Abstract
Meningitis is an inflammation of the meninges and subarachnoid space that can also involve the brain cortex and parenchyma. It can be acquired spontaneously in the community — community-acquired bacterial meningitis — or in the hospital as a complication of invasive procedures or head trauma (nosocomial bacterial meningitis). Despite advances in treatment and vaccinations, community-acquired bacterial meningitis remains one of the most important infectious diseases worldwide. Streptococcus pneumoniae and Neisseria meningitidis are the most common causative bacteria and are associated with high mortality and morbidity; vaccines targeting these organisms, which have designs similar to the successful vaccine that targets Haemophilus influenzae type b meningitis, are now being used in many routine vaccination programmes. Experimental and genetic association studies have increased our knowledge about the pathogenesis of bacterial meningitis. Early antibiotic treatment improves the outcome, but the growing emergence of drug resistance as well as shifts in the distribution of serotypes and groups are fuelling further development of new vaccines and treatment strategies. Corticosteroids were found to be beneficial in high-income countries depending on the bacterial species. Further improvements in the outcome are likely to come from dampening the host inflammatory response and implementing preventive measures, especially the development of new vaccines.
Similar content being viewed by others
Introduction
Meningitis is an inflammation of the meninges and subarachnoid space that can also involve the brain cortex and parenchyma owing to the close anatomical relationship between the cerebrospinal fluid (CSF) and the brain (Fig. 1). Per definition, bacterial meningitis is an infection of the CSF-filled subarachnoid space. Inflammation of the meninges and subarachnoid space leads to the classic triad of meningitis symptoms — headache, fever and neck stiffness — and to pleocytosis (an increased cell count, particularly of leukocytes) in the CSF1. Involvement of the brain cortex and parenchyma, because of either direct inflammation or vascular complications, might result in behavioural changes, focal neurological abnormalities and impairment of consciousness1, which are typically considered symptoms of encephalitis. Acute meningitis can be caused by a wide variety of infectious agents, but can also be a manifestation of non-infectious diseases2. Bacterial meningitis is considered the most severe form of this disease; the routes of exposure are mainly respiratory, but can be enteric, as is the case in listerial infection. Meningitis can be acquired spontaneously in the community — community-acquired bacterial meningitis2 — or in the hospital as a complication of invasive procedures or head trauma (nosocomial bacterial meningitis)3.
Mid-sagittal view of the brain showing the meninges: the dura mater, the subarachnoid mater and the pia mater. The meninges and cerebrospinal fluid (CSF) are in close anatomical relation with the cerebral cortex and brain parenchyma. Bacteria can reach the meninges through the blood–CSF barrier.
Despite the existence of antibiotic therapies, acute bacterial meningitis causes substantial morbidity and mortality, both in high-income and low-income countries4,5. Bacterial meningitis is an emergency situation and individuals with suspected disease require immediate evaluation and treatment. In this Primer, we provide an overview of community-acquired bacterial meningitis, focusing on the epidemiology, disease mechanisms, diagnosis, screening, prevention and management.
Epidemiology
H. influenzae, S. pneumoniae and N. meningitidis
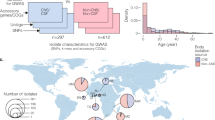
Bacterial meningitis is associated with high mortality and morbidity worldwide, with an estimated 16 million cases in 2013, causing 1.6 million years lived with disability each year6. Globally, the pathogens implicated in this disease vary somewhat and incidence varies widely between regions. Africa is the region with the highest meningitis disease burden; before the introduction of a vaccine (Box 1), the estimated incidence of invasive disease due to Haemophilus influenzae type b (Hib) infection was 46 per 100,000 population per year among children <5 years of age and Streptococcus pneumoniae (pneumococcus) infection was 38 per 100,000 population per year; Neisseria meningitidis (meningococcus) infection was >1,000 per 100,000 per year among all ages during epidemics4. The overall rates of community-acquired bacterial meningitis caused by specific aetiologies in Africa remain unclear owing to a lack of diagnostic tools. Incidence rates of community-acquired bacterial meningitis in high-income areas (such as Europe, the United States and Australia) are 1–3 per 100,000 population per year4. In the Netherlands, the incidence of adult meningitis declined from 1.72 to 0.94 per 100,000 per year from 2007 to 2014; S. pneumoniae caused 72% of episodes7. Reported case fatality rates are high and vary with patient age, causative pathogen and country income5,8. Meningitis caused by S. pneumoniae has the highest case fatality rates: 20–37% in high-income countries and up to 51% in low-income countries8. Case fatality rates for meningococcal meningitis are distinctly lower, in the range of 3–10% worldwide5,9.
The epidemiology of community-acquired bacterial meningitis is changing as prevention measures become increasingly used4. The most common causes of bacterial meningitis are S. pneumoniae and N. meningitidis, with varying prevalence depending on age group and region8,10. Pneumococcal meningitis is in general more common than meningococcal meningitis in children <5 years of age and in the elderly (≥65 years of age), whereas meningococcal meningitis is more frequent among older children, adolescents and young adults11. Pneumococcal conjugate vaccines, which are routinely used in most high-income countries and increasingly in developing countries, have reduced the rates of pneumococcal meningitis not only among vaccinated young children but also among age groups that are not targeted for vaccination through reduced transmission of invasive strains (herd protection)7,12. In the past, Hib caused the majority of bacterial meningitis in children <12 months of age and approximately 50% of all Hib meningitis cases occurred in children <5 years of age13. Now, most national immunization programmes for infants include a vaccine that provides coverage against Hib, rendering Hib-driven meningitis unusual in areas with high immunization coverage4.
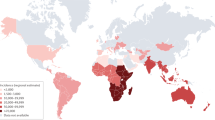
In Africa, epidemics of meningococcal disease occur in a well-defined region — the meningitis belt4 (Fig. 2). This sub-Saharan area from Senegal to Ethiopia is prone to intermittent epidemics of meningococcal meningitis, with rates reaching nearly 1% of the population in the worst outbreaks4. Epidemics were usually caused by serogroup A N. meningitidis, but a vaccination programme, initiated in 2010, against this bacterium has reduced its incidence14. Epidemics caused by serogroup X (2006–2010), serogroup W (2010–2011) and serogroup C (2015) have also been reported15–17.
The meningitis belt is a sub-Saharan African region that has experienced recurring large epidemics of meningococcal meningitis for over a century. Areas at high epidemic risk are marked in orange. The disease incidence and outbreak history in Niger, a typical meningitis belt country, have been extensively studied180. For example, the climate in Niger is dry, with an average yearly rainfall of 300 mm and a wintry warm, dry and dusty wind (the Harmattan). Outbreaks typically begin at the onset of the dry season in January and end abruptly at the start of the rainy season in May or June180,200. This distinct seasonality is striking and might be correlated with numerous factors, including the drying effect of the weather on mucous membranes and the seasonal transmission of respiratory viruses, although this has not been shown definitively200. Adapted from Ref. 201, which was produced using data from the WHO (http://www.cdc.gov/travel-static/yellowbook/2016/map_3-11.pdf).
Certain serotypes or serogroups of the leading pathogens that cause bacterial meningitis have been shown to have a higher ability to cause severe disease than others. Thirteen serogroups of meningococcus have been identified, although six account for the majority of disease (serogroups A–C and serogroups W–Y). Although at least 94 pneumococcal serotypes have been identified, the currently available 10-valent and 13-valent vaccine formulations cover the serotypes that cause ≥70% of cases in most areas of the world18. Serotype 2, a non-vaccine serotype, recently emerged as a common cause of pneumococcal meningitis among children in Bangladesh19. Pneumococcal meningitis typically does not occur in outbreaks, although periods of hyperendemicity (that is, of high and continuous incidence) caused by serotype 1 pneumococcus have been observed in Burkina Faso and Ghana, with high case fatality rates of >40%20.
Other pathogens
Among other aetiologies are group B streptococcus21, a leading cause of meningitis in infants <3 months of age, and Listeria monocytogenes22, which is most commonly seen in infants8. For group B streptococcus, serotype III is the most likely serotype to cause meningitis in infants23; a nationwide surveillance study in the Netherlands showed the emergence of a serotype III group B streptococcus with a genotype belonging to clonal complex 17 (Ref. 21). Aerobic Gram-negative (for example, Escherichia coli) meningitis occurs especially in neonates, the elderly and debilitated or diabetic people8,24.
In pig-farming countries in Asia, Streptococcus suis (group R haemolytic streptococcus) is the most common cause of meningitis25. Large case series of S. suis serotype 2 meningitis (and rarely infective endocarditis and septicaemia) were reported in Hong Kong, Thailand, China and Vietnam25. The precise epidemiology remains unclear, but S. suis infection is correlated with occupational contact with pigs or pork. Splenectomized patients are particularly susceptible to infection by capsulated Gram-positive organisms, such as S. suis26. Although rarely fatal, this strain is commonly associated with bilateral permanent deafness.
Risk factors
In addition to age, other factors have been linked to community-acquired bacterial meningitis risk. Immunocompromised individuals are at increased risk for meningitis caused by S. pneumoniae and L. monocytogenes8,27. T cell deficiencies, such as those caused by HIV infection, increase the risk of bacterial meningitis by approximately eightfold, despite the widespread use of combined antiretroviral therapy28. Genetic risk factors (for example, deficiencies in complement components and asplenia)29 and social and behavioural factors (for example, smoking)30 have been associated with increased risk of meningococcal meningitis. Outbreaks of meningococcal meningitis have occurred among college students, participants in the annual Muslim pilgrimage of Hajj and recently in the United States among men who have sex with men31. Certain ethnic groups have been shown to have higher rates of bacterial meningitis; for example, American Indian and Alaska Native children have higher Hib meningitis rates than the general US population32. All leading aetiologies are spread by human-to-human transmission, except for L. monocytogenes22,33, which is food-borne8.
Mechanisms/pathophysiology
Bacteria can reach the subarachnoid space through the bloodstream or through the spread of infections from contiguous sites, such as the paranasal sinuses or mastoid of the inner ear. Blood-borne pathogen invasion is assumed to be the main route of subarachnoid space entry; this multistep process involves mucosal colonization followed by invasion, survival and replication of the bacteria in the bloodstream and eventual traversal of the blood–brain barrier.
Colonization of the host by the pathogen
Pathogens such as S. pneumoniae, N. meningitidis, group B streptococci and E. coli initially colonize epithelial surfaces either in the respiratory tract (S. pneumoniae and N. meningitidis) or in the gastrointestinal or lower genital tract (group B streptococci and E. coli) before advancing to the bloodstream. The molecular and cellular events underlying colonization and epithelial invasion have been reviewed in detail elsewhere34–37. The exact mechanisms why bacterial bloodstream infections occur in some individuals but not in others are unclear, but seem to depend on a complex interplay between environmental factors (for example, prior influenza virus infection, smoking or alcohol abuse) and genetic factors of the host and pathogen29,34. Host factors that confer susceptibility to invasive infection by meningeal pathogens include congenital asplenia, complement deficiency, immunosuppressive treatment and antibody deficiency38 (Table 1). Furthermore, other genetic variations have recently been linked to predisposition to pneumococcal disease (for example, single-nucleotide polymorphisms (SNPs) in NFKBIA (which encodes nuclear factor-κB (NF-κB) inhibitor-α (IκBα)), or deficiencies in IL-1 receptor-associated kinase 4 (IRAK4) as well as myeloid differentiation primary response protein 88 (MYD88)) and meningococcal disease (for example, SNPs in pattern recognition receptor (PRR) genes, such as Toll-like receptor 9 (TLR9))29,39–41.
Regarding the pathogens, multilocus sequence typing of N. meningitidis has demonstrated that strains associated with asymptomatic carriage are highly genetically diverse, as the bacterial genome undergoes horizontal gene exchange and recombinant events while the bacteria colonize the nasopharynx, whereas only a limited number of genotypes, known as hyperinvasive lineages, are linked with invasive disease11,42. To some extent, the same is true for S. pneumoniae43, group B streptococci44 and E. coli45. The 94 capsular serotypes of S. pneumoniae have been shown to differ greatly in nasopharyngeal carriage rate, disease incidence and severity; serotypes 3, 6A, 6B, 9N and 19F seem to be associated with an increased risk for fatal disease43. Using complementary approaches comprising serotyping, multilocus sequence typing, cell culture and animal experiments, a serotype III, ST-17 group B streptococcal clone has recently been shown to be hypervirulent, accounting for the majority of neonatal group B streptococcal infections in the Netherlands44. Given the many E. coli serotypes (>80 serologically unique capsular (K) antigens), it is striking that K1 E. coli strains possessing K1 are predominant (approximately 80%) among isolates from neonates with E. coli meningitis45.
Survival within the bloodstream
Once the bacteria reach the bloodstream, they have to withstand the bactericidal environment of the blood. The polysaccharide capsules of N. meningitidis, S. pneumoniae, H. influenzae, group B streptococci and E. coli are anti-phagocytic and act as inert shields, inhibiting surface deposition of opsonins, especially complement factors46,47 (Fig. 3). Meningococcal capsular polysaccharides can also attenuate surface deposition of the complement component C4-binding protein (C4bp), thereby limiting complement-mediated direct bacterial killing48. In addition to the capsule, an array of bacterial surface molecules target specific complement components to reduce complement-mediated bacterial clearance49 (Fig. 3). N. meningitidis can directly bind to factor H (fH), which is the main regulator of alternative complement activation, through surface molecules, including fH-binding protein (fHbp), neisserial surface protein A (NspA) and porin B38,50. The increase in the environmental temperature that occurs as the bacteria change habitat from the nasopharynx to the bloodstream has been identified as a ‘danger signal’ for N. meningitidis, which prompts an upregulation of capsular biosynthesis and fHbp expression, thus enhancing its capacity to withstand complement attack51. Similarly, S. pneumoniae has many surface proteins that interact with and deplete complement, as well as inhibit the complement cascade (Fig. 3).
After initiation of bacteraemia, the pathogens must evade opsonophagocytosis and/or membrane attack complex (MAC)-induced lysis. Microbial factors that are involved in the inhibition of complement activation and bacterial killing include the polysaccharide capsule, the pneumococcal surface proteins PspA and PspC and the toxin pneumolysin (Ply) of Streptococcus pneumoniae. For Neisseria meningitidis, the polysaccharide capsule, the outer membrane proteins factor H-binding protein (fHbp), neisserial surface protein A (NspA) and porin B as well as the autotransporter Na1P are essential factors for host avoidance. Ply, which is released during bacterial autolysis, can activate the classical (via direct binding to nonspecific IgM or IgG3) and lectin (via interaction with l-ficolin) complement pathways, thereby diverting complement away from the bacterial surface202 and leading to complement depletion within the pneumococcal environment. By contrast, PspA can reduce C-reactive protein-mediated, complement factor C1q-dependent classical pathway of complement activation203. fHbp, NspA and porin B of N. meningitidis38,50 and PspC of S. pneumoniae can interact with factor H, interfering with the alternative pathway of complement activation. In addition, Na1P cleaves human complement C3, which facilitates C3b degradation and depletion. Bacterial invasion of the cerebrospinal fluid (CSF) requires pathogen adhesion to the endothelial surface. The initial adhesion step is mediated by the binding of bacterial adhesins (PspA for S. pneumoniae and type IV pili (Tfp) and porin A for N. meningitidis) to the laminin receptor (LR)61. In the case of S. pneumoniae, bacterial neuraminidase A (NanA) can also promote adherence and invasion through its laminin G-like lectin domain204. Binding of S. pneumoniae to the vascular wall seems to activate the underlying endothelial cells, specifically increasing the amount of platelet-activating factor receptor (PAFR) on the endothelial surface205. PAFR then binds to its proposed binding partner phosphorylcholine (PCho), which is displayed on the surface of S. pneumoniae, and internalizes the pneumococcus, enabling it to cross the endothelium intracellularly and move from the bloodstream into the CSF. PAFR activation has also been proposed to cause an upregulation of the polyimmunoglobulin receptor and CD31 on endothelial cells; the two receptors jointly facilitate the crossing of S. pneumoniae across the blood–CSF barrier (not shown)206. In addition to LR, the immunoglobulin superfamily member CD147 is a crucial host receptor for the primary attachment of N. meningitidis. Following primary adhesion (step 1), Tfp mediates the recruitment and activation of the β2-adrenoceptor (step 2), finally leading to the organization of the so-called cortical plaques (step 3), which ultimately results in the opening of the inter-endothelial junctions and paracellular transmigration of N. meningitidis.
Outer membrane protein A (OmpA), a major surface protein in E. coli, confers resistance against the serum bactericidal activity by binding to C4bp, which is an endogenous inhibitor of the classical and lectin pathways52. The group B streptococcal capsular polysaccharide contains a terminal α2,3-linked N-acetylneuraminic acid, which is identical to the most common sialic acid of many surface glycans of human cells (an example of molecular mimicry). The presence of sialic acid on human cell surfaces is essential for complement regulator fH-mediated protection against self-attack by complement. Thus, by displaying sialic acid on its own surface, the group B streptococcus impairs surface deposition of opsonin-activated C3 and protects itself from clearance53. In addition, sialylated capsular polysaccharides of group B streptococci can engage inhibitory receptors, such as sialic acid-recognizing immunoglobulin superfamily lectin 9 (Siglec9) on host leukocytes, thereby downregulating their immune responsiveness54. Similar to E. coli, group B streptococci also have evolved mechanisms that facilitate survival within phagocytic cells. For instance, the same operon containing the gene (cylE) that encodes the β-haemolysin cytotoxin has been linked to the production of a carotenoid pigment that can detoxify reactive oxygen species, shielding the bacteria against several antimicrobial weapons of leukocytes. Finally, besides coping with host defence mechanisms, to survive in the bloodstream, microbial invaders have to exploit the host iron pool. Iron is essential for bacterial physiological processes, such as DNA replication, transcription, metabolism and energy generation55. Hence, bacteria have developed diverse iron uptake mechanisms, including surface-associated haem uptake pathways and high-affinity iron-scavenging siderophores55.
Entry into the central nervous system
Prolonged high levels of bacteraemia are shown to favour bacterial penetration into the subarachnoid space for S. pneumoniae, group B streptococci and E. coli in humans and experimental animals, presumably by directly increasing the likelihood that bacteria interact with the endothelial cells of the blood–CSF barrier38. Post-capillary venules and veins within the subarachnoid and perivascular spaces might be the primary bacterial entry sites. Evidence in support of this assumption includes the fact that bacteria were found post-mortem in the arachnoid mater and pia mater of patients and laboratory animals with meningococcal or pneumococcal meningitis56,57. In addition, post-capillary venules and veins belong to the ‘leaky’ part of the vascular tree of the brain and are in close proximity to the CSF58. Finally, the shear stress in these vessels, defined as the tangential force exerted on the vascular wall by a moving fluid, is lower than in capillaries or arterioles59. Low shear stress has been determined to be paramount for the intimate contact between N. meningitidis and the host endothelial cells60.
S. pneumoniae and N. meningitidis. The initial adhesion step seems to be mediated by the binding of bacterial adhesins, such as the outer membrane protein porin A for N. meningitidis and the pneumococcal surface protein PspA for S. pneumoniae, to the laminin receptors expressed on brain endothelial cells61. The adhesion process is described in Fig. 3 and involves several factors, including the platelet-activating factor receptor (PAFR) on the endothelial cell surfaces, ultimately facilitating transcellular (involving receptor-mediated mechanisms) passage of S. pneumoniae and paracellular (following barrier disruption) passage of N. meningitidis through the blood–brain barrier. This process in N. meningitidis includes the pilus components PilE and PilV, which mediate bacterial adhesion by interacting with the immunoglobulin superfamily member CD147 on the host endothelial cells62. Following primary adhesion, the meningococcal type IV pilus mediates the recruitment and activation of the β2-adrenoceptor, triggering the organization of specific molecular complexes named cortical plaques in the corresponding underlying cytoplasm63. Plaque formation is accompanied by the local stimulation of actin polymerization, resulting in the formation of membrane protrusions that protect bacterial colonies from the complement-mediated lysis and opsonophagocytosis in the blood. Cortical plaques also stimulate the opening of the inter-endothelial junctions, which allows N. meningitidis to migrate to the CSF by slipping through adjacent cells (a paracellular route)64.
Other pathogens. E. coli K1 binding to and invasion of the brain endothelium is thought to involve several bacterial proteins, including the type 1 fimbrial adhesion protein FimH, cytotoxic necrotizing factor 1 (CNF1), invasion of brain endothelial cell proteins (Ibe) and OmpA. FimH (presumably via interacting with endothelial CD48) and CNF1 (via co-opting the 37/67 kDa laminin receptor) can induce cytoskeletal rearrangements through the activation of the GTPase RHOA, ultimately leading to bacterial invasion. In addition, binding of IbeA and OmpA to their respective putative receptors, vimentin or polypyrimidine tract-binding protein (PTB)-associated splicing factor (PSF; also known as SFPQ) and Ecgp96 (a β-form of the heat shock protein gp96 that is expressed on human brain-derived endothelial cells), can trigger the activation of RAC1, another member of the RHO GTPase family, thereby contributing to cytoskeletal rearrangements and bacterial internalization47,65,66. Ecgp96 also forms complexes with TLR2 and type 1 angiotensin II receptor (AT1AR)67,68. This interaction can activate protein kinase Cα (PKCα), which in turn can associate with vascular endothelial (VE)-cadherin at the endothelial tight junctions. This interaction promotes the dissociation of β-catenin from the endothelial tight junctions, which increases endothelial permeability. Thus, E. coli seems to be capable of invading the central nervous system (CNS) via the blood–brain barrier by both transcellular and paracellular pathways.
Group B streptococci possess many virulence factors that can promote bacterial interaction with the brain endothelium, including lipoteichoic acid, β-haemolysin, serine-rich repeat proteins and hypervirulent group B streptococcus adhesin laminin-binding protein (LMB), bacterial surface adhesin of group B streptococcus (BsaB), streptococcal fibronectin-binding protein A (SfbA) and the pilus tip protein PilA. LMB, BsaB and SfbA function by associating with extracellular matrix components, such as fibronectin44,47,69, whereas PilA can bind to collagen, which promotes its interaction with endothelial α2β1-integrins. This interaction leads to the activation of focal adhesion kinase and a subsequent intracellular signalling pathway that, among other effects, can result in phosphoinositide 3-kinase-dependent actin rearrangement and bacterial uptake70. Moreover, group B streptococci can induce the expression of the host transcriptional repressor Snail1, which impedes the expression of tight junction genes. This process involves bacterial cell wall components and extracellular signal-regulated kinase 1 and 2 (ERK1/2)–mitogen-activated protein kinase (MAPK)-dependent signalling and can cause disruption of the blood–brain barrier, facilitating the paracellular passage of group B streptococci into the CNS71.
Immune activation in bacterial meningitis
Once the pathogens reach the CSF, they are likely to survive because host defences in the subarachnoid space seem to be ineffective against encapsulated bacteria72. This immune deficit might be owing to the virtual absence of soluble PRRs, such as complement proteins, which bind to the surface of pathogens and mark them for phagocytosis. Moreover, complement deposition can cause lysis of susceptible bacterial species, namely, Neisseria spp., through membrane attack complex (MAC) formation. However, in normal CSF, complement levels are too low (100–1,000-fold lower than in blood) to exert substantial antibacterial activity. Blood complement proteins are prevented from entering the CSF by the blood–brain barrier, which acts like a molecular sieve to restrict the passage of large molecules and immunocompetent cells73. Even in the presence of high levels of inflammation (as in bacterial meningitis), which abnormally increases the permeability of the blood–brain barrier, complement levels remain substantially below those in the blood. As a consequence, when pathogens succeed in invading the CSF, they can grow efficiently and achieve a high population density within hours38.
PRRs. With increased bacterial density, the pathogens begin to die in response to various stress conditions, such as nutrient deprivation and antibiotic treatment74. As a result, bacterial fragments can accumulate within the CSF. Their interaction with surface-bound or intracellular PRRs that are expressed by immunocompetent cells can trigger the host immune response (Fig. 4). Unlike in the brain parenchyma, functionally active macrophages, dendritic cells and mast cells are present in sufficient numbers in tissues lining the CSF. Each of these cell types is a potential sentinel of bacterial invasion. However, animal studies addressing this topic are scarce. In a rat model of pneumococcal meningitis, depletion of meningeal and perivascular macrophages was associated with increased bacterial titres and decreased leukocyte counts in the CSF75. In a rabbit model, macrophage depletion was ineffective in dampening meningeal inflammation and neuronal injury, which questions the role of these cells as major initiators of inflammation in bacterial meningitis76. Cells of the arachnoid mater and pia mater are also capable of producing and releasing a vast variety of pro-inflammatory factors upon exposure to N. meningitidis, S. pneumoniae and E. coli K1 (Ref. 77), and might therefore detect pathogens within the CSF.
Streptococcus pneumoniae pathogen-associated molecular patterns (PAMPs) can activate immune cells within the cerebrospinal fluid (CSF) through membrane-bound Toll-like receptor 1/2 heterodimers (TLR1/2), TLR4 and TLR9, as well as cytosolic nucleotide-binding oligomerization domain (NOD)-like receptors, NOD2, NOD-, LRR and pyrin domain-containing 3 (NLRP3) and other yet unidentified pattern recognition receptors (PRRs). Pneumococcal lipoteichoid acid (LTA), pneumolysin (Ply) and DNA are recognized by TLR1/2 (Refs 207,208), TLR4 (Ref. 209) and TRL9, respectively, whereas internalized peptidoglycan (PG) and muramyl dipeptide (MDP) are recognized by NOD2. PG might also be sensed by TLR1/2, although this has not been definitively shown; similarly, the detection of Ply by TLR4 is also under debate83. Notably, TLR9, which is localized within endosomes, has only been shown to recognize S. pneumoniae DNA in vitro210. However, experiments in mice carrying a single point mutation in UNC93B1, which encodes a multi-pass transmembrane protein required for several TLRs211,212, have suggested that one or more of these receptors have a key role in pneumococcal sensing within the CSF (U.K., unpublished observations). Meningococcal porin B (PorB), Lip antigen and Neisseria hia/hsf homologue (NhhA) are recognized by TLR1/2, lipooligosaccharide (LOS) by TLR4 and DNA by TLR9. These interactions result in the activation of the enzyme caspase 1 (Casp1), which catalyses the conversion of the pro-form of IL-1 family cytokines into the active molecule, and of transcription factors, such as nuclear factor-κB (NF-κB), which in turn regulates the production of diverse pro-inflammatory factors (including neutrophil chemokines CXC-chemokine ligand 1 (CXCL1), CXCL2 and CXCL5 and the anaphylatoxin C5a). Indeed, long-lasting NF-κB activation is detectable in the brains of infected mice, especially in areas of heavy inflammation and along penetrating cortical vessels. Moreover, pharmacological blockade of NF-κB markedly attenuated pneumococci-induced inflammation in a rat model213,214. Consequently, large numbers of polymorphonuclear leukocytes (PMNs) are recruited. These infiltrating cells can release numerous cytotoxic products, including reactive oxygen species (ROS) or reactive nitrogen species (RNS) that can cause necrotic cell death. Damaged bacterial cells can release alarm signals (so-called damage-associated molecular patterns (DAMPs)), including myeloid-related protein 14 (MRP14) and high-mobility group box 1 (HMGB1), which can fuel inflammation by interacting with PRRs, such as TLR4 and receptor for advanced glycation end products (RAGE).
For N. meningitidis, our understanding of the inflammatory process within the subarachnoid space has been largely limited by its human host specificity, hampering the ability to reproduce the infection in animals64. By contrast, well-characterized animal models of S. pneumoniae, group B streptococci and E. coli meningitis are available that closely recapitulate human disease74. With respect to S. pneumoniae, the seminal studies involved the injection of pneumococcal cell wall components into the CSF of laboratory animals to provoke clinical signs of meningitis78,79. Indeed, high pneumococcal cell wall concentrations have been associated with functional clinical outcomes, as determined by the Glasgow Outcome Scale, in patients with pneumococcal meningitis80. In the past 15 years, a handful of PRRs have been identified that can detect S. pneumoniae within the CSF38. These include surface-bound TLRs and cytosolic nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), which function as sensors for cytoplasmic pathogen-associated molecular patterns (PAMPs; Fig. 4). NLRs are likely to be required to achieve maximum inflammation against S. pneumoniae, as NOD2-deficient mice exhibited substantially lower levels of inflammatory mediators in the brain than wild-type mice following intracerebral S. pneumoniae administration38,81. Accordingly, S. pneumoniae-induced inflammation activation in vitro depends on the presence of NOD2 (but not NOD1)82.
Another potential sensor of pneumococcal infection of the subarachnoid space is NOD-, LRR- and pyrin domain-containing 3 (NLRP3). In cell culture experiments, NLRP3 activation was induced through pore-building bacterial toxins, such as pneumolysin (Ply)83,84. In a mouse meningitis model, NLRP3 deficiency was associated with a markedly diminished immune response to pneumococcal infection, and NLRP3-dependent secretion of IL-1β into the CSF was substantially lower following infection with Ply-deficient rather than with Ply-producing pneumococci85. Moreover, in patients with bacterial meningitis, the levels of IL-1β and IL-18 in the CSF are related to complications and unfavourable disease outcomes86.
Activation of the complement cascade. The activation of TLRs and NLRs leads to the activation of inflammatory transcription factors, in particular, NF-κB. In turn, complement proteins are among the most crucial inflammatory mediators that are produced upon NF-κB activation, particularly in pneumococcal meningitis. The complement system consists of >30 regulators and receptors and serves two main functions: to kill bacteria, either directly via MAC formation or by labelling them for phagocytosis (for example, C1q or C3b), and to enhance the inflammatory response via the release of anaphylatoxins (for example, C3a or C5a) or the terminal complement complex (soluble C5b-9)87.
Analyses of CSF samples collected from patients with pneumococcal meningitis showed that C5a and C5b-9 concentrations were markedly increased during the acute stage of the disease and correlated positively with CSF leukocyte counts and disease severity88,89. Similarly, in a rabbit model of pneumococcal meningitis, C5-derived chemotactic activity largely accounted for the accumulation of neutrophils in the CSF90. In a mouse model of pneumococcal meningitis, C5a deficiency was associated with a drastic reduction in CSF pleocytosis and brain cytokine production88. Moreover, treatment of infected, wild-type (that is, complement sufficient) mice with anti-C5 antibodies and an antibiotic 24 hours after infection was protective against meningitis-induced brain damage, presumably through its anti-inflammatory action88. Combined treatment with anti-C5 antibodies and dexamethasone has been reported to improve survival in severe experimental pneumococcal meningitis91.
Of note, the effect of anti-C5 antibodies in bacterial meningitis other than pneumococcal meningitis is unclear and needs to be carefully evaluated before considering their use in patients. For example, treatment of patients with paroxysmal nocturnal haemoglobinuria or atypical haemolytic uraemic syndrome with an anti-C5 antibody (eculizumab) was associated with an increased risk for fungal and bacterial infections, primarily meningococcal infections92. Thus, an alternative approach might be to use anti-C5a antagonists that are more selective than eculizumab, which have been shown to inhibit the potentially harmful effects of N. meningitidis-induced C5a formation while preserving complement-mediated meningococcal killing via MAC93.
Brain injury
Histopathological studies document a wide range of brain injuries associated with bacterial meningitis in humans, including brain oedema formation, hydrocephalus, petechial haemorrhages, necrotic lesions in cortical and subcortical structures and loss of myelinated fibres in the white matter94,95. In addition, a recent autopsy study uncovered mild-to-moderate hippocampal apoptosis in 26 out of 37 (70%) cases96. By contrast, no significant differences in the number of apoptotic cells in the hippocampus were detected between patients with meningitis and control patients in a more recent study94. In animal models of the disease, the occurrence and degree of neuronal apoptosis depend on multiple factors, such as the age, strain and species of the animal used as well as the causative pathogen97–99.
The mechanisms underlying hippocampal apoptosis are not fully identified; in experimental bacterial meningitis, apoptotic cell death is thought to occur through caspase-dependent or mitochondrial cytochrome c-induced, apoptosis-inducing factor-dependent signalling events, depending on the time after infection and the causative pathogen95,100. Thus, treatment with caspase inhibitors might rescue only a fraction of the stressed hippocampal neurons. Of note, broad-spectrum caspase inhibitors, such as z-VAD-fmk, can also dampen inflammation by blocking the production of IL-1β and IL-18. Neutrophilic inflammation is a well-established contributor to meningitis-related tissue injury38. Neutrophils are armed with a collection of chemical weapons, such as oxidants and proteases. Their release cannot only be harmful to the pathogen but also to the host itself38. Besides neutrophils, microglial cells and astrocytes are a potential source of cytotoxic factors. In mouse astrocytes, Ply can initiate the release of the excitotoxic amino acid glutamate101. Similarly, mouse microglial cells can release toxic nitrogen species as well as pro-inflammatory cytokines upon exposure to Ply38. However, there is still no direct in vivo evidence (for example, no studies using microglia depletion models) to support the involvement of these cell types in brain pathology due to meningitis.
In this context, in post-mortem studies, S. pneumoniae has been detected in the subarachnoid, perivascular and ventricular spaces, but generally not (except in the rare case of abscess formation) within the brain parenchyma102. Moreover, leukocyte infiltration into the brain parenchyma has only been observed during late infection and in the direct vicinity of the fluid-filled spaces94. This distribution pattern argues against a dominant role of direct bacterial-derived and host-derived toxin-induced cytotoxicity in meningitis-related brain damage. Instead, brain damage is likely to be mediated to a greater extent by pathological changes in the vasculature103. The predominant findings in patients are vasculitis (an arterial narrowing due to severe inflammation of the vessel wall) and/or vasospasm, causing cerebral hypoperfusion and ischaemia104,105. Cerebral infarction can also occur as a result of thrombosis, embolization or a combination of both106,107.
Diagnosis, screening and prevention
Clinical presentation
Bacterial meningitis is a medical emergency: early recognition and immediate treatment are essential108. Neonates with bacterial meningitis often present with nonspecific signs and symptoms, such as poor feeding, irritability, hypertonia or hypotonia and respiratory distress8. Fever and seizures affect <40% and <35% of infected babies, respectively109. Patients with bacterial meningitis beyond the neonatal age commonly present with headache, photophobia, nausea and vomiting110.
Patients with bacterial meningitis often show signs of coexisting systemic compromise (that is, meningococcal or pneumococcal sepsis)1, which is associated with poor disease outcome. Furthermore, one systematic assessment of the development of early symptoms in children and adolescents with meningococcal disease (including sepsis) in the United Kingdom showed that rash, impaired consciousness and the other typical meningitis signs develop late in the pre-hospital illness, if at all, implicating that physicians should be aware that early recognition of bacterial meningitis can be difficult111.
A prospective nationwide study of 1,268 adults with community-acquired bacterial meningitis in the Netherlands showed that classic features of meningitis, such as headache (83% of patients), neck stiffness (74%), fever (≥38 °C; 74%) and impairment of consciousness (defined as a score of <14 on the Glasgow Coma Scale; 71%), were present in a high proportion of patients7. However, the ‘classic triad’ signs (neck stiffness, fever and altered mental status) were reported in only 41% of patients1. Other possible symptoms include focal neurological deficits, such as aphasia and hemiparesis, seizures or cranial nerve palsies7. Petechial skin rash, which is usually considered the hallmark of meningococcal infection, can also be observed in pneumococcal meningitis1.
Meningeal irritation manifests at physical examination as neck stiffness, the Kernig sign (painful knee extension after flexing the thigh with the hip and knee at 90° angles) and the Brudzinski sign (reactive hip and knee flexure when the neck is flexed)108. Neck stiffness is tested by passively flexing the neck, and if the manoeuver is painful and the chin cannot be brought to the chest. Notably, a prospective study of 297 adults in the United States showed that these signs do not accurately identify patients with meningitis, as they all showed poor sensitivity (5–30%) and high specificity (68–95%); meningitis was defined as >6 leukocytes per μl of CSF112. Doctors rely on multiple tests to diagnose meningitis, and the combination of test results and clinical characteristics indicates which further investigations will be appropriate.
Differential diagnosis
Bacterial meningitis is difficult to diagnose, as many illnesses share its symptoms. The differential diagnosis includes brain abscess113, tuberculous meningitis, viral encephalitis or septic encephalopathy, as well as benign conditions such as aseptic (that is, non-bacterial) meningitis or sinus infection. Depending on the setting, malaria, arboviral infections, HIV-related and parasitic infections of the CNS and mumps should be considered. Most patients with suspected bacterial meningitis will eventually receive a different diagnosis. In the United Kingdom, in a prospective study of 388 children with suspected bacterial meningitis, only 3% were actually affected, whereas 62% had a non-CNS infection and 16% had a non-infectious condition114. Similar results were found in a Swiss study in patients of all ages115.
Making the diagnosis
Several diagnostic algorithms have been developed to help predict the likelihood of bacterial meningitis116,117. Most of these algorithms aim to discriminate bacterial from aseptic (viral) meningitis in paediatric populations118, and can be used in patients with suspected acute bacterial meningitis to determine whether a patient needs further diagnostic studies (for example, CSF analysis) or immediate therapy117. CSF examination is essential to confirm or rule out bacterial meningitis and to identify other non-bacterial CNS infections or inflammatory neurological diseases included in the differential diagnosis. Typical CSF and serum characteristics that are assessed in the diagnosis of bacterial, viral and tuberculous meningitis are provided in Table 2. However, the physician first needs to verify whether the lumbar puncture is safe for the patient. A space-occupying intracranial lesion or diffuse brain oedema, which both cause a substantial brain shift, results in an increased risk of cerebral herniation when a lumbar puncture is performed108. A history of CNS lesions, new-onset seizures, focal neurological deficits, an immunocompromised state (for example, HIV/AIDS infection or immunosuppressive medication after organ transplantation) or a moderate-to-severe impairment of consciousness have been identified as predictors of abnormalities on cranial CT119. Other contraindications for immediate lumbar puncture are coagulation disorders, septic shock and respiratory failure2. Thus, cranial imaging (by CT scan) might be indicated to rule out this possibility. However, because preliminary imaging delays treatment and can worsen outcomes, physicians must select who needs it and who can immediately and safely undergo lumbar puncture2.
Classic abnormalities of the CSF in bacterial meningitis include pleocytosis (mainly of polymorphic leukocytes), low glucose concentration and increased protein levels117, which are signs of a self-propelling inflammatory response in the subarachnoid space caused by the accelerating bacterial growth. These three parameters are individual predictors of bacterial meningitis120, and at least one was present in 96% of 1,268 patients with community-acquired bacterial meningitis in a prospective cohort7. However, in neonates with bacterial meningitis, CSF examination often does not show an increased leukocyte count121. A measurement of CSF lactate concentration can be performed using a widely available, cheap and rapid diagnostic test that differentiates between bacterial and viral meningitis, although it has limited usefulness in patients who have been pre-treated with antibiotics before the lumbar puncture or with other CNS diseases in the differential diagnosis117,121. Thus, because CSF examination is not definitive, if bacterial meningitis is suspected, antimicrobial therapy should be started immediately and maintained until CSF culture results are negative8.
The gold standard for the diagnosis of bacterial meningitis is CSF culture, which is positive in 50–90% of patients (although the yield is lower when CSF is collected once antibiotic treatment has started) depending on the causative pathogen8. The specific bacteria can be identified by blood cultures in 50–80% of cases, making blood cultures a valuable, readily available alternative diagnostic tool8. CSF Gram staining is also a well-validated and rapid method to detect bacteria in the CSF, with a reported yield of 70–90% and 30–90% for pneumococcal and meningococcal meningitis, respectively8.
PCR on CSF has been increasingly used for the diagnosis of bacterial meningitis117,122, with real-time PCR reported to have better sensitivity, through-put, speed and specificity than conventional PCR123. The reported sensitivity of conventional PCR was 79–100% and 91–100% for S. pneumoniae and N. meningitidis, respectively8. The specificity of a multiplex PCR approach simultaneously detecting S. pneumoniae, N. meningitidis and H. influenzae DNA was generally high (95–100% for all microorganisms)124. PCR was shown to have incremental value compared with CSF culture and Gram stain, as it can provide a diagnosis when conventional methods fail125. For example, PCR is especially useful in patients who started antibiotic treatment before the lumbar puncture, as in these individuals, CSF and blood cultures are often negative. In several countries, such as the United Kingdom and Spain, PCR has become the standard (if not the only) method for confirmation of meningococcal disease126,127. Furthermore, false-negative PCR results are uncommon (about 5% of cases)8. Disadvantages of PCR compared with CSF culture include the lack of an isolate on which traditional antimicrobial susceptibility testing, serogrouping and serotyping can be performed. However, PCR assays are now available for both serogrouping and serotyping, and are used for surveillance and vaccine evaluation128.
Antigen and immunochromatographic tests provide tools for rapid identification of the pathogen8,109. Latex agglutination testing in CSF has a widely varying reported sensitivity depending on the causative pathogen: for example, 59–100% and 22–93% for S. pneumoniae and N. meningitidis, respectively8. Furthermore, the sensitivity of latex agglutination tests was shown to drop considerably in patients who had started treatment before undergoing lumbar puncture129. The efficacy of immunochromatographic antigen testing in CSF was assessed in large studies in children with suspected acute bacterial meningitis130. These studies showed a 98.6–100% sensitivity and a 99.3–100% specificity for pneumococcal meningitis8, although false-positive results have been reported in patients with meningitis due to other streptococcal species109.
C-reactive protein and pro-calcitonin have been advocated as diagnostic serum markers, enabling differentiation between bacterial and viral meningitis117. Both C-reactive protein and pro-calcitonin are acute-phase inflammation proteins that are stimulated by cytokines (for example, IL-1, IL-2, IL-6 and tumour necrosis factor) that play an important part in the pathophysiology of bacterial meningitis. However, although these markers have the potential to differentiate between bacterial and viral infection in general, their values in patients with suspected bacterial meningitis have not yet been studied117.
Severely immunocompromised patients, such as untreated HIV-positive individuals or patients who have received bone marrow or solid organ transplantation, can develop bacterial meningitis, but might present with only a minimal inflammatory response28,131,132. In these patients, CSF Gram stain might be especially important, showing high bacterial loads with minimal leukocytosis. Although the majority of these patients will eventually be diagnosed with pneumococcal meningitis, uncommon pathogens should also be suspected8.
Vaccine-based prevention
Bacterial meningitis is in part a preventable disease, as vaccines are available against the most common causative pathogens4 (Table 3). The first generation of meningococcal, pneumococcal and Hib vaccines were made from purified capsular polysaccharides4. Although the vaccines were useful, their effectiveness among children <2 years of age was limited. Contemporary vaccine formulations were made with capsular polysaccharides conjugated to carrier proteins; protein–polysaccharide vaccines trigger a T cell-dependent immune response, which can be elicited even in young infants. By contrast, vaccines that target N. meningitidis serogroup B are made with protein antigens.
Hib conjugate vaccines. Hib conjugate vaccines, first used in 1987 in the United States, are now available from several manufacturers as either a monovalent vaccine or as part of a polyvalent vaccine that includes some combination of pertussis, diphtheria, tetanus, polio, meningococcal disease or hepatitis B antigens4. Hib and pneumococcal conjugate vaccines are given to infants in 3–4 dose series4, whereas older children require fewer doses. This vaccine has been successful in reducing the incidence of Hib infection in the susceptible childhood population (Box 1).
Pneumococcal conjugate vaccines. A pneumococcal conjugate vaccine targeting the seven serotypes that cause the most severe pneumococcal infections in high-income countries was first licensed in 2000. In late 2009 and early 2010, second-generation conjugate vaccines targeting 10 or 13 serotypes became available, with the 13-valent vaccine replacing the earlier 7-valent version4. The 10-valent and 13-valent formulations both include an antigen that targets serotype 1, a frequent cause of meningitis in many low-income and middle-income countries18. Some national immunization policies recommend pneumococcal vaccines for adults who are at higher risk of pneumococcal infection because of older age or immunocompromising or chronic medical conditions such as sickle cell disease133. The 13-valent vaccine prevented invasive pneumococcal disease and pneumonia caused by the covered serotypes among the elderly population in a large clinical trial134. The 23-valent pneumococcal polysaccharide vaccine, available since 1983, is more often recommended for adults, although the 13-valent conjugate vaccine is now also licensed for adults and recommended routinely for adults ≥65 years of age in the United States135.
Meningococcal vaccines. Of the 12 known meningococcal serogroups, vaccines are available for serogroups A, B, C, Y and W, either in single (A, B or C) or multiple (A/C, A/C/Y or A/C/Y/W) serogroup-targeting versions. Conjugate vaccines are used for routine disease prevention, whereas either conjugate or polysaccharide versions are used for emergency control of outbreaks or epidemics. Vaccination of high-risk individuals (for example, those with asplenia or complement deficiency) is common, but universal vaccination policies vary between countries. In the United Kingdom, serogroup C vaccination has been implemented in England and Wales since 1998 and serogroup B vaccination in 2015 (see ‘Outlook’ section)136. As part of this programme, meningococcal vaccines targeting serogroups B and C are given routinely to all infants, and teenagers receive a booster dose with an A/C/Y/W conjugate vaccine136. In the United States, where meningococcal disease rates are low overall in the general population but somewhat higher in adolescents and young adults, a 4-valent vaccine targeting serogroups A, C, Y and W is recommended in children between 11 and 12 years of age, with a booster dose at 16 years of age; a more permissive recommendation for serogroup B vaccine has been given by a recent policy, which states that the vaccine may be used for individuals 16–23 years of age who are at increased risk of meningococcal disease137. MenAfriVac (Serum Institute of India Pvt. Ltd, Pune, India), a serogroup A monovalent conjugate vaccine specifically made to be affordable for low-income settings, was first introduced in Burkina Faso in 2010 (Ref. 138). The WHO recommends MenAfriVac vaccination to target all individuals 1–29 years of age in all meningitis belt countries and the establishment of routine vaccination programmes for children 9–18 months of age138. Early reports on serogroup A disease control in the meningitis belt (Fig. 2) are promising14.
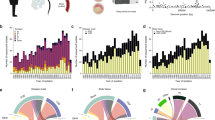
Effect of vaccine use on meningitis rates. Hib and pneumococcal conjugate vaccine programmes have resulted in substantial drops in the rates of meningitis caused by the covered bacterial strains12,139 (Fig. 5). Pneumococcal conjugate vaccines reduced pneumococcal carriage, transmission and meningitis not only in vaccinated children but also in older non-vaccinated individuals via herd protection7,12,139. However, the widespread use of the 7-valent pneumococcal vaccine led to an increase in pneumococcal diseases caused by bacterial strains that were not covered by the vaccine, a phenomenon known as replacement disease140; whether a similar effect will occur with the 10-valent and 13-valent vaccines remains unclear.
Incidence (left axis, bars) and average age at diagnosis (right axis, line) are shown for patients with bacterial meningitis in the United States from 1971 to 2007 (Refs 10,215–217). The decreasing proportion of disease caused by Haemophilus influenzae type b (Hib) and, later, Streptococcus pneumoniae (SP) and the increase in overall age of remaining cases show the effect of routine infant vaccination programmes. GSB, group B streptococcus; LM, Listeria monocytogenes; NM, Neisseria meningitidis.
Vaccination policy and advocacy. Pneumococcal and Hib conjugate vaccines are recommended by the WHO for routine use in infant immunization programmes globally and are widely used13,141. Meningococcal vaccines are not routinely recommended for use in countries where rates are generally low, but are increasingly used in epidemic-prone areas or risk groups with higher rates of endemic disease. Conjugate vaccines targeting group B streptococcal infections are in development21. Group B streptococcal vaccines in development are being designed for use in pregnant women to protect newborns through transplacental antibody transfer.
Meningococcal vaccine campaigns are used to control disease outbreaks. Updated guidelines from the WHO specify when mass vaccination campaigns should be ordered to fight epidemic meningitis in Africa142: when the number of suspected meningitis cases has reached five in a week or has doubled over a 3-week period in a population of <30,000 people, or when the number has reached ten in a week in populations of 30,000–100,000 people, mass vaccination should then start as soon as possible and within 4 weeks. For refugee populations or institutional outbreaks, vaccination is recommended when two cases of meningococcal meningitis are detected within a week142.
Chemoprophylaxis
Antibiotic prophylaxis is recommended for individuals who have had close contact with patients with meningococcal meningitis or bacteraemia (for example, household members) immediately after exposure109,143. Although the risk of nosocomial meningitis in patients with basilar skull fracture is high3, antibiotic prophylaxis has shown no clear benefit in these patients3. Several guidelines recommend universal screening for rectovaginal colonization by group B streptococci in pregnant women at 35–37 weeks of gestation, with intrapartum antibiotic prophylaxis for patients who test positive. This strategy has decreased neonatal early-onset group B streptococcal meningitis in the United States144, but a similar risk-based chemoprophylaxis in the Netherlands did not prove beneficial21.
Management
Given the high mortality of acute bacterial meningitis, treatment should be started in suspected cases even before the diagnosis can be confirmed109. The vital functions of the patient should be evaluated and weighted with the degree of suspicion for bacterial meningitis. Blood cultures and blood tests should be performed immediately143. Collection of CSF for examination and culture should be done as soon as possible. Even if a head CT scan is indicated before CSF collection, antimicrobial therapy should not be withheld, as a delay can result in a higher probability of adverse clinical outcomes145.
Antibiotic therapy
Empirical antibiotic therapy should be bactericidal and achieve adequate CSF levels. Both these parameters depend on lipid solubility, molecular size and protein-binding capacity of the antibiotic and on the patient's degree of meningeal inflammation143 (Table 4). The choice of the initial empirical antibiotics should be based on age, local epidemiological patterns of pneumococcal resistance and the need to add amoxicillin or ampicillin against L. monocytogenes143. Treatment for neonatal bacterial meningitis should cover at least E. coli and Streptococcus agalactiae8; indeed, outbreaks of extended-spectrum β-lactamase-positive E. coli meningitis in neonatal wards have been described146. Listeria spp. are resistant to cephalosporin; thus, amoxicillin or ampicillin should be given to all immunosuppressed patients with meningitis, including pregnant women or patients >60 years of age143. Once the causative pathogen and its specific antimicrobial susceptibility have been determined, the antibiotic therapy must be optimized for targeted treatment (Fig. 6).
Examples of first-line and alternative antibiotic therapies for bacterial meningitis based on the causative pathogen and its in vitro susceptibility test. Alternative therapies are prescribed if there are contraindications to the recommended treatment. MIC, minimum inhibitory concentration.
Penicillin resistance among S. pneumoniae strains has been increasing worldwide, changing the initial therapy of patients with bacterial meningitis in several parts of the world143. In March 2008, the Clinical and Laboratory Standards Institute (CLSI) revised the susceptibility breakpoints of penicillin against S. pneumoniae. In pneumococcal meningitis, penicillin resistance occurs when the minimum inhibitory concentration (MIC) is ≥0.2 μg per ml, and third-generation cephalosporin resistance is defined as an MIC of ≥2 μg per ml147. According to these criteria, the prevalence of penicillin resistance was 9% in 2010 in Europe148,149 and 35% in 2012 and 2013 in the United States, where 21% of the cases had multidrug resistance (defined as resistance to at least two other classes of antibiotics besides penicillin)150. Because of the variable geographical distribution of penicillin resistance, it is important to know the regional patterns when deciding on local empirical antibiotic therapy143. Penicillin resistance is also associated with decreased susceptibility to other antibiotics. Thus, guidelines from the Infectious Diseases Society of America (IDSA) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) recommend vancomycin and a third-generation cephalosporin as part of the initial treatment; however, in countries where the prevalence of cephalosporin-resistant pneumococcus is <1%, ceftriaxone alone is appropriate109,151. Empirical therapy should consist of vancomycin combined with either cefotaxime or ceftriaxone in areas with cephalosporin resistance109 (Fig. 6).
N. meningitidis strains with reduced susceptibility to penicillin have been associated with increased risk of poor disease outcome in children with meningococcal meningitis152. Strains with reduced susceptibility to penicillin resistance accounted for nearly 12% of strains in 2011 in the United States, although <1% were fully resistant153; however, all isolates remain susceptible to ceftriaxone, which is, therefore, the empirical antibiotic of choice in suspected meningococcal meningitis.
L. monocytogenes should be suspected in neonates, adults >50 years of age and immunosuppressed individuals. In these groups, amoxicillin or ampicillin should be added to the empirical therapy, as cephalosporins have no activity against Listeria spp.143. Retrospective clinical data indicate that gentamicin can reduce mortality in listerial meningitis154. However, another study showed that adding an aminoglycoside (such as gentamicin) to the treatment was associated with increased rates of kidney injury and mortality155.
International guidelines on the duration of treatment109 recommend 7–10-day treatment for H. influenzae or N. meningitidis meningitis, a 10–14-day treatment for S. pneumoniae meningitis143,151 and a prolonged, 21-day treatment for L. monocytogenes meningitis109. Efficacy and safety of 5-day versus 10-day ceftriaxone regimens were compared in a multi-country randomized study involving 1,004 children with bacterial meningitis156. No significant difference was found between groups in bacteriological failures (none of the patients in both groups had persistent positive CSF cultures 6–40 days after starting therapy) or relapses (2 out of 496 patients (0.4%) in the 5-day group versus 0 out of 508 patients (0%) in the 10-day group (risk difference: −0.4 (95% CI: −0.15–0.96)); however, the sample sizes of aetiological subgroups were relatively small, so caution is advised when extrapolating these results.
Adjunctive therapies
Despite antibiotic therapy, the mortality for bacterial meningitis has remained at 20–25% for several decades2. The high morbidity and mortality prompted the investigation of several adjunctive therapies in animal models38, which, unfortunately, have delivered poor performances in subsequent clinical trials so far, with the exception of steroids. The common goal of adjunctive therapies is to reduce inflammation-related neuronal death and brain damage. Bactericidal antibiotics lyse pathogens, causing the release of pro-inflammatory bacterial components that trigger the host immune response, which in turn contributes to brain damage. Some of the failed adjunctive treatments include therapeutic hypothermia, which resulted in increased mortality157, paracetamol, which did not lead to any improved outcomes158, and glycerol, which did not reduce or sometimes even increased death or neurological morbidity159,160.
Following several paediatric studies161,162, a large multi-centre European randomized controlled trial in adults showed a significant reduction of unfavourable outcomes and death in patients who were treated with adjunctive dexamethasone (an anti-inflammatory corticosteroid) in addition to antibiotic therapy compared with patients who only received antibiotics, with the most striking results observed in the subgroup of patients with pneumococcal meningitis163. A post-hoc analysis of 25 studies using several different antibiotics showed that dexamethasone mainly reduced deaths in pneumococcal meningitis by preventing systemic complications164. Other meta-analyses showed that corticosteroids significantly reduced hearing loss and neurological complications but not overall mortality161,165,166, whereas subgroup analyses showed corticosteroid-associated reduction of severe hearing loss in children with H. influenzae meningitis and mortality in adults with S. pneumoniae meningitis161. Notably, corticosteroids had no beneficial effects for patients in low-income countries165, most likely because patients present late in the course of the disease, when the inflammatory process in the CNS has already started. The results from the aforementioned studies imply that the effect and efficacy of adjunctive dexamethasone depend on the type of meningeal pathogen, the age of the patient and the income level of the countries. Dexamethasone is the only adjunctive therapy that has been advocated by the IDSA and ESCMID guidelines109,151.
Available evidence suggests that, in high-income countries, dexamethasone treatment should be started with or even before the first dose of antibiotics2 and continued for 4 days in both children and adults (although with different dose regimens)2,109. Guidelines recommend suspending dexamethasone treatment if the bacterial meningitis diagnosis is not confirmed or if the causative pathogen is other than H. influenzae or S. pneumoniae (although some experts advise dexamethasone to be continued irrespective of the meningeal pathogen)109. In patients with bacterial meningitis and severe sepsis or septic shock, the survival benefit provided by corticosteroid treatment outweighs the potential risks of high-dose steroid administration in sepsis143.
Intensive care
Patients with bacterial meningitis should be admitted to an intensive care unit, where the patient's consciousness and the development of complications, such as brain infarctions, hydrocephalus and seizures, can be closely watched2. Such heightened medical care can improve the outcome, but nevertheless a fulminant course might inevitably result in permanent damage or brain death. Furthermore, some patients seem to be refractory to treatment and their condition can escalate to a major systemic infection.
Occasionally, CT scanning can provide important direction for treatment. In patients with clinical deterioration, serial CT scanning might show cortical and sylvian fissure effacement, compression of the ventricles and eventually obliteration of the basal cisterns. Some experts would then advocate the standard treatment for intracranial pressure167: high doses of corticosteroids, osmotic diuretics, decompressive craniectomy and ventriculostomy in case of hydrocephalus. However, no conclusive evidence supports this treatment option and such aggressive strategies might be more harmful than beneficial168. Nevertheless, in case of impeding brain herniation, placement of an intracranial pressure monitor is advised, followed by continuous osmotic therapy to reduce high intracranial pressure169. Hypertonic saline might also control the commonly observed hyponatraemia170. Serial CT scanning can also show the development of hydrocephalus, which, in a prospective case series, was diagnosed in 5% of patients171. In case of hydrocephalus, repeated lumbar punctures or placement of an external lumbar drain can reduce intracranial pressure. Invasive procedures should be withheld in patients with mild enlargement of the ventricular system without clinical deterioration. Seizures, particularly focal, can be attributed to focal oedema, early cortical venous thrombosis and cerebral infarction from occlusion of penetrating branches that are encased by the basal purulent exudate172.
Palliative care (supportive care only) might be necessary owing to a poor neurological prognosis. However, early withdrawal of therapy in patients with preserved brainstem reflexes is inappropriate, as these seemingly hopeless patients can actually survive and some fully recover173. In one prospective cohort study, 3% of patients with bacterial meningitis had a score of 3 on the Glasgow Coma Scale (that is, totally unresponsive)174, and although such low levels of consciousness are commonly associated with high morbidity and mortality, as many as 20% of these patients will make a full recovery174, stressing the need for continued supportive care.
Quality of life
Individuals who have had bacterial meningitis (particularly pneumococcal meningitis) are at high risk of neurological complications that affect their quality of life175–178. About half of survivors experience focal neurological deficits, such as hearing loss, epilepsy and cognitive impairment179–182. A meta-analysis showed that the risk of major sequelae was twice as high in low-income countries compared with high-income countries181. A systematic review (which combined data from high-income and low-income countries) of bacterial meningitis complications in 18,183 children (in which the most common pathogen was Hib) showed that the most common were hearing loss (in 34% of patients), epileptic seizures (13%), motor deficits (12%), cognitive defects (9%) and hydrocephalus (7%)181. Post-meningitis complications have a relevant economic burden on health care systems179–182.
Patients with S. pneumoniae, H. influenzae and S. suis meningitis often develop hearing loss, probably because bacterial products and inflammatory mediators spread from the meninges directly to the cochlea25,183–185. Otitis media is an important risk factor for meningitis; it is found in up to 55% of patients with pneumococcal meningitis with hearing loss184 and might require invasive treatment. In patients with meningitis and hearing loss, obliteration of the cochlear lumen might follow the meningitis episode and has been associated with decreased success rates of cochlear implant surgery186. Thus, early identification of hearing loss is crucial and screening is advised before discharging a patient.
Survivors of bacterial meningitis are at high risk of cognitive impairment (reduced processing speed)175, which can be observed in approximately one-third of patients who have had pneumococcal or meningococcal meningitis177. In Denmark, a nationwide population-based cohort study showed that bacterial meningitis during childhood negatively affects educational level178.
Outlook
Despite advances in prevention and treatment, bacterial meningitis remains one of the most widespread and lethal infectious diseases worldwide. Prevention of disease and early initiation of the appropriate treatment in patients with suspected or proven bacterial meningitis are the key factors to reduce morbidity and mortality. Furthermore, as drug resistance spreads and serotype and serogroup incidences shift, novel antibiotic and adjuvant treatment strategies must be developed38, although growing evidence supports the decrease of antibiotic resistance for pneumococci since the implementation of conjugate vaccines139. Vaccination programmes and health education are needed to prevent the disease. Extending the use of the available conjugate vaccines in Africa and Asia, where the burden of acute bacterial meningitis is the greatest, will contribute to defeating the disease globally. Herd protection plays a major part in the effectiveness of conjugate vaccines12,139,187, helping to protect infants who are too young to be fully immunized and the elderly who have poor immunological response to vaccination7.
In view of the changing epidemiology, uniform surveillance systems should be implemented in many countries to monitor the effect of conjugate vaccines on serotype incidences, including emerging strains that are not covered by current vaccines. Molecular epidemiology of bacterial strains is key: whole-genome sequencing has been highly valuable in tracking the emergence, virulence and pathophysiology of these bacterial agents188–190. Thus, surveillance studies will also need to evaluate the effects of different bacterial genotypes on the clinical outcome191. Meanwhile, improved protein vaccines with broad coverage are needed192. In the United Kingdom, a nationwide vaccination campaign using a multicomponent meningococcal B protein vaccine193,194 was launched in September 2015 and an assessment on disease prevention is eagerly awaited136.
The pathophysiological mechanisms of bacterial meningitis are complex. In the 1980s, studies on twins and adopted children showed that genetic factors are major determinants in the development of infectious diseases, including meningitis29,195. The association between genetic factors and susceptibility and outcome of invasive meningococcal and pneumococcal disease has been confirmed by ‘extreme phenotype’ and case–control studies29. However, unbiased genome-wide association studies that take into account gene–gene interactions between host and pathogen could reveal new targets for vaccine development and treatment191. These types of studies require large numbers of patients, so joined efforts among research groups and countries should be sought; afterward, genetic, functional and experimental validation will be needed to distinguish real from spurious results.
Experimental animal models are essential to unravel the pathophysiology of pneumococcal meningitis and to evaluate new treatment strategies. The main goal for new therapies will be dampening the inflammatory response, and the targets with the highest therapeutic potential belong to the signalling cascades that regulate damage mediated by reactive oxygen species and reactive nitrogen species196, caspase inhibition197, complement system activation88 or vascular integrity198. Animal studies of new treatments should adhere to current standards of comparative experimental research191. These studies should include a standard treatment arm that consists of antibiotics plus dexamethasone (the current standard treatment strategy) and should be designed to detect a relevant clinical outcome, which is convincing enough to justify a clinical trial. Blocking the complement cascade seems to be the most promising strategy91. Clinical evaluation of complement-blocking therapies should be facilitated by the pharmaceutical industry.
New and more-specific anti-inflammatory treatments are urgently needed. Randomized controlled trials are crucial to establish efficacy, safety and treatment modalities of new drugs against bacterial meningitis199. To maximize the results, these clinical trials should be conducted on an international scale. Thus, international networks on clinical research in neurological infectious diseases, using uniform diagnostic and enrolment criteria and research standards, need to be established. In clinical practice, early treatment (ideally within 1 hour of presentation), implementation of adjunctive dexamethasone therapy and intense supportive care might contribute to improvement in the prognosis of patients with bacterial meningitis.
References
van de Beek, D. et al. Clinical features and prognostic factors in adults with bacterial meningitis. N. Engl. J. Med. 351, 1849–1859 (2004).
van de Beek, D., de Gans, J., Tunkel, A. R. & Wijdicks, E. F. Community-acquired bacterial meningitis in adults. N. Engl. J. Med. 354, 44–53 (2006).
van de Beek, D., Drake, J. M. & Tunkel, A. R. Nosocomial bacterial meningitis. N. Engl. J. Med. 362, 146–154 (2010).
McIntyre, P. B., O'Brien, K. L., Greenwood, B. & van de Beek, D. Effect of vaccines on bacterial meningitis worldwide. Lancet 380, 1703–1711 (2012). A review on the available vaccines against the most common pathogens of bacterial meningitis and the effect of their introduction on the epidemiology of bacterial meningitis.
Lucas, M. J., Brouwer, M. C. & van de Beek, D. Neurological sequelae of bacterial meningitis. J. Infect. 73, 18–27 (2016).
Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 743–800 (2015).
Bijlsma, M. W. et al. Community-acquired bacterial meningitis in adults in the Netherlands, 2006–14: a prospective cohort study. Lancet Infect. Dis. 16, 339–347 (2016). A nationwide prospective study on the epidemiology, clinical features and outcome of bacterial meningitis in the Netherlands in 2006–2014.
Brouwer, M. C., Tunkel, A. R. & van de Beek, D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin. Microbiol. Rev. 23, 467–492 (2010). A review on the epidemiology of bacterial meningitis.
Heckenberg, S. G. et al. Clinical features, outcome, and meningococcal genotype in 258 adults with meningococcal meningitis: a prospective cohort study. Medicine (Baltimore) 87, 185–192 (2008).
Thigpen, M. C. et al. Bacterial meningitis in the United States, 1998–2007. N. Engl. J. Med. 364, 2016–2025 (2011). A surveillance study on bacterial meningitis in the United States in 1998–2007.
Bijlsma, M. W. et al. Epidemiology of invasive meningococcal disease in the Netherlands, 1960–2012: an analysis of national surveillance data. Lancet Infect. Dis. 14, 805–812 (2014).
Hsu, H. E. et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N. Engl. J. Med. 360, 244–256 (2009).
World Health Organization. Haemophilus influenzae type b (Hib) vaccination WHO position paper: July 2013 — recommendations. Vaccine 31, 6168–6169 (2013).
Daugla, D. M. et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study [corrected]. Lancet 383, 40–47 (2014).
Xie, O., Pollard, A. J., Mueller, J. E. & Norheim, G. Emergence of serogroup X meningococcal disease in Africa: need for a vaccine. Vaccine 31, 2852–2861 (2013).
Burki, T. Meningitis outbreak in Niger is an urgent warning. Lancet Infect. Dis. 15, 1011 (2015).
Cibrelus, L. et al. Serogroup W meningitis outbreak at the subdistrict level, Burkina Faso, 2012. Emerg. Infect. Dis. 21, 2063–2066 (2015).
Johnson, H. L. et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med 7, e1000348 (2010).
Saha, S. K. et al. Streptococcus pneumoniae serotype-2 childhood meningitis in Bangladesh: a newly recognized pneumococcal infection threat. PLoS ONE 7, e32134 (2012).
Yaro, S. et al. Epidemiological and molecular characteristics of a highly lethal pneumococcal meningitis epidemic in Burkina Faso. Clin. Infect. Dis. 43, 693–700 (2006).
Bekker, V., Bijlsma, M. W., van de Beek, D., Kuijpers, T. W. & van der Ende, A. Incidence of invasive group B streptococcal disease and pathogen genotype distribution in newborn babies in the Netherlands over 25 years: a nationwide surveillance study. Lancet Infect. Dis. 14, 1083–1089 (2014).
Koopmans, M. M. et al. Listeria monocytogenes sequence type 6 and increased rate of unfavorable outcome in meningitis: epidemiologic cohort study. Clin. Infect. Dis. 57, 247–253 (2013).
Lingani, C. et al. Meningococcal meningitis surveillance in the African meningitis belt, 2004–2013. Clin. Infect. Dis. 61, S410–S415 (2015).
Saez-Llorens, X. & McCracken, G. H. Jr. Bacterial meningitis in children. Lancet 361, 2139–2148 (2003).
van Samkar, A., Brouwer, M. C., Schultsz, C., van der Ende, A. & van de Beek, D. Streptococcus suis meningitis: a systematic review and meta-analysis. PLoS Negl Trop. Dis. 9, e0004191 (2015).
Wertheim, H. F. et al. Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS ONE 4, e5973 (2009).
Costerus, J. M., Brouwer, M. C., van der Ende, A. & van de Beek, D. Community-acquired bacterial meningitis in adults with cancer or a history of cancer. Neurology 86, 860–866 (2016).
van Veen, K. E., Brouwer, M. C., van der Ende, A. & van de Beek, D. Bacterial meningitis in patients with HIV: a population-based prospective study. J. Infect. 72, 362–368 (2016).
Brouwer, M. C. et al. Host genetic susceptibility to pneumococcal and meningococcal disease: a systematic review and meta-analysis. Lancet Infect. Dis. 9, 31–44 (2009).
Fischer, M. et al. Tobacco smoke as a risk factor for meningococcal disease. Pediatr. Infect. Dis. J. 16, 979–983 (1997).
Kamiya, H. et al. Meningococcal disease among men who have sex with men — United States, January 2012–June 2015. MMWR Morb. Mortal. Wkly Rep. 64, 1256–1257 (2015).
Singleton, R. et al. Impact of immunizations on the disease burden of American Indian and Alaska Native children. Arch. Pediatr. Adolesc. Med. 163, 446–453 (2009).
Brouwer, M. C., van de Beek, D., Heckenberg, S. G., Spanjaard, L. & de Gans, J. Community-acquired Listeria monocytogenes meningitis in adults. Clin. Infect. Dis. 43, 1233–1238 (2006).
Bogaert, D., De Groot, R. & Hermans, P. W. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4, 144–154 (2004).
Hill, D. J., Griffiths, N. J., Borodina, E. & Virji, M. Cellular and molecular biology of Neisseria meningitidis colonization and invasive disease. Clin. Sci. (Lond.) 118, 547–564 (2010).
Landwehr-Kenzel, S. & Henneke, P. Interaction of Streptococcus agalactiae and cellular innate immunity in colonization and disease. Front. Immunol. 5, 519 (2014).
Vila, J. et al. Escherichia coli: an old friend with new tidings. FEMS Microbiol. Rev. 8 March 2016 [epub ahead of print].
Mook-Kanamori, B. B., Geldhoff, M., van der Poll, T. & van de Beek, D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin. Microbiol. Rev. 24, 557–591 (2011). A review on the pathogenesis and pathophysiology of pneumococcal meningitis.
Picard, C. et al. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 89, 403–425 (2010).
Chapman, S. J. et al. NFKBIZ polymorphisms and susceptibility to pneumococcal disease in European and African populations. Genes Immun. 11, 319–325 (2010).
Kloek, A. T. et al. Exome array analysis of susceptibility to pneumococcal meningitis. Sci. Rep. 6, 29351 (2016).
Read, R. C. Neisseria meningitidis ; clones, carriage, and disease. Clin. Microbiol. Infect. 20, 391–395 (2014).
Weinberger, D. M. et al. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin. Infect. Dis. 51, 692–699 (2010).
Tazi, A. et al. The surface protein HvgA mediates group B streptococcus hypervirulence and meningeal tropism in neonates. J. Exp. Med. 207, 2313–2322 (2010).
Kim, K. S. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat. Rev. Neurosci. 4, 376–385 (2003).
Koedel, U., Klein, M. & Pfister, H. W. New understandings on the pathophysiology of bacterial meningitis. Curr. Opin. Infect. Dis. 23, 217–223 (2010). A review on the pathophysiology of bacterial meningitis.
Doran, K. S. et al. Host–pathogen interactions in bacterial meningitis. Acta Neuropathol. 131, 185–209 (2016). A review on host–pathogen interactions in bacterial meningitis.
Agarwal, S., Vasudhev, S., DeOliveira, R. B. & Ram, S. Inhibition of the classical pathway of complement by meningococcal capsular polysaccharides. J. Immunol. 193, 1855–1863 (2014).
Johswich, K. O. et al. Invasive potential of nonencapsulated disease isolates of Neisseria meningitidis. Infect. Immun. 80, 2346–2353 (2012).
Lewis, L. A. et al. Factor H-dependent alternative pathway inhibition mediated by porin B contributes to virulence of Neisseria meningitidis. mBio 4, e00339-13 (2013).
Loh, E. et al. Temperature triggers immune evasion by Neisseria meningitidis. Nature 502, 237–240 (2013).
Mittal, R., Krishnan, S., Gonzalez-Gomez, I. & Prasadarao, N. V. Deciphering the roles of outer membrane protein A extracellular loops in the pathogenesis of Escherichia coli K1 meningitis. J. Biol. Chem. 286, 2183–2193 (2011).
Carlin, A. F. et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113, 3333–3336 (2009).
Ali, S. R. et al. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J. Exp. Med. 211, 1231–1242 (2014).
Sheldon, J. R., Laakso, H. A. & Heinrichs, D. E. Iron acquisition strategies of bacterial pathogens. Microbiol. Spectr.http://dx.doi.org/10.1128/microbiolspec.VMBF-0010-2015 (2016).
Iovino, F., Orihuela, C. J., Moorlag, H. E., Molema, G. & Bijlsma, J. J. Interactions between blood-borne Streptococcus pneumoniae and the blood–brain barrier preceding meningitis. PLoS ONE 8, e68408 (2013).
Ridpath, A. D. et al. Postmortem diagnosis of invasive meningococcal disease. Emerg. Infect. Dis. 20, 453–455 (2014).
Owens, T., Bechmann, I. & Engelhardt, B. Perivascular spaces and the two steps to neuroinflammation. J. Neuropathol. Exp. Neurol. 67, 1113–1121 (2008).
Wong, A. D. et al. The blood–brain barrier: an engineering perspective. Front. Neuroeng. 6, 7 (2013).
Mairey, E. et al. Cerebral microcirculation shear stress levels determine Neisseria meningitidis attachment sites along the blood–brain barrier. J. Exp. Med. 203, 1939–1950 (2006).
Orihuela, C. J. et al. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J. Clin. Invest. 119, 1638–1646 (2009).
Bernard, S. C. et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat. Med. 20, 725–731 (2014).
Coureuil, M. et al. Meningococcus hijacks a β2-adrenoceptor/β-arrestin pathway to cross brain microvasculature endothelium. Cell 143, 1149–1160 (2010).
Coureuil, M., Bourdoulous, S., Marullo, S. & Nassif, X. Invasive meningococcal disease: a disease of the endothelial cells. Trends Mol. Med. 20, 571–578 (2014).
Maruvada, R. & Kim, K. S. IbeA and OmpA of Escherichia coli K1 exploit Rac1 activation for invasion of human brain microvascular endothelial cells. Infect. Immun. 80, 2035–2041 (2012).
Dando, S. J. et al. Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev. 27, 691–726 (2014).
Krishnan, S., Chen, S., Turcatel, G., Arditi, M. & Prasadarao, N. V. Regulation of Toll-like receptor 2 interaction with Ecgp96 controls Escherichia coli K1 invasion of brain endothelial cells. Cell. Microbiol. 15, 63–81 (2013).
Norton, J. P. & Mulvey, M. A. Toxin–antitoxin systems are important for niche-specific colonization and stress resistance of uropathogenic Escherichia coli. PLoS Pathog. 8, e1002954 (2012).
Doran, K. S. et al. Blood–brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J. Clin. Invest. 115, 2499–2507 (2005).
Banerjee, A. et al. Bacterial Pili exploit integrin machinery to promote immune activation and efficient blood–brain barrier penetration. Nat. Commun. 2, 462 (2011).
Kim, B. J. et al. Bacterial induction of Snail1 contributes to blood–brain barrier disruption. J. Clin. Invest. 125, 2473–2483 (2015).
Koedel, U., Scheld, W. M. & Pfister, H. W. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect. Dis. 2, 721–736 (2002).
Pachter, J. S., de Vries, H. E. & Fabry, Z. The blood–brain barrier and its role in immune privilege in the central nervous system. J. Neuropathol. Exp. Neurol. 62, 593–604 (2003).
Chiavolini, D., Pozzi, G. & Ricci, S. Animal models of Streptococcus pneumoniae disease. Clin. Microbiol. Rev. 21, 666–685 (2008).
Trostdorf, F. et al. Reduction of meningeal macrophages does not decrease migration of granulocytes into the CSF and brain parenchyma in experimental pneumococcal meningitis. J. Neuroimmunol. 99, 205–210 (1999).
Polfliet, M. M. et al. Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. J. Immunol. 167, 4644–4650 (2001).
Fowler, M. I., Weller, R. O., Heckels, J. E. & Christodoulides, M. Different meningitis-causing bacteria induce distinct inflammatory responses on interaction with cells of the human meninges. Cell. Microbiol. 6, 555–567 (2004).
Tuomanen, E., Tomasz, A., Hengstler, B. & Zak, O. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J. Infect. Dis. 151, 535–540 (1985).
Tuomanen, E. I., Saukkonen, K., Sande, S., Cioffe, C. & Wright, S. D. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J. Exp. Med. 170, 959–969 (1989).
Schneider, O., Michel, U., Zysk, G., Dubuis, O. & Nau, R. Clinical outcome in pneumococcal meningitis correlates with CSF lipoteichoic acid concentrations. Neurology 53, 1584–1587 (1999).
Liu, X., Chauhan, V. S., Young, A. B. & Marriott, I. NOD2 mediates inflammatory responses of primary murine glia to Streptococcus pneumoniae. Glia 58, 839–847 (2010).
Opitz, B. et al. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J. Biol. Chem. 279, 36426–36432 (2004).
McNeela, E. A. et al. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 6, e1001191 (2010).
Witzenrath, M. et al. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. J. Immunol. 187, 434–440 (2011).
Hoegen, T. et al. The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal cathepsin B release. J. Immunol. 187, 5440–5451 (2011).
Geldhoff, M. et al. Inflammasome activation mediates inflammation and outcome in humans and mice with pneumococcal meningitis. BMC Infect. Dis. 13, 358 (2013).
Morgan, B. P. & Harris, C. L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 14, 857–877 (2015).
Woehrl, B. et al. Complement component 5 contributes to poor disease outcome in humans and mice with pneumococcal meningitis. J. Clin. Invest. 121, 3943–3953 (2011).
Mook-Kanamori, B. B., Brouwer, M. C., Geldhoff, M., Ende, A. & van de Beek, D. Cerebrospinal fluid complement activation in patients with pneumococcal and meningococcal meningitis. J. Infect. 68, 542–547 (2014).
Ernst, J. D., Hartiala, K. T., Goldstein, I. M. & Sande, M. A. Complement (C5)-derived chemotactic activity accounts for accumulation of polymorphonuclear leukocytes in cerebrospinal fluid of rabbits with pneumococcal meningitis. Infect. Immun. 46, 81–86 (1984).
Kasanmoentalib, E. S., Valls Seron, M., Morgan, B. P., Brouwer, M. C. & van de Beek, D. Adjuvant treatment with dexamethasone plus anti-C5 antibodies improves outcome of experimental pneumococcal meningitis: a randomized controlled trial. J. Neuroinflammation 12, 149 (2015).
Benamu, E. & Montoya, J. G. Infections associated with the use of eculizumab: recommendations for prevention and prophylaxis. Curr. Opin. Infect. Dis. 29, 319–329 (2016).
Sprong, T. et al. Inhibition of C5a-induced inflammation with preserved C5b–9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood 102, 3702–3710 (2003).
Engelen-Lee, J. Y., Brouwer, M. C., Aronica, E. & van de Beek, D. Pneumococcal meningitis: clinical–pathological correlations (meningene-path). Acta Neuropathol. Commun. 4, 26 (2016).
Gerber, J. & Nau, R. Mechanisms of injury in bacterial meningitis. Curr. Opin. Neurol. 23, 312–318 (2010).
Nau, R., Soto, A. & Bruck, W. Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J. Neuropathol. Exp. Neurol. 58, 265–274 (1999).
Braun, J. S. et al. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat. Med. 5, 298–302 (1999).
Grandgirard, D., Steiner, O., Tauber, M. G. & Leib, S. L. An infant mouse model of brain damage in pneumococcal meningitis. Acta Neuropathol. 114, 609–617 (2007).
Klein, M. et al. Protein expression pattern in experimental pneumococcal meningitis. Microbes Infect. 8, 974–983 (2006).
Weber, J. R. & Tuomanen, E. I. Cellular damage in bacterial meningitis: an interplay of bacterial and host driven toxicity. J. Neuroimmunol. 184, 45–52 (2007).
Wippel, C. et al. Bacterial cytolysin during meningitis disrupts the regulation of glutamate in the brain, leading to synaptic damage. PLoS Pathog. 9, e1003380 (2013).
Guarner, J. et al. Neutrophilic bacterial meningitis: pathology and etiologic diagnosis of fatal cases. Mod. Pathol. 26, 1076–1085 (2013).
Schaper, M. et al. Cerebral vasculature is the major target of oxidative protein alterations in bacterial meningitis. J. Neuropathol. Exp. Neurol. 61, 605–613 (2002).
Vergouwen, M. D., Schut, E. S., Troost, D. & van de Beek, D. Diffuse cerebral intravascular coagulation and cerebral infarction in pneumococcal meningitis. Neurocrit. Care 13, 217–227 (2010).
Schut, E. S. et al. Delayed cerebral thrombosis after initial good recovery from pneumococcal meningitis. Neurology 73, 1988–1995 (2009).
Kastenbauer, S. & Pfister, H. W. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain 126, 1015–1025 (2003).
Lucas, M. J., Brouwer, M. C. & van de Beek, D. Delayed cerebral thrombosis in bacterial meningitis: a prospective cohort study. Intensive Care Med. 39, 866–871 (2013).
Fitch, M. T. & van de Beek, D. Emergency diagnosis and treatment of adult meningitis. Lancet Infect. Dis. 7, 191–200 (2007).
van de Beek, D. et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin. Microbiol. Infect. 22, S37–S62 (2016). These are the latest clinical guidelines on bacterial meningitis published by the ESCMID.
Kim, K. S. Acute bacterial meningitis in infants and children. Lancet Infect. Dis. 10, 32–42 (2010).
Thompson, M. J. et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet 367, 397–403 (2006).
Thomas, K. E., Hasbun, R., Jekel, J. & Quagliarello, V. J. The diagnostic accuracy of Kernig's sign, Brudzinski's sign, and nuchal rigidity in adults with suspected meningitis. Clin. Infect. Dis. 35, 46–52 (2002).
Brouwer, M. C., Tunkel, A. R., McKhann, G. M. 2nd & van de Beek, D. Brain abscess. N. Engl. J. Med. 371, 447–456 (2014).
Attia, J., Hatala, R., Cook, D. J. & Wong, J. G. The rational clinical examination. Does this adult patient have acute meningitis? JAMA 282, 175–181 (1999).
Tissot, F., Prod'hom, G., Manuel, O. & Greub, G. Impact of round-the-clock CSF Gram stain on empirical therapy for suspected central nervous system infections. Eur. J. Clin. Microbiol. Infect. Dis. 34, 1849–1857 (2015).
Hasbun, R. et al. Risk score for identifying adults with CSF pleocytosis and negative CSF Gram stain at low risk for an urgent treatable cause. J. Infect. 67, 102–110 (2013).
Brouwer, M. C., Thwaites, G. E., Tunkel, A. R. & van de Beek, D. Dilemmas in the diagnosis of acute community-acquired bacterial meningitis. Lancet 380, 1684–1692 (2012). A review on diagnostics in patients with suspected bacterial meningitis.
Nigrovic, L. E. et al. Clinical prediction rule for identifying children with cerebrospinal fluid pleocytosis at very low risk of bacterial meningitis. JAMA 297, 52–60 (2007).
Hasbun, R., Abrahams, J., Jekel, J. & Quagliarello, V. J. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N. Engl. J. Med. 345, 1727–1733 (2001).
Spanos, A., Harrell, F. E. Jr & Durack, D. T. Differential diagnosis of acute meningitis. An analysis of the predictive value of initial observations. JAMA 262, 2700–2707 (1989).
Garges, H. P. et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics 117, 1094–1100 (2006).
Vuong, J. et al. Development of real-time PCR methods for the detection of bacterial meningitis pathogens without DNA extraction. PLoS ONE 11, e0147765 (2016).
Darton, T. et al. Severity of meningococcal disease associated with genomic bacterial load. Clin. Infect. Dis. 48, 587–594 (2009).
Corless, C. E. et al. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J. Clin. Microbiol. 39, 1553–1558 (2001).
Parent du Châtelet, I. et al. Bacterial meningitis in Burkina Faso: surveillance using field-based polymerase chain reaction testing. Clin. Infect. Dis. 40, 17–25 (2005).
Heinsbroek, E. et al. Added value of PCR-testing for confirmation of invasive meningococcal disease in England. J. Infect. 67, 385–390 (2013).
Munoz-Almagro, C. et al. Polymerase chain reaction for diagnosis and serogrouping of meningococcal disease in children. Diagn. Microbiol. Infect. Dis. 63, 148–154 (2009).
Centers for Disease Control and Prevention & World Health Organization. Laboratory methods for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. WHOhttp://apps.who.int/iris/bitstream/10665/70765/1/WHO_IVB_11.09_eng.pdf (2011).
Nigrovic, L. E., Kuppermann, N., McAdam, A. J. & Malley, R. Cerebrospinal latex agglutination fails to contribute to the microbiologic diagnosis of pretreated children with meningitis. Pediatr. Infect. Dis. J. 23, 786–788 (2004).
Saha, S. K. et al. Rapid diagnosis of pneumococcal meningitis: implications for treatment and measuring disease burden. Pediatr. Infect. Dis. J. 24, 1093–1098 (2005).
van Veen, K. E., Brouwer, M. C., van der Ende, A. & van de Beek, D. Bacterial meningitis in solid organ transplant recipients: a population-based prospective study. Transpl. Infect. Dis. 18, 674–680 (2016).
van Veen, K. E., Brouwer, M. C., van der Ende, A. & van de Beek, D. Bacterial meningitis in hematopoietic stem cell transplant recipients: a population-based prospective study. Bone Marrow Transplant.http://dx.doi.org/10.1038/bmt.2016.181 (2016).
Adriani, K. S., Brouwer, M. C., van der Ende, A. & van de Beek, D. Bacterial meningitis in adults after splenectomy and hyposplenic states. Mayo Clin. Proc. 88, 571–578 (2013).
Bonten, M. J. et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 372, 1114–1125 (2015).
Kobayashi, M. et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly Rep. 64, 944–947 (2015).
Ladhani, S. N. et al. Enter B and W: two new meningococcal vaccine programmes launched. Arch. Dis. Child. 101, 91–95 (2016).
MacNeil, J. R. et al. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb. Mortal. Wkly Rep. 64, 1171–1176 (2015).
[No authors listed.] Meningococcal A conjugate vaccine: updated guidance, February 2015. Wkly Epidemiol. Rec. 90, 57–62 (2015).
von Gottberg, A. et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N. Engl. J. Med. 371, 1889–1899 (2014).
Feikin, D. R. et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med. 10, e1001517 (2013).
World Health Organization. Pneumococcal vaccines WHO position paper — 2012 — recommendations. Vaccine 30, 4717–4718 (2012).
[No authors listed.] Revised guidance on meningitis outbreak response in sub-Saharan Africa. Wkly Epidemiol. Rec. 89, 580–586 (2014).
van de Beek, D., Brouwer, M. C., Thwaites, G. E. & Tunkel, A. R. Advances in treatment of bacterial meningitis. Lancet 380, 1693–1702 (2012). A review on the treatment of bacterial meningitis.
Simonsen, K. A., Anderson-Berry, A. L., Delair, S. F. & Davies, H. D. Early-onset neonatal sepsis. Clin. Microbiol. Rev. 27, 21–47 (2014).
McMillan, D. A., Lin, C. Y., Aronin, S. I. & Quagliarello, V. J. Community-acquired bacterial meningitis in adults: categorization of causes and timing of death. Clin. Infect. Dis. 33, 969–975 (2001).
Moissenet, D. et al. Meningitis caused by Escherichia coli producing TEM-52 extended-spectrum β-lactamase within an extensive outbreak in a neonatal ward: epidemiological investigation and characterization of the strain. J. Clin. Microbiol. 48, 2459–2463 (2010).
Auburtin, M. et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit. Care Med. 34, 2758–2765 (2006).
Auburtin, M. et al. Pneumococcal meningitis in the intensive care unit: prognostic factors of clinical outcome in a series of 80 cases. Am. J. Respir. Crit. Care Med. 165, 713–717 (2002).
Navarro-Torne, A. et al. Risk factors for death from invasive pneumococcal disease, Europe, 2010. Emerg. Infect. Dis. 21, 417–425 (2015).
Richter, S. S. et al. Changes in pneumococcal serotypes and antimicrobial resistance after introduction of the 13-valent conjugate vaccine in the United States. Antimicrob. Agents Chemother. 58, 6484–6489 (2014).
Tunkel, A. R. et al. Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. 39, 1267–1284 (2004).
Luaces Cubells, C., Garcia Garcia, J. J., Roca Martinez, J. & Latorre Otin, C. L. Clinical data in children with meningococcal meningitis in a Spanish hospital. Acta Paediatr. 86, 26–29 (1997).
Harcourt, B. H. et al. Population-based surveillance of Neisseria meningitidis antimicrobial resistance in the United States. Open Forum Infect. Dis. 2, ofv117 (2015).
Mylonakis, E., Hohmann, E. L. & Calderwood, S. B. Central nervous system infection with Listeria monocytogenes. 33 years’ experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore) 77, 313–336 (1998).
Mitja, O. et al. Predictors of mortality and impact of aminoglycosides on outcome in listeriosis in a retrospective cohort study. J. Antimicrob. Chemother. 64, 416–423 (2009).
Molyneux, E. et al. 5 versus 10 days of treatment with ceftriaxone for bacterial meningitis in children: a double-blind randomised equivalence study. Lancet 377, 1837–1845 (2011).
Mourvillier, B. et al. Induced hypothermia in severe bacterial meningitis: a randomized clinical trial. JAMA 310, 2174–2183 (2013).
Pelkonen, T. et al. Slow initial β-lactam infusion and oral paracetamol to treat childhood bacterial meningitis: a randomised, controlled trial. Lancet Infect. Dis. 11, 613–621 (2011).
Molyneux, E. M. et al. Glycerol and acetaminophen as adjuvant therapy did not affect the outcome of bacterial meningitis in Malawian children. Pediatr. Infect. Dis. J. 33, 214–216 (2014).
Peltola, H. et al. Adjuvant glycerol and/or dexamethasone to improve the outcomes of childhood bacterial meningitis: a prospective, randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 45, 1277–1286 (2007).
Brouwer, M. C., McIntyre, P., Prasad, K. & van de Beek, D. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst. Rev. 9, CD004405 (2015).
Fitch, M. T. & van de Beek, D. Drug insight: steroids in CNS infectious diseases — new indications for an old therapy. Nat. Clin. Pract. Neurol. 4, 97–104 (2008).
de Gans, J., van de Beek, D. & European Dexamethasone in Adulthood Bacterial Meningitis Study. Dexamethasone in adults with bacterial meningitis. N. Engl. J. Med. 347, 1549–1556 (2002). A landmark randomized clinical study on adjunctive dexamethasone in bacterial meningitis.
van de Beek, D. & de Gans, J. Dexamethasone and pneumococcal meningitis. Ann. Intern. Med. 141, 327 (2004).
van de Beek, D. et al. Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol. 9, 254–263 (2010).
van de Beek, D., de Gans, J., McIntyre, P. & Prasad, K. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect. Dis. 4, 139–143 (2004).
Lindvall, P. et al. Reducing intracranial pressure may increase survival among patients with bacterial meningitis. Clin. Infect. Dis. 38, 384–390 (2004).
Brouwer, M. C., Wijdicks, E. F. & van de Beek, D. What's new in bacterial meningitis. Intensive Care Med. 42, 415–417 (2016).
van de Beek, D., Weisfelt, M., de Gans, J., Tunkel, A. R. & Wijdicks, E. F. Drug insight: adjunctive therapies in adults with bacterial meningitis. Nat. Clin. Pract. Neurol. 2, 504–516 (2006).
Brouwer, M. C., van de Beek, D., Heckenberg, S. G., Spanjaard, L. & de Gans, J. Hyponatraemia in adults with community-acquired bacterial meningitis. QJM 100, 37–40 (2007).
Kasanmoentalib, E. S., Brouwer, M. C., van der Ende, A. & van de Beek, D. Hydrocephalus in adults with community-acquired bacterial meningitis. Neurology 75, 918–923 (2010).
Zoons, E. et al. Seizures in adults with bacterial meningitis. Neurology 70, 2109–2115 (2008).
Muralidharan, R., Mateen, F. J. & Rabinstein, A. A. Outcome of fulminant bacterial meningitis in adult patients. Eur. J. Neurol. 21, 447–453 (2014).
Lucas, M. J., Brouwer, M. C., van der Ende, A. & van de Beek, D. Outcome in patients with bacterial meningitis presenting with a minimal Glasgow Coma Scale score. Neurol. Neuroimmunol. Neuroinflamm. 1, e9 (2014).
van de Beek, D. et al. Cognitive impairment in adults with good recovery after bacterial meningitis. J. Infect. Dis. 186, 1047–1052 (2002).
Weisfelt, M. et al. Dexamethasone and long-term outcome in adults with bacterial meningitis. Ann. Neurol. 60, 456–468 (2006).
Hoogman, M., van de Beek, D., Weisfelt, M., de Gans, J. & Schmand, B. Cognitive outcome in adults after bacterial meningitis. J. Neurol. Neurosurg. Psychiatry 78, 1092–1096 (2007).
Roed, C. et al. Educational achievement and economic self-sufficiency in adults after childhood bacterial meningitis. JAMA 309, 1714–1721 (2013). A nationwide population-based cohort study using national registries of Danish-born children who were diagnosed with bacterial meningitis in 1977–2007, showing that bacterial meningitis in childhood is associated with lower educational achievement and economic self-sufficiency in adult life.
Portnoy, A. et al. Estimating costs of care for meningitis infections in low- and middle-income countries. Vaccine 33 (Suppl. 1), A240–A247 (2015).
Sridhar, S. et al. Global incidence of serogroup B invasive meningococcal disease: a systematic review. Lancet Infect. Dis. 15, 1334–1346 (2015).
Edmond, K. et al. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect. Dis. 10, 317–328 (2010). A systematic review on the disease burden of bacterial meningitis.
Watt, J. P. et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet 374, 903–911 (2009).
Heckenberg, S. G., Brouwer, M. C., van der Ende, A., Hensen, E. F. & van de Beek, D. Hearing loss in adults surviving pneumococcal meningitis is associated with otitis and pneumococcal serotype. Clin. Microbiol. Infect. 18, 849–855 (2012).
Worsoe, L., Caye-Thomasen, P., Brandt, C. T., Thomsen, J. & Ostergaard, C. Factors associated with the occurrence of hearing loss after pneumococcal meningitis. Clin. Infect. Dis. 51, 917–924 (2010).
Karppinen, M. et al. Hearing impairment after childhood bacterial meningitis dependent on etiology in Luanda, Angola. Int. J. Pediatr. Otorhinolaryngol. 79, 1820–1826 (2015).
van Loon, M. C. et al. Magnetic resonance imaging in the evaluation of patients with sensorineural hearing loss caused by meningitis: implications for cochlear implantation. Otol. Neurotol. 34, 845–854 (2013).
Bijlsma, M. W., Brouwer, M. C., Spanjaard, L., van de Beek, D. & van der Ende, A. A decade of herd protection after introduction of meningococcal serogroup C conjugate vaccination. Clin. Infect. Dis. 59, 1216–1221 (2014).
Croucher, N. J. et al. Selective and genetic constraints on pneumococcal serotype switching. PLoS Genet. 11, e1005095 (2015).
Chewapreecha, C. et al. Dense genomic sampling identifies highways of pneumococcal recombination. Nat. Genet. 46, 305–309 (2014).
Piet, J. R. et al. Streptococcus pneumoniae arginine synthesis genes promote growth and virulence in pneumococcal meningitis. J. Infect. Dis. 209, 1781–1791 (2014).
van de Beek, D. Progress and challenges in bacterial meningitis. Lancet 380, 1623–1624 (2012).
Black, S., Pizza, M., Nissum, M. & Rappuoli, R. Toward a meningitis-free world. Sci. Transl Med. 4, 123ps5 (2012).
Gossger, N. et al. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA 307, 573–582 (2012).
Santolaya, M. E. et al. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet 379, 617–624 (2012).
Brouwer, M. C., Read, R. C. & van de Beek, D. Host genetics and outcome in meningococcal disease: a systematic review and meta-analysis. Lancet Infect. Dis. 10, 262–274 (2010).
Klein, M., Koedel, U., Pfister, H. W. & Kastenbauer, S. Meningitis-associated hearing loss: protection by adjunctive antioxidant therapy. Ann. Neurol. 54, 451–458 (2003).
Koedel, U. et al. Role of caspase-1 in experimental pneumococcal meningitis: evidence from pharmacologic caspase inhibition and caspase-1-deficient mice. Ann. Neurol. 51, 319–329 (2002).
Brouwer, M. C. et al. Plasminogen activator inhibitor-1 influences cerebrovascular complications and death in pneumococcal meningitis. Acta Neuropathol. 127, 553–564 (2014).
Kasanmoentalib, E. S., Brouwer, M. C. & van de Beek, D. Update on bacterial meningitis: epidemiology, trials and genetic association studies. Curr. Opin. Neurol. 26, 282–288 (2013).
Roberts, L. Infectious disease. An ill wind, bringing meningitis. Science 320, 1710–1715 (2008).
MacNeil, J. R. & Meyer, S. A. Chapter 3: infections related to travel: meningococcal disease. CDChttp://www.nc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/meningococcal-disease (updated 10 July 2015).
Ali, Y. M. et al. Human l-ficolin, a recognition molecule of the lectin activation pathway of complement, activates complement by binding to pneumolysin, the major toxin of Streptococcus pneumoniae. PLoS ONE 8, e82583 (2013).
Mukerji, R. et al. Pneumococcal surface protein A inhibits complement deposition on the pneumococcal surface by competing with the binding of C-reactive protein to cell-surface phosphocholine. J. Immunol. 189, 5327–5335 (2012).
Uchiyama, S. et al. The surface-anchored NanA protein promotes pneumococcal brain endothelial cell invasion. J. Exp. Med. 206, 1845–1852 (2009).
Iovino, F., Brouwer, M. C., van de Beek, D., Molema, G. & Bijlsma, J. J. Signalling or binding: the role of the platelet-activating factor receptor in invasive pneumococcal disease. Cell. Microbiol. 15, 870–881 (2013).
Iovino, F., Seinen, J., Henriques-Normark, B. & van Dijl, J. M. How does Streptococcus pneumoniae invade the brain?. Trends Microbiol. 24, 307–315 (2016).
Koedel, U. et al. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J. Immunol. 170, 438–444 (2003).
Yoshimura, A. et al. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163, 1–5 (1999).
Malley, R. et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl Acad. Sci. USA 100, 1966–1971 (2003).
Klein, M. et al. Innate immunity to pneumococcal infection of the central nervous system depends on Toll-like receptor (TLR) 2 and TLR4. J. Infect. Dis. 198, 1028–1036 (2008).
Tabeta, K. et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 7, 156–164 (2006).
Oldenburg, M. et al. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science 337, 1111–1115 (2012).
Koedel, U., Bayerlein, I., Paul, R., Sporer, B. & Pfister, H. W. Pharmacologic interference with NF-κB activation attenuates central nervous system complications in experimental pneumococcal meningitis. J. Infect. Dis. 182, 1437–1445 (2000).
Rupprecht, T. A. et al. Complement C1q and C3 are critical for the innate immune response to Streptococcus pneumoniae in the central nervous system. J. Immunol. 178, 1861–1869 (2007).
Schuchat, A. et al. Active bacterial core surveillance of the emerging infections program network. Emerg. Infect. Dis. 7, 92–99 (2001).
Wenger, J. D. et al. Bacterial meningitis in the United States, 1986: report of a multistate surveillance study. J. Infect. Dis. 162, 1316–1323 (1990).
Fraser, D. W., Geil, C. C. & Feldman, R. A. Bacterial meningitis in Bernalillo County, New Mexico: a comparison with three other American populations. Am. J. Epidemiol. 100, 29–34 (1974).
Owen, E. P. et al. A complement C5 gene mutation, c.754G>A:p. A252T, is common in the Western Cape, South Africa and found to be homozygous in seven percent of Black African meningococcal disease cases. Mol. Immunol. 64, 170–176 (2015).
Davila, S. et al. Genome-wide association study identifies variants in the CFH region associated with host susceptibility to meningococcal disease. Nat. Genet. 42, 772–776 (2010).
Biebl, A. et al. Confirmation of host genetic determinants in the CFH region and susceptibility to meningococcal disease in a Central European study sample. Pediatr. Infect. Dis. J. 34, 1115–1117 (2015).
Lundbo, L. F. et al. Mannose-binding lectin gene, MBL2, polymorphisms are not associated with susceptibility to invasive pneumococcal disease in children. Clin. Infect. Dis. 59, e66–e71 (2014).
Vardar, F. et al. Association between mannose binding lectin polymorphisms and predisposition to bacterial meningitis. Turk J. Pediatr. 49, 270–273 (2007).
Dunstan, S. J. et al. LTA4H genotype is associated with susceptibility to bacterial meningitis but is not a critical determinant of outcome. PLoS ONE 10, e0118789 (2015).
Adriani, K. S. et al. Genetic variation in the β2-adrenocepter gene is associated with susceptibility to bacterial meningitis in adults. PLoS ONE 7, e37618 (2012).
Adriani, K. S. et al. Common polymorphisms in the complement system and susceptiblity to bacterial meningitis. J. Infect. 66, 255–262 (2013).
Sanders, M. S. et al. Single nucleotide polymorphisms in TLR9 are highly associated with susceptibility to bacterial meningitis in children. Clin. Infect. Dis. 52, 475–480 (2011).
Brouwer, M. C., Baas, F., van der Ende, A. & van de Beek, D. Genetic variation and cerebrospinal fluid levels of mannose binding lectin in pneumococcal meningitis patients. PLoS ONE 8, e65151 (2013).
Lundbo, L. F. et al. Genetic variation in NFKBIE is associated with increased risk of pneumococcal meningitis in children. EBioMedicine 3, 93–99 (2016).
Sanders, M. S., van Well, G. T., Ouburg, S., Morre, S. A. & van Furth, A. M. Genetic variation of innate immune response genes in invasive pneumococcal and meningococcal disease applied to the pathogenesis of meningitis. Genes Immun. 12, 321–334 (2011).
Thwaites, G. E. et al. Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet 360, 1287–1292 (2002).
World Health Organization. Media center: immunization coverage fact sheet. WHOhttp://who.int/mediacentre/factsheets/fs378/en/ (accessed 30 March 2016).
World Health Organization. Immunizations, vaccines, and biologicals: meningococcal meningitis. WHOhttp://who.int/immunization/diseases/meningitis/en/ (accessed 30 March 2016).
Centers for Disease Control and Prevention. Vaccine recommendations of the ACIP: menincococcal ACIP vaccine recommendations. CDChttp://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/mening.html (accessed 30 March 2016).
Acknowledgements
This work was supported by grants from the European Research Council (ERC; Starting Grant (proposal/contract 281156)) and the Netherlands Organization for Health Research and Development (ZonMw; NWO-Vidi grant 2010 (proposal/contract 016.116.358)), both to D.v.d.B., as well as from the German Research Foundation (KO-1974/5-1, 6–1 and 7–1) and the Else Kröner-Fresenius Stiftung (2013_A239), both to U.K. This work is a collaborative project from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Infectious Diseases of the Brain (ESGIB). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Contributions
Introduction (D.v.d.B.); Epidemiology (C.G.W.); Mechanisms/pathophysiology (U.K.); Diagnosis, screening and prevention (M.B. and C.G.W.); Management (R.H., D.v.d.B. and E.W.); Quality of life (M.B.); Outlook (D.v.d.B.); Overview of Primer (D.v.d.B.). All authors listed are in alphabetical order except for D.v.d.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
van de Beek, D., Brouwer, M., Hasbun, R. et al. Community-acquired bacterial meningitis. Nat Rev Dis Primers 2, 16074 (2016). https://doi.org/10.1038/nrdp.2016.74
Published:
DOI: https://doi.org/10.1038/nrdp.2016.74
This article is cited by
-
Nociceptors and Macrophages in Bacterial Meningitis: Partners in Crime?
Neuroscience Bulletin (2024)
-
Pericytes are protective in experimental pneumococcal meningitis through regulating leukocyte infiltration and blood–brain barrier function
Journal of Neuroinflammation (2023)
-
Determinants of mortality among pediatric patients admitted to Wolaita Sodo University Comprehensive Specialized Hospital with acute bacterial meningitis, Southern Ethiopia: an unmatched case–control study
BMC Pediatrics (2023)
-
The clinical characteristics and therapeutic outcomes of adult patients with community-acquired spontaneous bacterial meningitis with a fulminant clinical course in Taiwan
BMC Infectious Diseases (2023)
-
High-risk factors associated with refractory childhood bacterial meningitis in Southwest China
BMC Pediatrics (2023)