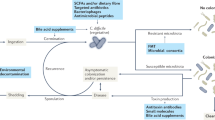
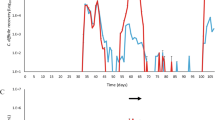
Abstract
Infection of the colon with the Gram-positive bacterium Clostridium difficile is potentially life threatening, especially in elderly people and in patients who have dysbiosis of the gut microbiota following antimicrobial drug exposure. C. difficile is the leading cause of health-care-associated infective diarrhoea. The life cycle of C. difficile is influenced by antimicrobial agents, the host immune system, and the host microbiota and its associated metabolites. The primary mediators of inflammation in C. difficile infection (CDI) are large clostridial toxins, toxin A (TcdA) and toxin B (TcdB), and, in some bacterial strains, the binary toxin CDT. The toxins trigger a complex cascade of host cellular responses to cause diarrhoea, inflammation and tissue necrosis — the major symptoms of CDI. The factors responsible for the epidemic of some C. difficile strains are poorly understood. Recurrent infections are common and can be debilitating. Toxin detection for diagnosis is important for accurate epidemiological study, and for optimal management and prevention strategies. Infections are commonly treated with specific antimicrobial agents, but faecal microbiota transplants have shown promise for recurrent infections. Future biotherapies for C. difficile infections are likely to involve defined combinations of key gut microbiota.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Hall, I. C. & O'Toole, E. Intestinal flora in new-born infants: with a description of a new pathogenic anaerobe, Bacillus difficilis. Am. J. Child. Dis. 49, 390–402 (1935).
Lyras, D. et al. Toxin B is essential for virulence of Clostridium difficile. Nature 458, 1176–1179 (2009).
Kuehne, S. A. et al. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467, 711–713 (2010).
Hafiz, S. & Oakley, C. L. Clostridium difficile: isolation and characteristics. J. Med. Microbiol. 9, 129–136 (1976).
Bartlett, J. G. Clostridium difficile: history of its role as an enteric pathogen and the current state of knowledge about the organism. Clin. Infect. Dis. 18, S265–S272 (1994). An exceptional overview of the early experiments demonstrating the involvement of C. difficile in (antibiotic-associated) colitis.
Kuijper, E. J., Coignard, B. & Tüll, P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin. Microbiol. Infect. 12 (Suppl. 6), 2–18 (2006).
Goorhuis, A. et al. Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin. Infect. Dis. 47, 1162–1170 (2008).
He, M. et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat. Genet. 45, 109–113 (2013). A large-scale whole-genome sequencing study that was the first to demonstrate the potential of the technique to trace the emergence of epidemic strains and relatedness between isolates.
Sebaihia, M. et al. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38, 779–786 (2006).
Minton, N. et al. The development of Clostridium difficile genetic systems. Anaerobe 10, 75–84 (2004).
Kuehne, S. A., Heap, J. T., Cooksley, C. M., Cartman, S. T. & Minton, N. P. ClosTron-mediated engineering of Clostridium. Methods Mol. Biol. 765, 389–407 (2011).
Freeman, J. et al. The changing epidemiology of Clostridium difficile infections. Clin. Microbiol. Rev. 23, 529–549 (2010).
Hensgens, M. P. et al. Clostridium difficile infection in the community: a zoonotic disease? Clin. Microbiol. Infect. 18, 635–645 (2012).
Leffler, D. A. & LaMont, J. T. Clostridium difficile infection. N. Engl. J. Med. 372, 1539–1548 (2015).
Yutin, N. & Galperin, M. Y. A genomic update on clostridial phylogeny: Gram-negative spore formers and other misplaced clostridia. Environ. Microbiol. 15, 2631–2641 (2013).
Nadon, C. A. et al. Development and application of MLVA methods as a tool for inter-laboratory surveillance. Euro. Surveill. 18, 20565 (2013).
Knetsch, C. W. et al. Current application and future perspectives of molecular typing methods to study Clostridium difficile infections. Euro. Surveill. 18, 20381 (2013). An updated review of typing methods for C. difficile.
Clements, A. C., Magalhaes, R. J., Tatem, A. J., Paterson, D. L. & Riley, T. V. Clostridium difficile PCR ribotype 027: assessing the risks of further worldwide spread. Lancet Infect. Dis. 10, 395–404 (1992).
Tickler, I. A. et al. Strain types and antimicrobial resistance patterns of Clostridium difficile isolates from the United States, 2011 to 2013. Antimicrob. Agents Chemother. 58, 4214–4218 (2014).
Bauer, M. P. et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet 377, 63–73 (2011). The first pan-European study of the epidemiology of CDI.
Baldan, R. et al. Clostridium difficile PCR ribotype 018, a successful epidemic genotype. J. Clin. Microbiol. 53, 2575–2580 (2015).
Pituch, H. et al. Hospital-based Clostridium difficile infection surveillance reveals high proportions of PCR ribotypes 027 and 176 in different areas of Poland, 2011 to 2013. Euro. Surveill. 20, 30025 (2015).
Lim, S. K. et al. Emergence of a ribotype 244 strain of Clostridium difficile associated with severe disease and related to the epidemic ribotype 027 strain. Clin. Infect. Dis. 58, 1723–1730 (2014).
Hensgens, M. P. et al. Diarrhoea in general practice: when should a Clostridium difficile infection be considered? Results of a nested case–control study. Clin. Microbiol. Infect. 20, O1067–O1074 (2014).
Eyre, D. W. et al. Diverse sources of C. difficile infection identified on whole-genome sequencing. N. Engl. J. Med. 369, 1195–1205 (2013).
Lessa, F. C. et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 372, 825–834 (2015).
Bouwknegt, M., Van, D. S. & Kuijper, E. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 372, 2368 (2015).
Hensgens, M. P. et al. Clostridium difficile infection in an endemic setting in the Netherlands. Eur. J. Clin. Microbiol. Infect. Dis. 30, 587–593 (2011).
Wilcox, M. H., Mooney, L., Bendall, R., Settle, C. D. & Fawley, W. N. A case–control study of community-associated Clostridium difficile infection. J. Antimicrob. Chemother. 62, 388–396 (2008).
Reveles, K. R., Lee, G. C., Boyd, N. K. & Frei, C. R. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001–2010. Am. J. Infect. Control 42, 1028–1032 (2014).
European Centre for Disease Prevention and Control. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals 2011–2012. ECDU[online], (2013).
Davies, K. A. et al. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect. Dis. 14, 1208–1219 (2014). A large pan-European study demonstrating the extent of missed diagnoses of CDI.
European Centre for Disease Prevention and Control. European surveillance of Clostridium difficile infections. Surveillance protocol version 2.1. ECDU[online], (2015).
Planche, T. D. et al. Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C. difficile infection. Lancet Infect. Dis. 13, 936–945 (2013). A large multicentre study demonstrating the importance of toxin detection as part of a diagnostic algorithm for CDI.
Knetsch, C. W. et al. Whole genome sequencing reveals potential spread of Clostridium difficile between humans and farm animals in the Netherlands, 2002 to 2011. Euro. Surveill. 19, 20954 (2014).
Zacharioudakis, I. M., Zervou, F. N., Pliakos, E. E., Ziakas, P. D. & Mylonakis, E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am. J. Gastroenterol. 110, 381–390 (2015).
Paredes-Sabja, D., Shen, A. & Sorg, J. A. Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends Microbiol. 22, 406–416 (2014). A comprehensive overview of C. difficile sporulation, including the role of bile acids in germination.
Deakin, L. J. et al. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect. Immun. 80, 2704–2711 (2012).
Theriot, C. M. & Young, V. B. Interactions between the gastrointestinal microbiome and Clostridium difficile. Annu. Rev. Microbiol. 69, 445–461 (2015).
Bhattacharjee, D. et al. Reexamining the germination phenotypes of several Clostridium difficile strains suggests another role for the CspC germinant receptor. J. Bacteriol. 198, 777–786 (2015).
Sorg, J. A. & Sonenshein, A. L. Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 190, 2505–2512 (2008).
Buffie, C. G. et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517, 205–208 (2015).
Theriot, C. M., Bowman, A. A. & Young, V. B. Antibiotic-induced alterations of the gut microbiota alter secondary bile acid production and allow for Clostridium difficile spore germination and outgrowth in the large intestine. mSphere 1, e00045-15 (2016).
Janoir, C., Pechine, S., Grosdidier, C. & Collignon, A. Cwp84, a surface-associated protein of Clostridium difficile, is a cysteine protease with degrading activity on extracellular matrix proteins. J. Bacteriol. 189, 7174–7180 (2007).
Merrigan, M. M. et al. Surface-layer protein A (SlpA) is a major contributor to host-cell adherence of Clostridium difficile. PLoS ONE 8, e78404 (2013).
Tasteyre, A., Barc, M. C., Collignon, A., Boureau, H. & Karjalainen, T. Role of FliC and FliD flagellar proteins of Clostridium difficile in adherence and gut colonization. Infect. Immun. 69, 7937–7940 (2001).
Spigaglia, P. et al. Surface-layer (S-layer) of human and animal Clostridium difficile strains and their behaviour in adherence to epithelial cells and intestinal colonization. J. Med. Microbiol. 62, 1386–1393 (2013).
Lin, Y. P., Kuo, C. J., Koleci, X., McDonough, S. P. & Chang, Y. F. Manganese binds to Clostridium difficile Fbp68 and is essential for fibronectin binding. J. Biol. Chem. 286, 3957–3969 (2011).
Kovacs-Simon, A. et al. Lipoprotein CD0873 is a novel adhesin of Clostridium difficile. J. Infect. Dis. 210, 274–284 (2014).
Tulli, L. et al. CbpA: a novel surface exposed adhesin of Clostridium difficile targeting human collagen. Cell. Microbiol. 15, 1674–1687 (2013).
Deneve, C., Delomenie, C., Barc, M. C., Collignon, A. & Janoir, C. Antibiotics involved in Clostridium difficile-associated disease increase colonization factor gene expression. J. Med. Microbiol. 57, 732–738 (2008).
Paredes-Sabja, D. & Sarker, M. R. Adherence of Clostridium difficile spores to Caco-2 cells in culture. J. Med. Microbiol. 61, 1208–1218 (2012).
Bordeleau, E. & Burrus, V. Cyclic-di-GMP signaling in the Gram-positive pathogen Clostridium difficile. Curr. Genet. 61, 497–502 (2015).
Purcell, E. B., McKee, R. W., McBride, S. M., Waters, C. M. & Tamayo, R. Cyclic diguanylate inversely regulates motility and aggregation in Clostridium difficile. J. Bacteriol. 194, 3307–3316 (2012).
Peltier, J. et al. Cyclic diGMP regulates production of sortase substrates of Clostridium difficile and their surface exposure through ZmpI protease-mediated cleavage. J. Biol. Chem. 290, 24453–24469 (2015).
Crowther, G. S. et al. Comparison of planktonic and biofilm-associated communities of Clostridium difficile and indigenous gut microbiota in a triple-stage chemostat gut model. J. Antimicrob. Chemother. 69, 2137–2147 (2014).
Dapa, T. & Unnikrishnan, M. Biofilm formation by Clostridium difficile. Gut Microbes. 4, 397–402 (2013).
Semenyuk, E. G. et al. Analysis of bacterial communities during C. difficile infection in the mouse. Infect. Immun. 83, 4383–4391 (2015).
Darkoh, C., DuPont, H. L., Norris, S. J. & Kaplan, H. B. Toxin synthesis by Clostridium difficile is regulated through quorum signaling. mBio 6, e02569 (2015).
Martin, M. J. et al. The agr locus regulates virulence and colonization genes in Clostridium difficile 027. J. Bacteriol. 195, 3672–3681 (2013).
Sun, X. & Hirota, S. A. The roles of host and pathogen factors and the innate immune response in the pathogenesis of Clostridium difficile infection. Mol. Immunol. 63, 193–202 (2015).
Cowardin, C. A. & Petri, W. A. Jr. Host recognition of Clostridium difficile and the innate immune response. Anaerobe 30, 205–209 (2014).
Ryan, A. et al. A role for TLR4 in Clostridium difficile infection and the recognition of surface layer proteins. PLoS Pathog. 7, e1002076 (2011).
Yoshino, Y. et al. Clostridium difficile flagellin stimulates Toll-like receptor 5, and toxin B promotes flagellin-induced chemokine production via TLR5. Life Sci. 92, 211–217 (2013).
Hasegawa, M. et al. Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. J. Immunol. 186, 4872–4880 (2011).
Giesemann, T., Guttenberg, G. & Aktories, K. Human α-defensins inhibit Clostridium difficile toxin B. Gastroenterology 134, 2049–2058 (2008).
Hing, T. C. et al. The antimicrobial peptide cathelicidin modulates Clostridium difficile-associated colitis and toxin A-mediated enteritis in mice. Gut 62, 1295–1305 (2013).
Abt, M. C. et al. Innate immune defenses mediated by two ILC subsets are critical for protection against acute Clostridium difficile infection. Cell Host Microbe 18, 27–37 (2015).
McQuade, R., Roxas, B., Viswanathan, V. K. & Vedantam, G. Clostridium difficile clinical isolates exhibit variable susceptibility and proteome alterations upon exposure to mammalian cationic antimicrobial peptides. Anaerobe 18, 614–620 (2012).
Ho, T. D. et al. Clostridium difficile extracytoplasmic function σ factor σV regulates lysozyme resistance and is necessary for pathogenesis in the hamster model of infection. Infect. Immun. 82, 2345–2355 (2014).
Ho, T. D. & Ellermeier, C. D. PrsW is required for colonization, resistance to antimicrobial peptides, and expression of extracytoplasmic function sigma factors in Clostridium difficile. Infect. Immun. 79, 3229–3238 (2011).
Suarez, J. M., Edwards, A. N. & McBride, S. M. The Clostridium difficile cpr locus is regulated by a noncontiguous two-component system in response to type A and B lantibiotics. J. Bacteriol. 195, 2621–2631 (2013).
McBride, S. M. & Sonenshein, A. L. The dlt operon confers resistance to cationic antimicrobial peptides in Clostridium difficile. Microbiology 157, 1457–1465 (2011).
Awad, M. M., Johanesen, P. A., Carter, G. P., Rose, E. & Lyras, D. Clostridium difficile virulence factors: Insights into an anaerobic spore-forming pathogen. Gut Microbes 5, 579–593 (2014).
Smits, W. K. Hype or hypervirulence: a reflection on problematic C. difficile strains. Virulence 4, 592–596 (2013).
Hammond, G. A. & Johnson, J. L. The toxigenic element of Clostridium difficile strain VPI 10463. Microb. Pathog. 19, 203–213 (1995).
Braun, V., Hundsberger, T., Leukel, P., Sauerborn, M. & von Eichel-Streiber, C. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene 181, 29–38 (1996).
Monot, M. et al. Clostridium difficile: new insights into the evolution of the pathogenicity locus. Sci. Rep. 5, 15023 (2015).
Mani, N. et al. Environmental response and autoregulation of Clostridium difficile TxeR, a sigma factor for toxin gene expression. J. Bacteriol. 184, 5971–5978 (2002).
Mani, N. & Dupuy, B. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proc. Natl Acad. Sci. USA 98, 5844–5849 (2001).
Dupuy, B., Govind, R., Antunes, A. & Matamouros, S. Clostridium difficile toxin synthesis is negatively regulated by TcdC. J. Med. Microbiol. 57, 685–689 (2008).
Warny, M. et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366, 1079–1084 (2005).
Tan, K. S., Wee, B. Y. & Song, K. P. Evidence for holin function of tcdE gene in the pathogenicity of Clostridium difficile. J. Med. Microbiol. 50, 613–619 (2001).
Govind, R. & Dupuy, B. Secretion of Clostridium difficile toxins A. and B. requires the holin-like protein TcdE. PLoS Pathog. 8, e1002727 (2012).
Olling, A. et al. Release of TcdA and TcdB from Clostridium difficile cdi 630 is not affected by functional inactivation of the tcdE gene. Microb. Pathog. 52, 92–100 (2012).
Karlsson, S. et al. Expression of Clostridium difficile toxins A and B and their sigma factor TcdD is controlled by temperature. Infect. Immun. 71, 1784–1793 (2003).
Karlsson, S., Lindberg, A., Norin, E., Burman, L. G. & Akerlund, T. Toxins, butyric acid, and other short-chain fatty acids are coordinately expressed and down-regulated by cysteine in Clostridium difficile. Infect. Immun. 68, 5881–5888 (2000).
Aldape, M. J., Packham, A. E., Nute, D. W., Bryant, A. E. & Stevens, D. L. Effects of ciprofloxacin on the expression and production of exotoxins by Clostridium difficile. J. Med. Microbiol. 62, 741–747 (2013).
Chilton, C. H. et al. Co-amoxiclav induces proliferation and cytotoxin production of Clostridium difficile ribotype 027 in a human gut model. J. Antimicrob. Chemother. 67, 951–954 (2012).
Dineen, S. S., McBride, S. M. & Sonenshein, A. L. Integration of metabolism and virulence by Clostridium difficile CodY. J. Bacteriol. 192, 5350–5362 (2010).
Antunes, A. et al. Global transcriptional control by glucose and carbon regulator CcpA in Clostridium difficile. Nucleic Acids Res. 40, 10701–10718 (2012).
Dupuy, B. & Sonenshein, A. L. Regulated transcription of Clostridium difficile toxin genes. Mol. Microbiol. 27, 107–120 (1998).
McKee, R. W., Mangalea, M. R., Purcell, E. B., Borchardt, E. K. & Tamayo, R. The second messenger cyclic Di-GMP regulates Clostridium difficile toxin production by controlling expression of sigD. J. Bacteriol. 195, 5174–5185 (2013).
El Meouche, I. et al. Characterization of the SigD regulon of C. difficile and its positive control of toxin production through the regulation of tcdR. PLoS ONE 8, e83748 (2013).
Mackin, K. E., Carter, G. P., Howarth, P., Rood, J. I. & Lyras, D. Spo0A differentially regulates toxin production in evolutionarily diverse strains of Clostridium difficile. PLoS ONE 8, e79666 (2013).
Rosenbusch, K. E., Bakker, D., Kuijper, E. J. & Smits, W. K. C. difficile 630Δerm Spo0A regulates sporulation, but does not contribute to toxin production, by direct high-affinity binding to target DNA. PLoS ONE 7, e48608 (2012).
Pettit, L. J. et al. Functional genomics reveals that Clostridium difficile Spo0A coordinates sporulation, virulence and metabolism. BMC Genomics 15, 160 (2014).
Knetsch, C. W. et al. Comparative analysis of an expanded Clostridium difficile reference strain collection reveals genetic diversity and evolution through six lineages. Infect. Genet. Evol. 12, 1577–1585 (2012).
Bouillaut, L., Dubois, T., Sonenshein, A. L. & Dupuy, B. Integration of metabolism and virulence in Clostridium difficile. Res. Microbiol. 166, 375–383 (2015).
Shen, A. Clostridium difficile toxins: mediators of inflammation. J. Innate. Immun. 4, 149–158 (2012).
Lyerly, D. M., Saum, K. E., MacDonald, D. K. & Wilkins, T. D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect. Immun. 47, 349–352 (1985).
Mitchell, T. J. et al. Effect of toxin A and B of Clostridium difficile on rabbit ileum and colon. Gut 27, 78–85 (1986).
Triadafilopoulos, G., Pothoulakis, C., O'Brien, M. J. & LaMont, J. T. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology 93, 273–279 (1987).
Riegler, M. et al. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J. Clin. Invest. 95, 2004–2011 (1995).
Savidge, T. C. et al. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 125, 413–420 (2003).
Chumbler, N. M. et al. Crystal structure of Clostridium difficile toxin A. Nat. Microbiol. 1, 15002 (2016). A structural study that provided insight into the mode of action of autoproteolytic activity and allosteric activation of the large clostridial toxins.
Pruitt, R. N. et al. Structural determinants of Clostridium difficile toxin A glucosyltransferase activity. J. Biol. Chem. 287, 8013–8020 (2012).
Papatheodorou, P., Zamboglou, C., Genisyuerek, S., Guttenberg, G. & Aktories, K. Clostridial glucosylating toxins enter cells via clathrin-mediated endocytosis. PLoS ONE 5, e10673 (2010).
Pruitt, R. N. & Lacy, D. B. Toward a structural understanding of Clostridium difficile toxins A and B. Front. Cell. Infect. Microbiol. 2, 28 (2012).
Greco, A. et al. Carbohydrate recognition by Clostridium difficile toxin A. Nat. Struct. Mol. Biol. 13, 460–461 (2006).
Murase, T. et al. Structural basis for antibody recognition in the receptor-binding domains of toxins A and B from Clostridium difficile. J. Biol. Chem. 289, 2331–2343 (2014).
Sauerborn, M., Leukel, P. & von Eichel-Streiber, C. The C-terminal ligand-binding domain of Clostridium difficile toxin A (TcdA) abrogates TcdA-specific binding to cells and prevents mouse lethality. FEMS Microbiol. Lett. 155, 45–54 (1997).
Genisyuerek, S. et al. Structural determinants for membrane insertion, pore formation and translocation of Clostridium difficile toxin B. Mol. Microbiol. 79, 1643–1654 (2011).
Olling, A. et al. The repetitive oligopeptide sequences modulate cytopathic potency but are not crucial for cellular uptake of Clostridium difficile toxin A. PLoS ONE 6, e17623 (2011).
Schorch, B. et al. LRP1 is a receptor for Clostridium perfringens TpeL toxin indicating a two-receptor model of clostridial glycosylating toxins. Proc. Natl Acad. Sci. USA 111, 6431–6436 (2014).
LaFrance, M. E. et al. Identification of an epithelial cell receptor responsible for Clostridium difficile TcdB-induced cytotoxicity. Proc. Natl Acad. Sci. USA 112, 7073–7078 (2015).
Yuan, P. et al. Chondroitin sulfate proteoglycan 4 functions as the cellular receptor for Clostridium difficile toxin B. Cell Res. 25, 157–168 (2015).
Terada, N. et al. Immunohistochemical study of NG2 chondroitin sulfate proteoglycan expression in the small and large intestines. Histochem. Cell Biol. 126, 483–490 (2006).
Shen, A. et al. Defining an allosteric circuit in the cysteine protease domain of Clostridium difficile toxins. Nat. Struct. Mol. Biol. 18, 364–371 (2011).
Egerer, M., Giesemann, T., Jank, T., Satchell, K. J. & Aktories, K. Auto-catalytic cleavage of Clostridium difficile toxins A and B depends on cysteine protease activity. J. Biol. Chem. 282, 25314–25321 (2015).
Savidge, T. C. et al. Host S-nitrosylation inhibits clostridial small molecule-activated glucosylating toxins. Nat. Med. 17, 1136–1141 (2011).
Lanis, J. M., Hightower, L. D., Shen, A. & Ballard, J. D. TcdB from hypervirulent Clostridium difficile exhibits increased efficiency of autoprocessing. Mol. Microbiol. 84, 66–76 (2012).
Just, I. et al. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J. Biol. Chem. 270, 13932–13936 (1995).
Just, I. et al. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375, 500–503 (1995).
Brito, G. A. et al. Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J. Infect. Dis. 186, 1438–1447 (2002).
Farrow, M. A. et al. Clostridium difficile toxin B-induced necrosis is mediated by the host epithelial cell NADPH oxidase complex. Proc. Natl Acad. Sci. USA 110, 18674–18679 (2013).
Slater, L. H. et al. Identification of novel host-targeted compounds that protect from anthrax lethal toxin-induced cell death. ACS Chem. Biol. 8, 812–822 (2013).
Tam, J. et al. Small molecule inhibitors of Clostridium difficile toxin B-induced cellular damage. Chem. Biol. 22, 175–185 (2015).
Bender, K. O. et al. A small-molecule antivirulence agent for treating Clostridium difficile infection. Sci. Transl. Med. 7, 306ra148 (2015).
Smith, S. M. et al. Ebselen and congeners inhibit NADPH oxidase 2-dependent superoxide generation by interrupting the binding of regulatory subunits. Chem. Biol. 19, 752–763 (2012).
Kuehne, S. A. et al. Importance of toxin A, toxin B, and CDT in virulence of an epidemic Clostridium difficile strain. J. Infect. Dis. 209, 83–86 (2014).
Carter, G. P. et al. Defining the roles of TcdA and TcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. mBio 6, e00551 (2015). A histopathological analysis of the effects of TcdA and TcdB in a mouse model of CDI.
Drudy, D., Fanning, S. & Kyne, L. Toxin A-negative, toxin B-positive Clostridium difficile. Int. J. Infect. Dis. 11, 5–10 (2007).
King, A. M., Mackin, K. E. & Lyras, D. Emergence of toxin A-negative, toxin B-positive Clostridium difficile strains: epidemiological and clinical considerations. Future Microbiol. 10, 1–4 (2015).
Gerding, D. N., Johnson, S., Rupnik, M. & Aktories, K. Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance. Gut Microbes 5, 15–27 (2014). A review of the different aspects of the binary toxin CDT.
Perelle, S., Gibert, M., Bourlioux, P., Corthier, G. & Popoff, M. R. Production of a complete binary toxin (actin-specific ADP-ribosyltransferase) by Clostridium difficile CD196. Infect. Immun. 65, 1402–1407 (1997).
Goncalves, C., Decre, D., Barbut, F., Burghoffer, B. & Petit, J. C. Prevalence and characterization of a binary toxin (actin-specific ADP-ribosyltransferase) from Clostridium difficile. J. Clin. Microbiol. 42, 1933–1939 (2004).
Stare, B. G., Delmee, M. & Rupnik, M. Variant forms of the binary toxin CDT locus and tcdC gene in Clostridium difficile strains. J. Med. Microbiol. 56, 329–335 (2007).
Carter, G. P. et al. Binary toxin production in Clostridium difficile is regulated by CdtR, a LytTR family response regulator. J. Bacteriol. 189, 7290–7301 (2007).
Metcalf, D. S. & Weese, J. S. Binary toxin locus analysis in Clostridium difficile. J. Med. Microbiol. 60, 1137–1145 (2011).
Sundriyal, A., Roberts, A. K., Shone, C. C. & Acharya, K. R. Structural basis for substrate recognition in the enzymatic component of ADP-ribosyltransferase toxin CDTa from Clostridium difficile. J. Biol. Chem. 284, 28713–28719 (2009).
Schwan, C. et al. Cholesterol- and sphingolipid-rich microdomains are essential for microtubule-based membrane protrusions induced by Clostridium difficile transferase (CDT). J. Biol. Chem. 286, 29356–29365 (2011).
Androga, G. O. et al. Infection With toxin A-negative, toxin B-negative, binary toxin-positive Clostridium difficile in a young patient with ulcerative colitis. J. Clin. Microbiol. 53, 3702–3704 (2015).
Eckert, C. et al. Prevalence and pathogenicity of binary toxin-positive Clostridium difficile strains that do not produce toxins A and B. New Microbes New Infect. 3, 12–17 (2015).
Hutton, M. L., Mackin, K. E., Chakravorty, A. & Lyras, D. Small animal models for the study of Clostridium difficile disease pathogenesis. FEMS Microbiol. Lett. 352, 140–149 (2014).
Lawley, T. D. & Young, V. B. Murine models to study Clostridium difficile infection and transmission. Anaerobe 24, 94–97 (2013).
Best, E. L., Freeman, J. & Wilcox, M. H. Models for the study of Clostridium difficile infection. Gut Microbes 3, 145–167 (2012).
Collignon, A. Methods for working with the mouse model. Methods Mol. Biol. 646, 229–237 (2010).
Lawley, T. D. et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 8, e1002995 (2012).
Steele, J., Feng, H., Parry, N. & Tzipori, S. Piglet models of acute or chronic Clostridium difficile illness. J. Infect. Dis. 201, 428–434 (2010).
Baines, S. D. et al. Mixed infection by Clostridium difficile in an in vitro model of the human gut. J. Antimicrob. Chemother. 68, 1139–1143 (2013).
Crowther, G. S. et al. Development and validation of a chemostat gut model to study both planktonic and biofilm modes of growth of Clostridium difficile and human microbiota. PLoS ONE 9, e88396 (2014).
Dobson, G., Hickey, C. & Trinder, J. Clostridium difficile colitis causing toxic megacolon, severe sepsis and multiple organ dysfunction syndrome. Intensive Care Med. 29, 1030 (2003).
Cohen, S. H. et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect. Control Hosp. Epidemiol. 31, 431–455 (2010).
Debast, S. B., Bauer, M. P. & Kuijper, E. J. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 20 (Suppl. 2), 1–26 (2014).
Yu, H. et al. Identification of toxemia in patients with Clostridium difficile infection. PLoS ONE 10, e0124235 (2015).
Slimings, C. & Riley, T. V. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J. Antimicrob. Chemother. 69, 881–891 (2014).
Kwok, C. S. et al. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am. J. Gastroenterol. 107, 1011–1019 (2012).
McDonald, E. G., Milligan, J., Frenette, C. & Lee, T. C. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern. Med. 175, 784–791 (2015).
Novack, L. et al. Acid suppression therapy does not predispose to Clostridium difficile infection: the case of the potential bias. PLoS ONE 9, e110790 (2014).
Tleyjeh, I. M. et al. Association between proton pump inhibitor therapy and Clostridium difficile infection: a contemporary systematic review and meta-analysis. PLoS ONE 7, e50836 (2012).
Bartlett, J. G. & Gerding, D. N. Clinical recognition and diagnosis of Clostridium difficile infection. Clin. Infect. Dis. 46 (Suppl. 1), S12–S18 (2008).
Zollner-Schwetz, I. et al. Role of Klebsiella oxytoca in antibiotic-associated diarrhea. Clin. Infect. Dis. 47, e74–e78 (2008).
Planche, T. & Wilcox, M. H. Diagnostic pitfalls in Clostridium difficile infection. Infect. Dis. Clin. North Am. 29, 63–82 (2015).
Polage, C. R. et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern. Med. 175, 1792–1801 (2015). Together with reference 34, this report confirms that reliance on molecular tests alone for diagnosing CDI will probably lead to overdiagnosis, overtreatment and increased health-care costs.
Crobach, M. J. et al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. (in the press).
Louie, T. J. et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin. Infect. Dis. 55 (Suppl. 2), S132–S142 (2012).
Eastwood, K., Else, P., Charlett, A. & Wilcox, M. Comparison of nine commercially available Clostridium difficile toxin detection assays, a real-time PCR assay for C. difficile tcdB, and a glutamate dehydrogenase detection assay to cytotoxin testing and cytotoxigenic culture methods. J. Clin. Microbiol. 47, 3211–3217 (2009).
Ota, K. V. & McGowan, K. L. Clostridium difficile testing algorithms using glutamate dehydrogenase antigen and C. difficile toxin enzyme immunoassays with C. difficile nucleic acid amplification testing increase diagnostic yield in a tertiary pediatric population. J. Clin. Microbiol. 50, 1185–1188 (2012).
Reigadas, E. et al. Missed diagnosis of Clostridium difficile infection; a prospective evaluation of unselected stool samples. J. Infect. 70, 264–272 (2015).
Faust, S. N. et al. Lack of evidence for an unmet need to treat Clostridium difficile infection in infants aged <2 years: expert recommendations on how to address this issue. Clin. Infect. Dis. 60, 912–918 (2015).
Schutze, G. E. & Willoughby, R. E. Clostridium difficile infection in infants and children. Pediatrics 131, 196–200 (2013).
Spina, A. et al. Spectrum of enteropathogens detected by the FilmArray GI Panel in a multicentre study of community-acquired gastroenteritis. Clin. Microbiol. Infect. 21, 719–728 (2015).
Bruijnesteijn van Coppenraet, L. E. et al. Case–control comparison of bacterial and protozoan microorganisms associated with gastroenteritis: application of molecular detection. Clin. Microbiol. Infect. 21, 592 (2015).
Curry, S. R. et al. Use of multilocus variable number of tandem repeats analysis genotyping to determine the role of asymptomatic carriers in Clostridium difficile transmission. Clin. Infect. Dis. 57, 1094–1102 (2013).
Kyne, L., Warny, M., Qamar, A. & Kelly, C. P. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 342, 390–397 (2000).
Donskey, C. J., Kundrapu, S. & Deshpande, A. Colonization versus carriage of Clostridium difficile. Infect. Dis. Clin. North Am. 29, 13–28 (2015).
Ziakas, P. D. et al. Asymptomatic carriers of toxigenic C. difficile in long-term care facilities: a meta-analysis of prevalence and risk factors. PLoS ONE 10, e0117195 (2015).
Riggs, M. M. et al. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin. Infect. Dis. 45, 992–998 (2007).
Loo, V. G. et al. Host and pathogen factors for Clostridium difficile infection and colonization. N. Engl. J. Med. 365, 1693–1703 (2011).
Samore, M. H., Venkataraman, L., DeGirolami, P. C., Arbeit, R. D. & Karchmer, A. W. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am. J. Med. 100, 32–40 (1996).
Enoch, D. A., Butler, M. J., Pai, S., Aliyu, S. H. & Karas, J. A. Clostridium difficile in children: colonisation and disease. J. Infect. 63, 105–113 (2011).
Furuichi, M. et al. Characteristics of Clostridium difficile colonization in Japanese children. J. Infect. Chemother. 20, 307–311 (2014).
Leibowitz, J., Soma, V. L., Rosen, L., Ginocchio, C. C. & Rubin, L. G. Similar proportions of stool specimens from hospitalized children with and without diarrhea test positive for Clostridium difficile. Pediatr. Infect. Dis. J. 34, 261–266 (2015).
Bergstrom, A. et al. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl. Environ. Microbiol. 80, 2889–2900 (2014).
Shim, J. K., Johnson, S., Samore, M. H., Bliss, D. Z. & Gerding, D. N. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet 351, 633–636 (1998).
Gerding, D. N. et al. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: a randomized clinical trial. JAMA 313, 1719–1727 (2015).
Brouwer, M. S. et al. Horizontal gene transfer converts non-toxigenic Clostridium difficile strains into toxin producers. Nat. Commun. 4, 2601 (2013).
Clabots, C. R., Johnson, S., Olson, M. M., Peterson, L. R. & Gerding, D. N. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J. Infect. Dis. 166, 561–567 (1992).
Lanzas, C., Dubberke, E. R., Lu, Z., Reske, K. A. & Grohn, Y. T. Epidemiological model for Clostridium difficile transmission in healthcare settings. Infect. Control Hosp. Epidemiol. 32, 553–561 (2011).
Vonberg, R. P. et al. Infection control measures to limit the spread of Clostridium difficile. Clin. Microbiol. Infect. 14 (Suppl. 5), 2–20 (2008).
Dubberke, E. R. et al. Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 update. Infect. Control Hosp. Epidemiol. 35 (Suppl. 2), S48–S65 (2014).
Surawicz, C. M. et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 108, 478–498 (2013).
Dancer, S. J. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin. Microbiol. Rev. 27, 665–690 (2014).
Feazel, L. M. et al. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J. Antimicrob. Chemother. 69, 1748–1754 (2014).
Barbut, F. How to eradicate Clostridium difficile from the environment. J. Hosp. Infect. 89, 287–295 (2015).
Johnson, S. et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin. Infect. Dis. 59, 345–354 (2014). This paper describes two large randomized studies and was the first paper to show the significantly improved outcome following vancomycin versus metronidazole therapy for CDI on an intent-to-treat basis.
Cornely, O. A., Miller, M. A., Louie, T. J., Crook, D. W. & Gorbach, S. L. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin. Infect. Dis. 55 (Suppl. 2), S154–S161 (2012).
Neal, M. D., Alverdy, J. C., Hall, D. E., Simmons, R. L. & Zuckerbraun, B. S. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann. Surg. 254, 423–427 (2011).
Bauer, M. P. et al. Renal failure and leukocytosis are predictors of a complicated course of Clostridium difficile infection if measured on day of diagnosis. Clin. Infect. Dis. 55 (Suppl. 2), S149–S153 (2012).
Kelly, C. P. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin. Microbiol. Infect. 18 (Suppl. 6), 21–27 (2012).
Cornely, O. A. et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect. Dis. 12, 281–289 (2012).
Louie, T. J. et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 364, 422–431 (2011). Together with reference 202, this study formed the basis for the addition of fidaxomicin as a therapeutic for the treatment of recurrent CDI.
D'Agostino Sr, R. B., Collins, S. H., Pencina, K. M., Kean, Y. & Gorbach, S. Risk estimation for recurrent Clostridium difficile infection based on clinical factors. Clin. Infect. Dis. 58, 1386–1393 (2014).
Goldenberg, J. Z. et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 5, CD006095 (2013).
van Nood, E. et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415 (2013). The first randomized controlled trial demonstrating the superiority of FMT over vancomycin for the treatment of patients with multiple recurrences of CDI.
Sokol, H. et al. Faecal microbiota transplantation in recurrent Clostridium difficile infection: recommendations from the French Group of Faecal microbiota Transplantation. Dig. Liver Dis. 48, 242–247 (2015).
Varier, R. U. et al. Cost-effectiveness analysis of fecal microbiota transplantation for recurrent Clostridium difficile infection. Infect. Control Hosp. Epidemiol. 36, 438–444 (2015).
Kump, P. K. et al. Recommendations for the use of faecal microbiota transplantation ‘stool transplantation’: consensus of the Austrian Society of Gastroenterology and Hepatology (OGGH) in cooperation with the Austrian Society of Infectious Diseases and Tropical Medicine. Z. Gastroenterol. 52, 1485–1492 (2014) (in German).
Youngster, I. et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 312, 1772–1778 (2014).
Petrof, E. O. et al. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome 1, 3 (2013).
Tvede, M., Tinggaard, M. & Helms, M. Rectal bacteriotherapy for recurrent Clostridium difficile-associated diarrhoea: results from a case series of 55 patients in Denmark 2000–2012. Clin. Microbiol. Infect. 21, 48–53 (2015).
Khanna, S. et al. A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent Clostridium difficile infection. J. Infect. Dis.http://dx.doi.org/10.1093/infdis/jiv766 (2016).
Wilcox, M. et al. Bezlotoxumab alone and with actoxumab for prevention of recurrant C. difficile infection in patients on standard of care antibiotics: integrated results of 2 Phase 3 studies (MODIFY I and MODIFY II). Open Forum Infect. Dis. [online], (2015).
Wilcox, M. H., Cunniffe, J. G., Trundle, C. & Redpath, C. Financial burden of hospital-acquired Clostridium difficile infection. J. Hosp. Infect. 34, 23–30 (1996).
Vonberg, R. P. et al. Costs of nosocomial Clostridium difficile-associated diarrhoea. J. Hosp. Infect. 70, 15–20 (2008).
Wiegand, P. N. et al. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J. Hosp. Infect. 81, 1–14 (2012). A systematic review of European data on CDI-related mortality, recurrence, length of hospital stay and cost.
Mitchell, B. G. & Gardner, A. Prolongation of length of stay and Clostridium difficile infection: a review of the methods used to examine length of stay due to healthcare associated infections. Antimicrob. Resist. Infect. Control 1, 14 (2012).
Ghantoji, S. S., Sail, K., Lairson, D. R., DuPont, H. L. & Garey, K. W. Economic healthcare costs of Clostridium difficile infection: a systematic review. J. Hosp. Infect. 74, 309–318 (2010).
Nanwa, N. et al. The economic impact of Clostridium difficile infection: a systematic review. Am. J. Gastroenterol. 110, 511–519 (2015).
Zimlichman, E. et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern. Med. 173, 2039–2046 (2013).
Levy, A. R. et al. Incidence and costs of Clostridium difficile infections in Canada. Open. Forum Infect. Dis. 2, ofv076 (2015).
Kwon, J. H., Olsen, M. A. & Dubberke, E. R. The morbidity, mortality, and costs associated with Clostridium difficile infection. Infect. Dis. Clin. North Am. 29, 123–134 (2015).
Vuong, N. N. et al. Use of PROMIS network to evaluate patient-reported health status associated with Clostridium difficile infection. Am. Soc. Microbiol. Abstr.[online], (2015).
Fimlaid, K. A. & Shen, A. Diverse mechanisms regulate sporulation sigma factor activity in the Firmicutes. Curr. Opin. Microbiol. 24, 88–95 (2015).
Battistuzzi, F. U., Feijao, A. & Hedges, S. B. A genomic timescale of prokaryote evolution: insights into the origin of methanogenesis, phototrophy, and the colonization of land. BMC. Evol. Biol. 4, 44 (2004).
Merrigan, M. et al. Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production. J. Bacteriol. 192, 4904–4911 (2010).
Burns, D. A., Heap, J. T. & Minton, N. P. The diverse sporulation characteristics of Clostridium difficile clinical isolates are not associated with type. Anaerobe 16, 618–622 (2010).
Borgmann, S. et al. Increased number of Clostridium difficile infections and prevalence of Clostridium difficile PCR ribotype 001 in southern Germany. Euro. Surveill. 13, 5 (2008).
Knetsch, C. W. et al. Genetic markers for Clostridium difficile lineages linked to hypervirulence. Microbiology 157, 3113–3123 (2011).
Eyre, D. W. et al. Emergence and spread of predominantly community-onset Clostridium difficile PCR ribotype 244 infection in Australia, 2010 to 2012. Euro. Surveill. 20, 21059 (2015).
Rea, M. C. et al. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc. Natl Acad. Sci. USA 107, 9352–9357 (2010).
Hopkins, M. J. & Macfarlane, G. T. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J. Med. Microbiol. 51, 448–454 (2002).
Bingley, P. J. & Harding, G. M. Clostridium difficile colitis following treatment with metronidazole and vancomycin. Postgrad. Med. J. 63, 993–994 (1987).
Gebhart, D. et al. A modified R-type bacteriocin specifically targeting Clostridium difficile prevents colonization of mice without affecting gut microbiota diversity. mBio 6, e02368-14 (2015).
Hargreaves, K. R. & Clokie, M. R. Clostridium difficile phages: still difficult? Front. Microbiol. 5, 184 (2014).
Rasko, D. A. & Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 9, 117–128 (2010).
Lowy, I. et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N. Engl. J. Med. 362, 197–205 (2010).
Martin, C. E. et al. Immunological evaluation of a synthetic Clostridium difficile oligosaccharide conjugate vaccine candidate and identification of a minimal epitope. J. Am. Chem. Soc. 135, 9713–9722 (2013).
Kandalaft, H. et al. Targeting surface-layer proteins with single-domain antibodies: a potential therapeutic approach against Clostridium difficile-associated disease. Appl. Microbiol. Biotechnol. 99, 8549–8562 (2015).
Abou Chakra, C. N., Pepin, J. & Valiquette, L. Prediction tools for unfavourable outcomes in Clostridium difficile infection: a systematic review. PLoS ONE 7, e30258 (2012).
Na, X. et al. A multi-center prospective derivation and validation of a clinical prediction tool for severe Clostridium difficile infection. PLoS ONE 10, e0123405 (2015).
Hensgens, M. P., Dekkers, O. M., Goorhuis, A., LeCessie, S. & Kuijper, E. J. Predicting a complicated course of Clostridium difficile infection at the bedside. Clin. Microbiol. Infect. 20, O301–O308 (2014).
Zilberberg, M. D., Reske, K., Olsen, M., Yan, Y. & Dubberke, E. R. Development and validation of a recurrent Clostridium difficile risk-prediction model. J. Hosp. Med. 9, 418–423 (2014).
European Commission. The 2015 Ageing Report. Underlying Assumptions and Projection Methodologies. European Economy[online], (2014).
Ortman, J. M., Velkoff, V. A. & Howard, H. An Aging Nation: The Older Population in the United States. US Census Bureau[online], (2014).
Tenover, F. C. et al. Comparison of strain typing results for Clostridium difficile isolates from North America. J. Clin. Microbiol. 49, 1831–1837 (2011).
Fawley, W. N. et al. Development and validation of an internationally-standardized, high-resolution capillary gel-based electrophoresis PCR-ribotyping protocol for Clostridium difficile. PLoS ONE 10, e0118150 (2015).
Baines, S. D., O'Connor, R., Saxton, K., Freeman, J. & Wilcox, M. H. Activity of vancomycin against epidemic Clostridium difficile strains in a human gut model. J. Antimicrob. Chemother. 63, 520–525 (2009).
Paredes-Sabja, D., Cofre-Araneda, G., Brito-Silva, C., Pizarro-Guajardo, M. & Sarker, M. R. Clostridium difficile spore-macrophage interactions: spore survival. PLoS ONE 7, e43635 (2012).
Spigaglia, P., Barbanti, F. & Mastrantonio, P. Multidrug resistance in European Clostridium difficile clinical isolates. J. Antimicrob. Chemother. 66, 2227–2234 (2011).
Peltier, J. et al. Genomic and expression analysis of the vanG-like gene cluster of Clostridium difficile. Microbiology 159, 1510–1520 (2013).
Ammam, F. et al. The functional vanG Cd cluster of Clostridium difficile does not confer vancomycin resistance. Mol. Microbiol. 89, 612–625 (2013).
Amy, J., Johanesen, P. & Lyras, D. Extrachromosomal and integrated genetic elements in Clostridium difficile. Plasmid 80, 97–110 (2015).
Hansen, L. H. & Vester, B. A cfr-like gene from Clostridium difficile confers multiple antibiotic resistance by the same mechanism as the cfr gene. Antimicrob. Agents Chemother. 59, 5841–5843 (2015).
Roberts, A. P., Johanesen, P. A., Lyras, D., Mullany, P. & Rood, J. I. Comparison of Tn5397 from Clostridium difficile, Tn916 from Enterococcus faecalis and the CW459tet(M) element from Clostridium perfringens shows that they have similar conjugation regions but different insertion and excision modules. Microbiology 147, 1243–1251 (2001).
Chong, P. M. et al. Proteomic analysis of a NAP1 Clostridium difficile clinical isolate resistant to metronidazole. PLoS ONE 9, e82622 (2014).
Lynch, T. et al. Characterization of a stable, metronidazole-resistant Clostridium difficile clinical isolate. PLoS ONE 8, e53757 (2013).
Leeds, J. A., Sachdeva, M., Mullin, S., Barnes, S. W. & Ruzin, A. In vitro selection, via serial passage, of Clostridium difficile mutants with reduced susceptibility to fidaxomicin or vancomycin. J. Antimicrob. Chemother. 69, 41–44 (2014).
Freeman, J. et al. Pan-European longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes. Clin. Microbiol. Infect. 21, 248.e9–248.e16 (2015).
Centers for Disease Control and Prevention. Threat Report 2013. CDC[online], (2013). A report from the CDC that qualifies C. difficile as an urgent antibiotic-resistance threat.
Spigaglia, P. et al. Fluoroquinolone resistance in Clostridium difficile isolates from a prospective study of C. difficile infections in Europe. J. Med. Microbiol. 57, 784–789 (2008).
Papatheodorou, P. et al. Lipolysis-stimulated lipoprotein receptor (LSR) is the host receptor for the binary toxin Clostridium difficile transferase (CDT). Proc. Natl Acad. Sci. USA 108, 16422–16427 (2011).
Martin, J., Monaghan, T. & Wilcox, M. H. Clostridium difficile infection: advances in epidemiology, diagnosis and understanding of transmission. Nat. Rev. Gastroenterol. Hepatol.http://dx.doi.org/10.1038/nrgastro.2016.25 (2016).
Le, M. A. et al. Hospital cost of Clostridium difficile infection including the contribution of recurrences in French acute-care hospitals. J. Hosp. Infect. 91, 117–122 (2015).
Gabriel, L. & Beriot-Mathiot, A. Hospitalization stay and costs attributable to Clostridium difficile infection: a critical review. J. Hosp. Infect. 88, 12–21 (2014).
Acknowledgements
This work was partially supported by a Vidi fellowship from the Netherlands Organization for Scientific Research and a Gisela Thier Fellowship from the Leiden University Medical Center to W.K.S.; a National Institutes of Health Award AI95755 and a Burroughs Wellcome Investigator in the Pathogenesis of Infectious Disease Award to D.B.L.; and a Future Fellowship FT120100779 of the Australian Research Council to D.L. The authors thank the ESCMID Study Group for Clostridium difficile (ESGCD) for their advice and suggestions and apologize to the authors whose work could not be cited due to restrictions imposed by the format of this Primer. W. Knetsch is acknowledged for drafting the illustration used in Box 1.
Author information
Authors and Affiliations
Contributions
Introduction (W.K.S.); Epidemiology (M.H.W., E.J.K. and W.K.S.); Mechanisms/pathophysiology (D.B.L., D.L. and W.K.S.); Diagnosis, screening and prevention (M.H.W., E.J.K. and W.K.S.); Management (M.H.W., E.J.K. and W.K.S.); Quality of life (M.H.W., E.J.K. and W.K.S.); Outlook (W.K.S. and E.J.K.); overview of the Primer (W.K.S.).
Corresponding author
Ethics declarations
Competing interests
W.K.S. has performed research for Cubist. D.L. has performed research for Immuron and Adenium Biotech. D.B.L. has performed research for MedImmune and Merck. M.H.W. has received consulting fees from Abbott, Actelion Pharmaceuticals, Astellas, AstraZeneca, Bayer, Cerexa, Cubist, Durata, The European Tissue Symposium, The Medicines Company, MedImmune, Merck, Motif Biosciences, Nabriva, Optimer, Paratek, Pfizer, Roche, Sanofi Pasteur, Seres Therapeutics, Summit Pharmaceuticals, and Synthetic Biologics. M.H.W. has also received lecture fees from Abbott, Alere, Astellas, AstraZeneca, Pfizer and Hoffmann La Roche, and received grant support from Abbott, Actelion, Astellas, bioMérieux, Cubist, Da Volterra, The European Tissue Symposium, Merck and Summit Pharmaceuticals. E.J.K. has performed research for Cubist, Novartis and Qiagen, and has participated in advisory forums of Astellas, Optimer, Actelion, Pfizer, Sanofi Pasteur and Seres Therapeutics. These companies had no role in the writing of this Primer.
Rights and permissions
About this article
Cite this article
Smits, W., Lyras, D., Lacy, D. et al. Clostridium difficile infection. Nat Rev Dis Primers 2, 16020 (2016). https://doi.org/10.1038/nrdp.2016.20
Published:
DOI: https://doi.org/10.1038/nrdp.2016.20
This article is cited by
-
The anti-inflammatory and anti-apoptotic effects of Achillea millefolium L. extracts on Clostridioides difficile ribotype 001 in human intestinal epithelial cells
BMC Complementary Medicine and Therapies (2024)
-
The burden of CDI in the United States: a multifactorial challenge
BMC Infectious Diseases (2023)
-
Emerging applications of phage therapy and fecal virome transplantation for treatment of Clostridioides difficile infection: challenges and perspectives
Gut Pathogens (2023)
-
Modulation of colonic immunometabolic responses during Clostridioides difficile infection ameliorates disease severity and inflammation
Scientific Reports (2023)
-
C. difficile intoxicates neurons and pericytes to drive neurogenic inflammation
Nature (2023)