeting NIH criteria has high sensitivity and mutation negative testing is reassuring in isolated cases with pigmentary features only. EBioMedicine 7, 212–220 (2016).
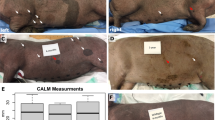
Nunley, K. S., Gao, F., Albers, A. C., Bayliss, S. J. & Gutmann, D. H. Predictive value of cafe au lait macules at initial consultation in the diagnosis of neurofibromatosis type 1. Arch. Dermatol. 145, 883–887 (2009).
DeBella, K., Szudek, J. & Friedman, J. M. Use of the National Institutes of Health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics 105, 608–614 (2000).
Ruggieri, M. & Huson, S. M. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology 56, 1433–1443 (2001).
Ruggieri, M. et al. The natural history of spinal neurofibromatosis: a critical review of clinical and genetic features. Clin. Genet. 87, 401–410 (2015).
Messiaen, L. et al. Clinical and mutational spectrum of neurofibromatosis type 1-like syndrome. JAMA 302, 2111–2118 (2009).
Trofatter, J. A. et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 72, 791–800 (1993).
Hulsebos, T. J. et al. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am. J. Hum. Genet. 80, 805–810 (2007).
Smith, M. J. et al. Mutations in LZTR1 add to the complex heterogeneity of schwannomatosis. Neurology 84, 141–147 (2015).
Huson, S. M., Compston, D. A., Clark, P. & Harper, P. S. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J. Med. Genet. 26, 704–711 (1989).
Verlinsky, Y. et al. Preimplantation diagnosis for neurofibromatosis. Reprod. Biomed. Online 4, 218–222 (2002).
De Raedt, T. et al. Elevated risk for MPNST in NF1 microdeletion patients. Am. J. Hum. Genet. 72, 1288–1292 (2003).
Listernick, R., Ferner, R. E., Liu, G. T. & Gutmann, D. H. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann. Neurol. 61, 189–198 (2007).
Listernick, R. et al. Late-onset optic pathway tumors in children with neurofibromatosis 1. Neurology 63, 1944–1946 (2004).
Listernick, R., Louis, D. N., Packer, R. J. & Gutmann, D. H. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic Pathway Glioma Task Force. Ann. Neurol. 41, 143–149 (1997). This is the first report from the Neurofibromatosis type 1 Optic Pathway Glioma Task Force, which outlines the recommended practices for the management of children with these tumours.
Fossali, E. et al. Renovascular disease and hypertension in children with neurofibromatosis. Pediatr. Nephrol. 14, 806–810 (2000).
Ferner, R. E. et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J. Med. Genet. 44, 81–88 (2007).
Listernick, R., Charrow, J., Greenwald, M. & Mets, M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J. Pediatr. 125, 63–66 (1994).
King, A., Listernick, R., Charrow, J., Piersall, L. & Gutmann, D. H. Optic pathway gliomas in neurofibromatosis type 1: the effect of presenting symptoms on outcome. Am. J. Med. Genet. A 122A, 95–99 (2003).
Listernick, R., Charrow, J. & Greenwald, M. Emergence of optic pathway gliomas in children with neurofibromatosis type 1 after normal neuroimaging results. J. Pediatr. 121, 584–587 (1992).
Prada, C. E. et al. The use of magnetic resonance imaging screening for optic pathway gliomas in children with neurofibromatosis type 1. J. Pediatr. 167, 851–856.e1 (2015).
Fisher, M. J. et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 14, 790–797 (2012). This is one of the first descriptions of risk factors that are important for vision loss in children with neurofibromatosis type 1-associated optic pathway glioma.
Mautner, V. F. et al. Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol. 10, 593–598 (2008).
Crawford, A. H. & Herrera-Soto, J. Scoliosis associated with neurofibromatosis. Orthop. Clin. North Am. 38, 553–562 (2007).
Heerva, E. et al. A controlled register-based study of 460 neurofibromatosis 1 patients: increased fracture risk in children and adults over 41 years of age. J. Bone Miner. Res. 27, 2333–2337 (2012).
Stevenson, D. A. et al. Approaches to treating NF1 tibial pseudarthrosis: consensus from the Children's Tumor Foundation NF1 Bone Abnormalities Consortium. J. Pediatr. Orthop. 33, 269–275 (2013). This consensus report describes the recommended management of tibial pseudarthrosis in children with neurofibromatosis type 1.
Hyman, S. L., Shores, A. & North, K. N. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology 65, 1037–1044 (2005).
Hyman, S. L., Arthur Shores, E. & North, K. N. Learning disabilities in children with neurofibromatosis type 1: subtypes, cognitive profile, and attention-deficit-hyperactivity disorder. Dev. Med. Child Neurol. 48, 973–977 (2006).
Mautner, V. F., Granstrom, S. & Leark, R. A. Impact of ADHD in adults with neurofibromatosis type 1: associated psychological and social problems. J. Atten. Disord. 19, 35–43 (2015).
Isenberg, J. C., Templer, A., Gao, F., Titus, J. B. & Gutmann, D. H. Attention skills in children with neurofibromatosis type 1. J. Child Neurol. 28, 45–49 (2013).
Lidzba, K., Granstrom, S., Lindenau, J. & Mautner, V. F. The adverse influence of attention-deficit disorder with or without hyperactivity on cognition in neurofibromatosis type 1. Dev. Med. Child Neurol. 54, 892–897 (2012).
Garg, S. et al. Neurofibromatosis type 1 and autism spectrum disorder. Pediatrics 132, e1642–e1648 (2013).
Morris, S. M. et al. Disease burden and symptom structure of autism in neurofibromatosis type 1: a study of the International NF1-ASD Consortium Team (INFACT). JAMA Psychiatry 73, 1276–1284 (2016). This international consortium study characterizes autism spectrum disorder in children with neurofibromatosis type 1.
Omrani, A. et al. HCN channels are a novel therapeutic target for cognitive dysfunction in neurofibromatosis type 1. Mol. Psychiatry 20, 1311–1321 (2015).
Bearden, C. E. et al. A randomized placebo-controlled lovastatin trial for neurobehavioral function in neurofibromatosis I. Ann. Clin. Transl Neurol. 3, 266–279 (2016).
van der Vaart, T. et al. Simvastatin for cognitive deficits and behavioural problems in patients with neurofibromatosis type 1 (NF1-SIMCODA): a randomised, placebo-controlled trial. Lancet Neurol. 12, 1076–1083 (2013).
Krab, L. C. et al. Effect of simvastatin on cognitive functioning in children with neurofibromatosis type 1: a randomized controlled trial. JAMA 300, 287–294 (2008). This is the first study to use a farnesyltransferase inhibitor to treat learning problems in children with neurofibromatosis type 1.
Payne, J. M. et al. A double-blind randomized placebo-controlled study of lovastatin for cognitive deficits in children with neurofibromatosis type 1. Neurology 87, 2575–2584 (2016).
Thomas, P. K. et al. Neurofibromatous neuropathy. Muscle Nerve 13, 93–101 (1990).
Stewart, D. R. et al. Diagnosis, management, and complications of glomus tumours of the digits in neurofibromatosis type 1. J. Med. Genet. 47, 525–532 (2010).
Leonard, J. R., Ferner, R. E., Thomas, N. & Gutmann, D. H. Cervical cord compression from plexiform neurofibromas in neurofibromatosis 1. J. Neurol. Neurosurg. Psychiatry 78, 1404–1406 (2007).
Tucker, T., Wolkenstein, P., Revuz, J., Zeller, J. & Friedman, J. M. Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology 65, 205–211 (2005).
Weiss, B. et al. Sirolimus for progressive neurofibromatosis type 1-associated plexiform neurofibromas: a neurofibromatosis Clinical Trials Consortium phase II study. Neuro Oncol. 17, 596–603 (2015).
Hirbe, A. C. & Gutmann, D. H. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 13, 834–843 (2014).
Dombi, E. et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N. Engl. J. Med. 375, 2550–2560 (2017).
Ferner, R. E., Hughes, R. A., Hall, S. M., Upadhyaya, M. & Johnson, M. R. Neurofibromatous neuropathy in neurofibromatosis 1 (NF1). J. Med. Genet. 41, 837–841 (2004).
Drouet, A. et al. Neurofibromatosis 1-associated neuropathies: a reappraisal. Brain 127, 1993–2009 (2004).
Lin, B. T., Weiss, L. M. & Medeiros, L. J. Neurofibroma and cellular neurofibroma with atypia: a report of 14 tumors. Am. J. Surg. Pathol. 21, 1443–1449 (1997).
Beert, E. et al. Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer 50, 1021–1032 (2011).
Evans, D. G. et al. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J. Med. Genet. 39, 311–314 (2002).
Uusitalo, E. et al. Distinctive cancer associations in patients with neurofibromatosis type 1. J. Clin. Oncol. 34, 1978–1986 (2016).
Ferner, R. E. & Gutmann, D. H. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 62, 1573–1577 (2002).
Warbey, V. S., Ferner, R. E., Dunn, J. T., Calonje, E. & O'Doherty, M. J. [18F]FDG PET/CT in the diagnosis of malignant peripheral nerve sheath tumours in neurofibromatosis type-1. Eur. J. Nucl. Med. Mol. Imaging 36, 751–757 (2009). This important study demonstrates the use of PET imaging for the diagnosis of MPNSTs associated with neurofibromatosis type 1.
Frustaci, S. et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J. Clin. Oncol. 19, 1238–1247 (2001).
Kroep, J. R. et al. First-line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: an EORTC soft tissue and bone sarcoma group study. Ann. Oncol. 22, 207–214 (2011).
Carli, M. et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J. Clin. Oncol. 23, 8422–8430 (2005).
Guillamo, J. S. et al. Prognostic factors of CNS tumours in neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain 126, 152–160 (2003). This paper provides a detailed description of brain tumours arising in children with neurofibromatosis type 1.
DiPaolo, D. P. et al. Neurofibromatosis type 1: pathologic substrate of high-signal-intensity foci in the brain. Radiology 195, 721–724 (1995).
Fisher, M. J. et al. Gender as a disease modifier in neurofibromatosis type 1 optic pathway glioma. Ann. Neurol. 75, 799–800 (2014).
Habiby, R., Silverman, B., Listernick, R. & Charrow, J. Precocious puberty in children with neurofibromatosis type 1. J. Pediatr. 126, 364–367 (1995).
Listernick, R., Darling, C., Greenwald, M., Strauss, L. & Charrow, J. Optic pathway tumors in children: the effect of neurofibromatosis type 1 on clinical manifestations and natural history. J. Pediatr. 127, 718–722 (1995).
Packer, R. J. et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J. Neurosurg. 86, 747–754 (1997).
Grill, J. et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann. Neurol. 45, 393–396 (1999).
Sharif, S. et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J. Clin. Oncol. 24, 2570–2575 (2006). This is the first study to demonstrate that radiation is associated with secondary malignancies in patients with neurofibromatosis type 1.
Mahdi, J. et al. A multi-institutional study of brainstem gliomas in children with neurofibromatosis type 1. Neurology (in the press).
Ullrich, N. J., Raja, A. I., Irons, M. B., Kieran, M. W. & Goumnerova, L. Brainstem lesions in neurofibromatosis type 1. Neurosurgery 61, 762–766 (2007).
Opocher, G., Conton, P., Schiavi, F., Macino, B. & Mantero, F. Pheochromocytoma in von Hippel–Lindau disease and neurofibromatosis type 1. Fam.Cancer 4, 13–16 (2005).
Walther, M. M., Herring, J., Enquist, E., Keiser, H. R. & Linehan, W. M. von Recklinghausen's disease and pheochromocytomas. J. Urol. 162, 1582–1586 (1999).
Pappachan, J. M., Raskauskiene, D., Sriraman, R., Edavalath, M. & Hanna, F. W. Diagnosis and management of pheochromocytoma: a practical guide to clinicians. Curr. Hypertens. Rep. 16, 442 (2014).
Miles, D. K. et al. Patterns of hematopoietic lineage involvement in children with neurofibromatosis type 1 and malignant myeloid disorders. Blood 88, 4314–4320 (1996).
Andersson, J. et al. NF1-associated gastrointestinal stromal tumors have unique clinical, phenotypic, and genotypic characteristics. Am. J. Surg. Pathol. 29, 1170–1176 (2005).
Salvi, P. F. et al. Gastrointestinal stromal tumors associated with neurofibromatosis 1: a single centre experience and systematic review of the literature including 252 cases. Int. J. Surg. Oncol. 2013, 398570 (2013).
Joensuu, H., Hohenberger, P. & Corless, C. L. Gastrointestinal stromal tumour. Lancet 382, 973–983 (2013).
Cambiaghi, S., Restano, L. & Caputo, R. Juvenile xanthogranuloma associated with neurofibromatosis 1: 14 patients without evidence of hematologic malignancies. Pediatr. Dermatol. 21, 97–101 (2004).
Cham, E., Siegel, D. & Ruben, B. S. Cutaneous xanthogranulomas, hepatosplenomegaly, anemia, and thrombocytopenia as presenting signs of juvenile myelomonocytic leukemia. Am. J. Clin. Dermatol. 11, 67–71 (2010).
Han, M. & Criado, E. Renal artery stenosis and aneurysms associated with neurofibromatosis. J. Vascular Surg. 41, 539–543 (2005).
Terry, A. R., Jordan, J. T., Schwamm, L. & Plotkin, S. R. Increased risk of cerebrovascular disease among patients with neurofibromatosis type 1: population-based approach. Stroke 47, 60–65 (2016).
Farmakis, S. G., Han, M., White, F. & Khanna, G. Neurofibromatosis 1 vasculopathy manifesting as a peripheral aneurysm in an adolescent. Pediatr. Radiol. 44, 1328–1331 (2014).
Koss, M., Scott, R. M., Irons, M. B., Smith, E. R. & Ullrich, N. J. Moyamoya syndrome associated with neurofibromatosis type 1: perioperative and long-term outcome after surgical revascularization. J. Neurosurg. Pediatr. 11, 417–425 (2013).
Vranceanu, A. M., Merker, V. L., Park, E. R. & Plotkin, S. R. Quality of life among children and adolescents with neurofibromatosis 1: a systematic review of the literature. J. Neurooncol. 122, 219–228 (2015).
Vranceanu, A. M., Merker, V. L., Park, E. & Plotkin, S. R. Quality of life among adult patients with neurofibromatosis 1, neurofibromatosis 2 and schwannomatosis: a systematic review of the literature. J. Neurooncol. 114, 257–262 (2013).
Garwood, M. M. et al. Physical, cognitive, and psychosocial predictors of functional disability and health-related quality of life in adolescents with neurofibromatosis-1. Pain Res. Treat. 2012, 975364 (2012). This important study describes the predictors of QOL and functional disability in adolescents with neurofibromatosis type 1.
Graf, A., Landolt, M. A., Mori, A. C. & Boltshauser, E. Quality of life and psychological adjustment in children and adolescents with neurofibromatosis type 1. J. Pediatr. 149, 348–353 (2006).
Oostenbrink, R. et al. Parental reports of health-related quality of life in young children with neurofibromatosis type 1: influence of condition specific determinants. J. Pediatr. 151, 182–186.e2 (2007).
Krab, L. C. et al. Health-related quality of life in children with neurofibromatosis type 1: contribution of demographic factors, disease-related factors, and behavior. J. Pediatr. 154, 420–425.e1 (2009).
Wolkenstein, P. et al. Impact of neurofibromatosis 1 upon quality of life in childhood: a cross-sectional study of 79 cases. Br. J. Dermatol. 160, 844–848 (2009). This report describes the effect of neurofibromatosis type 1 on QOL in children with neurofibromatosis type 1.
Wolters, P. L. et al. Pain interference in youth with neurofibromatosis type 1 and plexiform neurofibromas and relation to disease severity, social-emotional functioning, and quality of life. Am. J. Med. Genet. A 167A, 2103–2113 (2015).
Martin, S. et al. Social–emotional functioning of children and adolescents with neurofibromatosis type 1 and plexiform neurofibromas: relationships with cognitive, disease, and environmental variables. J. Pediatr. Psychol. 37, 713–724 (2012).
Engel, G. L. The need for a new medical model: a challenge for biomedicine. Science 196, 129–136 (1977).
Page, P. Z. et al. Impact of neurofibromatosis 1 on quality of life: a cross-sectional study of 176 American cases. Am. J. Med. Genet. A 140A, 1893–1898 (2006).
Wolkenstein, P., Zeller, J., Revuz, J., Ecosse, E. & Leplege, A. Quality-of-life impairment in neurofibromatosis type 1: a cross-sectional study of 128 cases. Arch. Dermatol. 137, 1421–1425 (2001).
Nutakki, K., Hingtgen, C. M., Monahan, P., Varni, J. W. & Swigonski, N. L. Development of the adult PedsQL neurofibromatosis type 1 module: initial feasibility, reliability and validity. Health Qual. Life Outcomes 11, 21 (2013). The study describes the development of a QOL assessment tool for patients with neurofibromatosis type 1.
Afridi, S. K., Leschziner, G. D. & Ferner, R. E. Prevalence and clinical presentation of headache in a National Neurofibromatosis 1 Service and impact on quality of life. Am. J. Med. Genet. A 167A, 2282–2285 (2015).
Crawford, H. A. et al. The impact of neurofibromatosis type 1 on the health and wellbeing of Australian adults. J. Genet. Couns. 24, 931–944 (2015).
Merker, V. L. et al. Relationship between whole-body tumor burden, clinical phenotype, and quality of life in patients with neurofibromatosis. Am. J. Med. Genet. A 164A, 1431–1437 (2014).
Kodra, Y. et al. Health-related quality of life in patients with neurofibromatosis type 1. A survey of 129 Italian patients. Dermatology 218, 215–220 (2009).
Granstrom, S., Langenbruch, A., Augustin, M. & Mautner, V. F. Psychological burden in adult neurofibromatosis type 1 patients: impact of disease visibility on body image. Dermatology 224, 160–167 (2012).
Cohen, J. S., Levy, H. P., Sloan, J., Dariotis, J. & Biesecker, B. B. Depression among adults with neurofibromatosis type 1: prevalence and impact on quality of life. Clin. Genet. 88, 425–430 (2015).
Weiss, B. et al. Sirolimus for non-progressive NF1-associated plexiform neurofibromas: an NF clinical trials consortium phase II study. Pediatr. Blood Cancer 61, 982–986 (2014).
Widemann, B. C. et al. Phase 2 randomized, flexible crossover, double-blinded, placebo-controlled trial of the farnesyltransferase inhibitor tipifarnib in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Neuro Oncol. 16, 707–718 (2014).
Hua, C. et al. Sirolimus improves pain in NF1 patients with severe plexiform neurofibromas. Pediatrics 133, e1792–e1797 (2014).
Halloran, J. et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience 223, 102–113 (2012).
Martin, S. et al. Acceptance and commitment therapy in youth with neurofibromatosis type 1 (NF1) and chronic pain and their parents: a pilot study of feasibility and preliminary efficacy. Am. J. Med. Genet. A 170A, 1462–1470 (2016).
Vranceanu, A. M., Merker, V. L., Plotkin, S. R. & Park, E. R. The relaxation response resiliency program (3RP) in patients with neurofibromatosis 1, neurofibromatosis 2, and schwannomatosis: results from a pilot study. J. Neurooncol. 120, 103–109 (2014).
Wolters, P. L. et al. Patient-reported outcomes in neurofibromatosis and schwannomatosis clinical trials. Neurology 81, S6–S14 (2013).
Plotkin, S. R. et al. Achieving consensus for clinical trials: the REiNS International Collaboration. Neurology 81, S1–S5 (2013).
Gutmann, D. H., Blakeley, J. O., Korf, B. R. & Packer, R. J. Optimizing biologically targeted clinical trials for neurofibromatosis. Expert Opin. Investig. Drugs 22, 443–462 (2013).
Brossier, N. M. & Gutmann, D. H. Improving outcomes for neurofibromatosis 1-associated brain tumors. Expert Rev. Anticancer Ther. 15, 415–423 (2015).
Terzi, Y. K. et al. Reproductive decisions after prenatal diagnosis in neurofibromatosis type 1: importance of genetic counseling. Genet. Couns. 20, 195–202 (2009).
Hersh, J. H. & American Academy of Pediatrics Committee on Genetics. Health supervision for children with neurofibromatosis. Pediatrics 121, 633–642 (2008).
Walker, L. et al. A prospective study of neurofibromatosis type 1 cancer incidence in the UK. Br. J. Cancer 95, 233–238 (2006).
Gutmann, D. H. et al. Gliomas presenting after age 10 in individuals with neurofibromatosis type 1 (NF1). Neurology 59, 759–761 (2002).
Seminog, O. O. & Goldacre, M. J. Risk of benign tumours of nervous system, and of malignant neoplasms, in people with neurofibromatosis: population-based record-linkage study. Br. J. Cancer 108, 193–198 (2012).
Blanchard, G. et al. Systematic MRI in NF1 children under six years of age for the diagnosis of optic pathway gliomas. Study and outcome of a French cohort. Eur. J. Paediatr. Neurol. 20, 275–281 (2016).
Listernick, R., Charrow, J., Greenwald, M. J. & Esterly, N. B. Optic gliomas in children with neurofibromatosis type 1. J. Pediatr. 114, 788–792 (1989).
Sharif, S. et al. Women with neurofibromatosis 1 are at a moderately increased risk of developing breast cancer and should be considered for early screening. J. Med. Genet. 44, 481–484 (2007).
Madanikia, S. A., Bergner, A., Ye, X. & Blakeley, J. O. Increased risk of breast cancer in women with NF1. Am. J. Med. Genet. A 158A, 3056–3060 (2012).
Madubata, C. C., Olsen, M. A., Stwalley, D. L., Gutmann, D. H. & Johnson, K. J. Neurofibromatosis type 1 and chronic neurological conditions in the United States: an administrative claims analysis. Genet. Med. 17, 36–42 (2015).
Perini, P. & Gallo, P. The range of multiple sclerosis associated with neurofibromatosis type 1. J. Neurol. Neurosurg. Psychiatry 71, 679–681 (2001).
Ostendorf, A. P., Gutmann, D. H. & Weisenberg, J. L. Epilepsy in individuals with neurofibromatosis type 1. Epilepsia 54, 1810–1814 (2013).
Leschziner, G. D., Golding, J. F. & Ferner, R. E. Sleep disturbance as part of the neurofibromatosis type 1 phenotype in adults. Am. J. Med. Genet. A 161A, 1319–1322 (2013).
Licis, A. K. et al. Prevalence of sleep disturbances in children with neurofibromatosis type 1. J. Child Neurol. 28, 1400–1405 (2013).
Acknowledgements
This work was partially funded by a grant from the US Department of Defense (W81XWH-12-1-0155 to B.R.K.).
Author information
Authors and Affiliations
Contributions
Introduction (B.R.K.); Epidemiology (K.J.J.); Mechanisms/pathophysiology (D.H.G.); Diagnosis, screening and prevention (R.E.F.); Management (R.H.L. and R.E.F.); Quality of life (P.L.W.); Outlook (B.R.K.); Overview of Primer (D.H.G.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Gutmann, D., Ferner, R., Listernick, R. et al. Neurofibromatosis type 1. Nat Rev Dis Primers 3, 17004 (2017). https://doi.org/10.1038/nrdp.2017.4
Published:
DOI: https://doi.org/10.1038/nrdp.2017.4
This article is cited by
-
The contribution of morbidity and unemployment for the reduced labor market participation of individuals with neurofibromatosis 1 in Finland
European Journal of Human Genetics (2024)
-
Schwann cell derived pleiotrophin stimulates fibroblast for proliferation and excessive collagen deposition in plexiform neurofibroma
Cancer Gene Therapy (2024)
-
Advances in pediatric gliomas: from molecular characterization to personalized treatments
European Journal of Pediatrics (2024)
-
Incidence of tethered cord syndrome in neurofibromatosis types 1 and 2 pediatric patients: a population-level analysis
Child's Nervous System (2024)
-
Efficacy and safety of selumetinib in patients with neurofibromatosis type 1 and inoperable plexiform neurofibromas: a systematic review and meta-analysis
Journal of Neurology (2024)