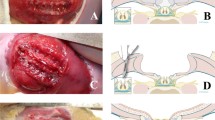
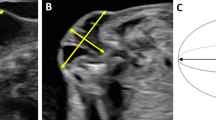
Abstract
Spina bifida is a birth defect in which the vertebral column is open, often with spinal cord involvement. The most clinically significant subtype is myelomeningocele (open spina bifida), which is a condition characterized by failure of the lumbosacral spinal neural tube to close during embryonic development. The exposed neural tissue degenerates in utero, resulting in neurological deficit that varies with the level of the lesion. Occurring in approximately 1 per 1,000 births worldwide, myelomeningocele is one of the most common congenital malformations, but its cause is largely unknown. The genetic component is estimated at 60–70%, but few causative genes have been identified to date, despite much information from mouse models. Non-genetic maternal risk factors include reduced folate intake, anticonvulsant therapy, diabetes mellitus and obesity. Primary prevention by periconceptional supplementation with folic acid has been demonstrated in clinical trials, leading to food fortification programmes in many countries. Prenatal diagnosis is achieved by ultrasonography, enabling women to seek termination of pregnancy. Individuals who survive to birth have their lesions closed surgically, with subsequent management of associated defects, including the Chiari II brain malformation, hydrocephalus, and urological and orthopaedic sequelae. Fetal surgical repair of myelomeningocele has been associated with improved early neurological outcome compared with postnatal operation. Myelomeningocele affects quality of life during childhood, adolescence and adulthood, posing a challenge for individuals, families and society as a whole. For an illustrated summary of this Primer, visit: http://go.nature.com/fK9XNa
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Meuli, M. et al. The spinal cord lesion in human fetuses with myelomeningocele: Implications for fetal surgery. J. Pediatr. Surg. 32, 448–452 (1997).
Oakeshott, P., Hunt, G. M., Poulton, A. & Reid, F. Open spina bifida: birth findings predict long-term outcome. . Arch. Dis. Child. 97, 474–476 (2012).
Yi, Y., Lindemann, M., Colligs, A. & Snowball, C. Economic burden of neural tube defects and impact of prevention with folic acid: a literature review. Eur. J. Pediatr. 170, 1391–1400 (2011).
Elwood, J. M., Little, J. & Elwood, J. H. Epidemiology and Control of Neural Tube Defects (Oxford Univ. Press, 1992).
International Center on Birth Defects. International Clearinghouse for Birth Defects Monitoring Systems (ICBDMS), Annual Report 2011 with Data for 2009 (International Center on Birth Defects, 2011).
Li, Z. et al. Extremely high prevalence of neural tube defects in a 4-county area in Shanxi Province, China. Birth Defects Res. A Clin. Mol. Teratol. 76, 237–240 (2006).
Moore, C. A. et al. Elevated rates of severe neural tube defects in a high-prevalence area in northern China. Am. J. Med. Genet. 73, 113–118 (1997).
Canfield, M. A. et al. Anencephaly and spina bifida among Hispanics: maternal, sociodemographic, and acculturation factors in the National Birth Defects Prevention Study. Birth Defects Res. A Clin. Mol. Teratol. 85, 637–646 (2009).
Cragan, J. D. et al. Surveillance for anencephaly and spina bifida and the impact of prenatal diagnosis – United States, 1985–1994. MMWR CDC Surveill. Summ. 44, 1–13 (1995).
Velie, E. M. & Shaw, G. M. Impact of prenatal diagnosis and elective termination on prevalence and risk estimates of neural tube defects in California, 1989–1991. Am. J. Epidemiol. 144, 473–479 (1996).
Dolk, H., Loane, M. & Garne, E. The prevalence of congenital anomalies in Europe. Adv. Exp. Med. Biol. 686, 349–364 (2010).
Carter, C. O. & Evans, K. A. Spina bifida and anencephalus in Greater London. J. Med. Genet. 10, 209–234 (1973).
Rampersaud, E., Melvin, E. C. & Speer, M. C. in Neural Tube Defects: From Origin to Treatment (ed. Wyszynski, D. F. ) 165–175 (Oxford Univ. Press, 2006).
Juriloff, D. M. & Harris, M. J. Hypothesis: the female excess in cranial neural tube defects reflects an epigenetic drag of the inactivating X chromosome on the molecular mechanisms of neural fold elevation. Birth Defects Res. A Clin. Mol. Teratol. 94, 849–855 (2012).
Leck, I. Causation of neural tube defects: clues from epidemiology. Br. Med. Bull. 30, 158–163 (1974).
Stothard, K. J., Tennant, P. W., Bell, R. & Rankin, J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA 301, 636–650 (2009).
Waller, D. K. et al. Prepregnancy obesity as a risk factor for structural birth defects. Arch. Pediatr. Adolesc. Med. 161, 745–750 (2007).
Carmichael, S. L., Rasmussen, S. A. & Shaw, G. M. Prepregnancy obesity: a complex risk factor for selected birth defects. Birth Defects Res. A Clin. Mol. Teratol. 88, 804–810 (2010). This paper provides a critical synoptic view of the importance and complexity of obesity as a risk factor for human birth defects.
Parker, S. E., Yazdy, M. M., Tinker, S. C., Mitchell, A. A. & Werler, M. M. The impact of folic acid intake on the association among diabetes mellitus, obesity, and spina bifida. Am. J. Obstet. Gynecol. 209, 239–238 (2013).
Agopian, A. J., Tinker, S. C., Lupo, P. J., Canfield, M. A. & Mitchell, L. E. Proportion of neural tube defects attributable to known risk factors. Birth Defects Res. A Clin. Mol. Teratol. 97, 42–46 (2013).
Stiefel, D., Copp, A. J. & Meuli, M. Fetal spina bifida: loss of neural function in utero. J. Neurosurg. 106, 213–221 (2007).
Harris, M. J. & Juriloff, D. M. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res. A Clin. Mol. Teratol. 88, 653–669 (2010). This article gives an overview of >200 genes in mice that when mutated or knocked out, give rise to a range of NTDs, confirming that various pathways are involved in neurulation.
Copp, A. J. & Greene, N. D. E. Genetics and development of neural tube defects. J. Pathol. 220, 217–230 (2010).
Harris, M. J. & Juriloff, D. M. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res. A Clin. Mol. Teratol. 79, 187–210 (2007).
Juriloff, D. M. & Harris, M. J. A consideration of the evidence that genetic defects in planar cell polarity contribute to the etiology of human neural tube defects. Birth Defects Res. A Clin. Mol. Teratol. 94, 824–840 (2012).
Murdoch, J. N. et al. Interactions between planar cell polarity genes cause diverse neural tube defects. Dis. Model. Mech. 7, 1153–1163 (2014).
Amorim, M. R., Lima, M. A., Castilla, E. E. & Orioli, I. M. Non-Latin European descent could be a requirement for association of NTDs and MTHFR variant 677C > T: a meta-analysis. Am. J. Med. Genet. A 143A, 1726–1732 (2007).
Pickell, L. et al. Methylenetetrahydrofolate reductase deficiency and low dietary folate increase embryonic delay and placental abnormalities in mice. Birth Defects Res. A Clin. Mol. Teratol. 85, 531–541 (2009).
Narisawa, A. et al. Mutations in genes encoding the glycine cleavage system predispose to neural tube defects in mice and humans. Hum. Mol. Genet. 21, 1496–1503 (2012).
Pai, Y. J. et al. Glycine decarboxylase deficiency causes neural tube defects and features of non-ketotic hyperglycinemia in mice. Nat. Commun. 6, 6388 (2015).
Robert, E. & Guidbaud, P. Maternal valproic acid and congenital neural tube defects. Lancet 320, 937 (1982).
Phiel, C. J. et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 276, 36734–36741 (2001).
Hendricks, K. A., Simpson, J. S. & Larsen, R. D. Neural tube defects along the Texas-Mexico border, 1993–1995. Am. J. Epidemiol. 149, 1119–1127 (1999).
Marasas, W. F. O. et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 134, 711–716 (2004).
Phelan, S. A., Ito, M. & Loeken, M. R. Neural tube defects in embryos of diabetic mice: role of the Pax-3gene and apoptosis. Diabetes 46, 1189–1197 (1997).
Müller, F. & O'Rahilly, R. The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at stage 12. Anat. Embryol. 176, 413–430 (1987).
Ybot-Gonzalez, P. et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development 134, 789–799 (2007).
Ybot-Gonzalez, P. et al. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of BMP signalling. Development 134, 3203–3211 (2007).
Van Straaten, H. W. M. & Copp, A. J. Curly tail: a 50-year history of the mouse spina bifida model. Anat. Embryol. 203, 225–237 (2001).
Schoenwolf, G. C. Histological and ultrastructural studies of secondary neurulation of mouse embryos. Am. J. Anat. 169, 361–374 (1984).
Wilson, V., Olivera-Martinez, I. & Storey, K. G. Stem cells, signals and vertebrate body axis extension. Development 136, 1591–1604 (2009). This paper reviews the important studies that have shown the existence of multipotent progenitor cells at the caudal extremity of the embryo, and that have determined the signalling pathways involved in secondary neurulation and formation of the lower body axis.
Finn, M. A. & Walker, M. L. Spinal lipomas: clinical spectrum, embryology, and treatment. Neurosurg. Focus 23, E10 (2007).
Barkovich, A. J. & Raybaud, C. Pediatric Neuroimaging (Lippincott Williams & Wilkins, 2011).
McLone, D. G. & Knepper, P. A. The cause of Chiari II malformation: a unified theory. Pediatr. Neurosci. 15, 1–12 (1989).
Juranek, J., Dennis, M., Cirino, P. T., El-Messidi, L. & Fletcher, J. M. The cerebellum in children with spina bifida and Chiari II malformation: quantitative volumetrics by region. Cerebellum 9, 240–248 (2010).
Fletcher, J. M. et al. Spinal lesion level in spina bifida: a source of neural and cognitive heterogeneity. J. Neurosurg. 102, 268–279 (2005).
Ware, A. L. et al. Anatomical and diffusion MRI of deep gray matter in pediatric spina bifida. Neuroimage Clin. 5, 120–127 (2014).
Treble-Barna, A. et al. Prospective and episodic memory in relation to hippocampal volume in adults with spina bifida myelomeningocele. Neuropsychology 29, 92–101 (2014).
Barkovich, A. J. & Norman, D. Anomalies of the corpus callosum: correlation with further anomalies of the brain. Am. J. Roentgenol. 151, 171–179 (1988).
Crawley, J. T. et al. Structure, integrity, and function of the hypoplastic corpus callosum in spina bifida myelomeningocele. Brain Connect. 4, 608–618 (2014).
Herweh, C. et al. DTI of commissural fibers in patients with Chiari II-malformation. Neuroimage 44, 306–311 (2009).
Erol, F. S., Ozturk, S., Akgun, B., Cakin, H. & Kaplan, M. How innocent is corpus callosum dysgenesis? Pediatr. Neurosurg. 49, 24–28 (2013).
Juranek, J. et al. Neocortical reorganization in spina bifida. Neuroimage 40, 1516–1522 (2008).
Del Bigio, M. R. Neuropathology and structural changes in hydrocephalus. Dev. Disabil. Res. Rev. 16, 16–22 (2010). This report integrates the results of animal and human studies to further our understanding of the effects of hydrocephalus on the brain.
Hasan, K. M. et al. White matter microstructural abnormalities in children with spina bifida myelomeningocele and hydrocephalus: a diffusion tensor tractography study of the association pathways. J. Magn. Reson. Imaging 27, 700–709 (2008).
Ou, X., Glasier, C. M. & Snow, J. H. Diffusion tensor imaging evaluation of white matter in adolescents with myelomeningocele and Chiari II malformation. Pediatr. Radiol. 41, 1407–1415 (2011).
Williams, V. J. et al. Examination of frontal and parietal tectocortical attention pathways in spina bifida meningomyelocele using probabilistic diffusion tractography. Brain Connect. 3, 512–522 (2013).
Hampton, L. E. et al. Hydrocephalus status in spina bifida: an evaluation of variations in neuropsychological outcomes. J. Neurosurg. Pediatr. 8, 289–298 (2011).
Treble, A., Juranek, J., Stuebing, K. K., Dennis, M. & Fletcher, J. M. Functional significance of atypical cortical organization in spina bifida myelomeningocele: relations of cortical thickness and gyrification with IQ and fine motor dexterity. Cereb. Cortex 23, 2357–2369 (2013).
Dennis, M., Salman, M. S., Juranek, J. & Fletcher, J. M. Cerebellar motor function in spina bifida meningomyelocele. Cerebellum 9, 484–498 (2010).
Treble-Barna, A., Kulesz, P. A., Dennis, M. & Fletcher, J. M. Covert orienting in three etiologies of congenital hydrocephalus: the effect of midbrain and posterior fossa dysmorphology. J. Int. Neuropsychol. Soc. 20, 268–277 (2014).
Hannay, H. J. et al. Auditory interhemispheric transfer in relation to patterns of partial agenesis and hypoplasia of the corpus callosum in spina bifida meningomyelocele. J. Int. Neuropsychol. Soc. 14, 771–781 (2008).
Taylor, H. B. et al. Motor contingency learning and infants with spina bifida. J. Int. Neuropsychol. Soc. 19, 206–215 (2013).
Dennis, M., Landry, S. H., Barnes, M. & Fletcher, J. M. A model of neurocognitive function in spina bifida over the life span. J. Int. Neuropsychol. Soc. 12, 285–296 (2006). This work provides a framework for understanding the variability in cognitive and motor outcomes for people with myelomeningocele, based on genetic, neurological and environmental factors.
Brock, D. J. H. & Sutcliffe, R. G. Early prenatal diagnosis of anencephaly. Lancet 300, 1252–1253 (1972).
Seller, M. J., Campbell, S., Coltart, T. M. & Singer, J. D. Early termination of anencephalic pregnancy after detection by raised alpha-fetoprotein levels. Lancet 302, 73 (1973).
[No authors listed.] Amniotic fluid acetylcholinesterase electrophoresis as a secondary test in the diagnosis of anencephaly and open spina bifida in early pregnancy. Report of the Collaborative Acetylcholinesterase Study. Lancet 318, 321–324 (1981).
Wald, N. J. et al. Maternal serum-alpha-fetoprotein measurement in antenatal screening for anencephaly and spina bifida in early pregnancy. Report of UK collaborative study on alpha-fetoprotein in relation to neural-tube defects. Lancet 309, 1323 (1977).
Wald, N. J. Prenatal screening for open neural tube defects and Down syndrome: three decades of progress. Prenat. Diag. 30, 619–621 (2010).
Campbell, S., Pryse-Davies, J., Coltart, T. M., Seller, M. J. & Singer, J. D. Ultrasound in the diagnosis of spina bifida. Lancet 305, 1065–1068 (1975).
Wald, N., Cuckle, H., Boreham, J. & Stirrat, G. Small biparietal diameter of fetuses with spina bifida: implications for antenatal screening. Br. J. Obstet. Gynaecol. 87, 219–221 (1980).
Biggio, J. R. Jr., Owen, J., Wenstrom, K. D. & Oakes, W. J. Can prenatal ultrasound findings predict ambulatory status in fetuses with open spina bifida? Am. J. Obstet. Gynecol. 185, 1016–1020 (2001).
Nicolaides, K. H., Gabbe, S. G., Campbell, S. & Guidetti, R. Ultrasound screening for spina bifida: cranial and cerebellar signs. Lancet 328, 72–74 (1986).
Nyberg, D. A., Mack, L. A., Hirsch, J. & Mahony, B. S. Abnormalities of fetal cranial contour in sonographic detection of spina bifida: evaluation of the “lemon” sign. Radiology 167, 387–392 (1988).
Penso, C., Redline, R. W. & Benacerraf, B. R. A sonographic sign which predicts which fetuses with hydrocephalus have an associated neural tube defect. J. Ultrasound Med. 6, 307–311 (1987).
Thiagarajah, S. et al. Early diagnosis of spina bifida: the value of cranial ultrasound markers. Obstet. Gynecol. 76, 54–57 (1990).
Bahlmann, F. et al. Cranial and cerebral signs in the diagnosis of spina bifida between 18 and 22 weeks of gestation: a German multicenter study. Prenat. Diag. 35, 228–235 (2014).
Blumenfeld, Z., Siegler, E. & Bronshtein, M. The early diagnosis of neural tube defects. Prenat. Diag. 13, 863–871 (1993).
Van den Hof, M. C., Nicolaides, K. H., Campbell, J. & Campbell, S. Evaluation of the lemon and banana signs in one hundred thirty fetuses with open spina bifida. Am. J. Obstet. Gynecol. 162, 322–327 (1990). This investigation demonstrates the importance of the cranial signs in prenatal ultrasonography screening for spina bifida.
Chitty, L. S. Ultrasound screening for fetal abnormalities. Prenat. Diag. 15, 1241–1257 (1995).
Rankin, J., Glinianaia, S., Brown, R. & Renwick, M. The changing prevalence of neural tube defects: a population-based study in the north of England, 1984–1996. Paediatr. Perinat. Epidemiol. 14, 104–110 (2000).
[No authors listed.] NHS Fetal Anomaly Screening Programme Standards. UK National Screening Committee[online], (2015).
Kennedy, D., Chitayat, D., Winsor, E. J. T., Silver, M. & Toi, A. Prenatally diagnosed neural tube defects: ultrasound, chromosome, and autopsy or postnatal findings in 212 cases. Am. J. Med. Genet. 77, 317–321 (1998). This work studies the underlying aetiology of NTDs and demonstrates the benefit of autopsy and other investigations in determining aetiology.
Buyukkurt, S. et al. Prenatal determination of the upper lesion level of spina bifida with three-dimensional ultrasound. Fetal Diagn. Ther. 33, 36–40 (2013).
Van Der Vossen, S. et al. Role of prenatal ultrasound in predicting survival and mental and motor functioning in children with spina bifida. Ultrasound Obstet. Gynecol. 34, 253–258 (2009).
Obican, S. G., Finnell, R. H., Mills, J. L., Shaw, G. M. & Scialli, A. R. Folic acid in early pregnancy: a public health success story. FASEB J. 24, 4167–4174 (2010). This paper nicely summarizes the very extensive literature on folic acid and NTDs, including data obtained from human genetic studies and from animal experiments.
Smithells, R. W., Sheppard, S. & Schorah, C. J. Vitamin deficiencies and neural tube defects. Arch. Dis. Child. 51, 944–950 (1976).
Smithells, R. W. et al. Apparent prevention of neural tube defects by periconceptional vitamin supplementation. Arch. Dis. Child. 56, 911–918 (1981).
MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 338, 131–137 (1991). This is the randomized clinical trial on which primary prevention of spina bifida by folic acid is based.
Czeizel, A. E. & Dudás, I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 327, 1832–1835 (1992).
Canfield, M. A. et al. Changes in the birth prevalence of selected birth defects after grain fortification with folic acid in the United States: findings from a multi-state population-based study. Birth Defects Res. A Clin. Mol. Teratol. 73, 679–689 (2005).
Cortes, F., Mellado, C., Pardo, R. A., Villarroel, L. A. & Hertrampf, E. Wheat flour fortification with folic acid: changes in neural tube defects rates in Chile. Am. J. Med. Genet. A 158A, 1885–1890 (2012).
Chen, L. T. & Rivera, M. A. The Costa Rican experience: reduction of neural tube defects following food fortification programs. Nutr. Rev. 62, S40–S43 (2004).
De Wals, P. et al. Reduction in neural-tube defects after folic acid fortification in Canada. N. Engl. J. Med. 357, 135–142 (2007).
Sayed, A. R., Bourne, D., Pattinson, R., Nixon, J. & Henderson, B. Decline in the prevalence of neural tube defects following folic acid fortification and its cost-benefit in South Africa. Birth Defects Res. A Clin. Mol. Teratol. 82, 211–216 (2008).
Safdar, O. Y., Al-Dabbagh, A. A., Abuelieneen, W. A. & Kari, J. A. Decline in the incidence of neural tube defects after the national fortification of flour (1997–2005). Saudi Med. J. 28, 1227–1229 (2007).
Pacheco, S. S., Braga, C., Souza, A. I. & Figueiroa, J. N. Effects of folic acid fortification on the prevalence of neural tube defects. Rev. Saude Publica 43, 565–571 (2009).
Ricks, D. J. et al. Peru's national folic acid fortification program and its effect on neural tube defects in Lima. Rev. Panam. Salud Pubica 32, 391–398 (2012).
Bower, C., D'Antoine, H. & Stanley, F. J. Neural tube defects in Australia: trends in encephaloceles and other neural tube defects before and after promotion of folic acid supplementation and voluntary food fortification. Birth Defects Res. A Clin. Mol. Teratol. 85, 269–273 (2009).
Oakley, G. P. Jr. Folic acid-preventable spina bifida: a good start but much to be done. Am. J. Prev. Med. 38, 569–570 (2010).
Heseker, H. B., Mason, J. B., Selhub, J., Rosenberg, I. H. & Jacques, P. F. Not all cases of neural-tube defect can be prevented by increasing the intake of folic acid. Br. J. Nutr. 102, 173–180 (2009).
Osterhues, A., Ali, N. S. & Michels, K. B. The role of folic acid fortification in neural tube defects: a review. Crit. Rev. Food Sci. Nutr. 53, 1180–1190 (2013).
Rintoul, N. E. et al. A new look at myelomeningoceles: functional level, vertebral level, shunting, and the implications for fetal intervention. Pediatrics 109, 409–413 (2002).
Kulkarni, A. V. et al. Endoscopic third ventriculostomy and choroid plexus cauterization in infants with hydrocephalus: a retrospective Hydrocephalus Clinical Research Network study. J. Neurosurg. Pediatr. 14, 224–229 (2014).
McComb, J. G. Spinal and cranial neural tube defects. Semin. Pediatr. Neurol. 4, 156–166 (1997).
Bauer, S. B. The management of the myelodysplastic child: a paradigm shift. BJU Int. 92, (Suppl. 1), 23–28 (2003).
Leibold, S., Ekmark, E. & Adams, R. C. Decision-making for a successful bowel continence program. Eur. J. Pediatr. Surg. 10, (Suppl. 1), 26–30 (2000).
Perez, M., Lemelle, J. L., Barthelme, H., Marquand, D. & Schmitt, M. Bowel management with antegrade colonic enema using a Malone or a Monti conduit – clinical results. Eur. J. Pediatr. Surg. 11, 315–318 (2001).
Adzick, N. S. Fetal surgery for myelomeningocele: trials and tribulations. Isabella Forshall Lecture. J. Pediatr. Surg. 47, 273–281 (2012).
Sutton, L. N. et al. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA 282, 1826–1831 (1999).
Bruner, J. P. et al. Fetal surgery for myelomeningocele and the incidence of shunt- dependent hydrocephalus. JAMA 282, 1819–1825 (1999).
Bouchard, S. et al. Correction of hindbrain herniation and anatomy of the vermis after in utero repair of myelomeningocele in sheep. J. Pediatr. Surg. 38, 451–458 (2003).
Quinn, T. M., Hubbard, A. M. & Adzick, N. S. Prenatal magnetic resonance imaging enhances fetal diagnosis. J. Pediatr. Surg. 33, 553–558 (1998).
Adzick, N. S. et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N. Engl. J. Med. 364, 993–1004 (2011). This prospective randomized clinical trial shows that prenatal repair reduces the need for a ventriculoperitoneal shunt and improves motor outcomes when compared with postnatal repair. Fetal and maternal risks relating to fetal surgery are quantified.
Rychik, J. et al. Acute cardiovascular effects of fetal surgery in the human. Circulation 110, 1549–1556 (2004).
Adzick, N. S., Sutton, L. N., Crombleholme, T. M. & Flake, A. W. Successful fetal surgery for spina bifida. Lancet 352, 1675–1676 (1998). This is the first report of successful fetal surgery for spina bifida in an early-gestation human fetus.
Tulipan, N., Hernanz-Schulman, M. & Bruner, J. P. Reduced hindbrain herniation after intrauterine myelomeningocele repair: a report of four cases. Pediatr. Neurosurg. 29, 274–278 (1998).
Black, M. M. & Matula, K. Essentials of Bayley Scales of Infant Development (Wiley, 1999).
Werner, E. F. et al. Evaluating the cost-effectiveness of prenatal surgery for myelomeningocele: a decision analysis. Ultrasound Obstet. Gynecol. 40, 158–164 (2012).
Moldenhauer, J. S. et al. Fetal myelomeningocele repair: the post-MOMS experience at the Children's Hospital of Philadelphia. Fetal Diagn. Ther. 37, 235–240 (2014).
Cohen, A. R. et al. Position statement on fetal myelomeningocele repair. Am. J. Obstet. Gynecol. 210, 107–111 (2014).
Greenley, R. N., Holmbeck, G. N., Zukerman, J. & Buck, C. F. in Neural Tube Defects: From Origin to Treatment (ed. Wyszynski, D. F. ) 307–324 (Oxford Univ. Press, 2006).
Kelly, L. M., Zebracki, K., Holmbeck, G. N. & Gershenson, L. Adolescent development and family functioning in youth with spina bifida. J. Pediatr. Rehabil. Med. 1, 291–302 (2008).
Singh, D. K. Families of children with spina bifida: a review. J. Dev. Phys. Disabil. 15, 37–55 (2003).
Murray, C. B. et al. A longitudinal examination of health-related quality of life in children and adolescents with spina bifida. J. Pediatr. Psychol.http://dx.doi.org/10.1093/jpepsy/jsu098 (2014).
Sawin, K. J. & Bellin, M. H. Quality of life in individuals with spina bifida: a research update. Dev. Disabil. Res. Rev. 16, 47–59 (2010). This report provides an up-to-date review of the research literature on quality of life in individuals with spina bifida.
Freeman, K. A., Smith, K., Adams, E., Mizokawa, S. & Neville-Jan, A. Is continence status associated with quality of life in young children with spina bifida? J. Pediatr. Rehabil. Med. 6, 215–223 (2013).
Cope, H. et al. Outcome and life satisfaction of adults with myelomeningocele. Disabil. Health J. 6, 236–243 (2013).
Dicianno, B. E., Gaines, A., Collins, D. M. & Lee, S. Mobility, assistive technology use, and social integration among adults with spina bifida. Am. J. Phys. Med. Rehabil. 88, 533–541 (2009).
Bellin, M. H. et al. Family satisfaction, pain, and quality-of-life in emerging adults with spina bifida: a longitudinal analysis. Am. J. Phys. Med. Rehabil. 92, 641–655 (2013).
Holmbeck, G. N. et al. A multimethod, multi-informant, and multidimensional perspective on psychosocial adjustment in preadolescents with spina bifida. J. Consult. Clin. Psychol. 71, 782–796 (2003).
Holmbeck, G. N. et al. Trajectories of psychosocial adjustment in adolescents with spina bifida: a 6-year, four-wave longitudinal follow-up. J. Consult. Clin. Psychol. 78, 511–525 (2010).This longitudinal study of young people with spina bifida reveals areas of psychological adjustment difficulties as well as areas of psychosocial resilience.
Shields, N., Taylor, N. F. & Dodd, K. J. Self-concept in children with spina bifida compared with typically developing children. Dev. Med. Child Neurol. 50, 733–743 (2008).
Blum, R. W., Resnick, M. D., Nelson, R. & St Germaine, A. Family and peer issues among adolescents with spina bifida and cerebral palsy. Pediatrics 88, 280–285 (1991).
Ellerton, M. L., Stewart, M. J., Ritchie, J. A. & Hirth, A. M. Social support in children with a chronic condition. Can. J. Nurs. Res. 28, 15–36 (1996).
Davis, B. E., Shurtleff, D. B., Walker, W. O., Seidel, K. D. & Duguay, S. Acquisition of autonomy skills in adolescents with myelomeningocele. Dev. Med. Child Neurol. 48, 253–258 (2006).
Friedman, D., Holmbeck, G. N., DeLucia, C., Jandasek, B. & Zebracki, K. Trajectories of autonomy development across the adolescent transition in children with spina bifida. Rehabil. Psychol. 54, 16–27 (2009).
Holmbeck, G. N. et al. Observed and perceived parental overprotection in relation to psychosocial adjustment in preadolescents with a physical disability: the mediational role of behavioral autonomy. J. Consult. Clin. Psychol. 70, 96–110 (2002).
Holmbeck, G. N., Greenley, R. N., Coakley, R. M., Greco, J. & Hagstrom, J. Family functioning in children and adolescents with spina bifida: an evidence-based review of research and interventions. J. Dev. Behav. Pediatr. 27, 249–277 (2006). This literature review focuses on basic psychosocial research and interventions that reveal the lack of interventions for individuals with spina bifida and their families, as compared with the number of interventions that are available for individuals with other chronic health conditions.
Costigan, C. L., Floyd, F. J., Harter, K. S. M. & McClintock, J. C. Family process and adaptation to children with mental retardation: Disruption and resilience in family problems-solving interactions. J. Fam. Psychol. 11, 515–529 (1997).
Ammerman, R. et al. Psychiatric symptomatology and family functioning in children and adolescents with spina bifida. J. Clin. Psychol. Med. Settings 5, 449–465 (1998).
Wiegner, S. & Donders, J. Predictors of parental distress after congenital disabilities. J. Dev. Behav. Pediatr. 21, 271–277 (2000).
Holmbeck, G. N., Coakley, R. M., Hommeyer, J. S., Shapera, W. E. & Westhoven, V. C. Observed and perceived dyadic and systemic functioning in families of preadolescents with spina bifida. J. Pediatr. Psychol. 27, 177–189 (2002).
Cappelli, M., McGarth, P. J., Daniels, T., Manion, I. & Schillinger, J. Marital quality of parents of children with spina bifida: a case-comparison study. J. Dev. Behav. Pediatr. 15, 320–326 (1994).
Holmbeck, G. N. et al. Maternal, paternal, and marital functioning in families of preadolescents with spina bifida. J. Pediatr. Psychol. 22, 167–181 (1997).
Spaulding, B. R. & Morgan, S. B. Spina bifida children and their parents: a population prone to family dysfunction? J. Pediatr. Psychol. 11, 359–374 (1986).
Vermaes, I. P., Janssens, J. M., Bosman, A. M. & Gerris, J. R. Parents’ psychological adjustment in families of children with spina bifida: a meta-analysis. BMC Pediatr. 5, 32–44 (2005).
Vermaes, I. P., Gerris, J. R. & Janssens, J. M. Parents’ social adjustment in families of children with spina bifida: a theory-driven review. J. Pediatr. Psychol. 32, 1214–1226 (2007).
Grosse, S., Flores, A., Ouyang, L., Robbins, J. & Tilford, J. Impact of spina bifida on parental caregivers: findings from a survey of Arkansas families. J. Child Fam. Stud. 18, 574–581 (2009).
Sawin, K. J. et al. The experience of parenting an adolescent with spina bifida. Rehabil. Nurs. 28, 173–185 (2003).
Macias, M., Clifford, S., Saylor, C. & Kreh, S. Predictors of parenting stress in families of children with spina bifida. Child. Health Care 30, 57–65 (2001).
Bellin, M. H., Bentley, K. J. & Sawin, K. J. Factors associated with the psychological and behavioral adjustment of siblings of youths with spina bifida. Fam. Syst. Health 27, 1–15 (2009).
Bowman, R. M., McLone, D. G., Grant, J. A., Tomita, T. & Ito, J. A. Spina bifida outcome: a 25-year prospective. Pediatr. Neurosurg. 34, 114–120 (2001).
Oakeshott, P., Hunt, G. M., Poulton, A. & Reid, F. Expectation of life and unexpected death in open spina bifida: a 40-year complete, non-selective, longitudinal cohort study. Dev. Med. Child Neurol. 52, 749–753 (2010).
Holmbeck, G. N., Bauman, L., Essner, B., Kelly, L. & Zebracki, K. in Launching into Adulthood: An Integrated Response to Support Transition of Youth with Chronic Health Conditions and Disabilities (ed. Lollar, D. ) 21–47 (Brookes, 2010).
Liptak, G. S., Kennedy, J. A. & Dosa, N. P. Youth with spina bifida and transitions: health and social participation in a nationally represented sample. J. Pediatr. 157, 584–588 (2010).
Sawyer, S. M. et al. Young people with spina bifida: transfer from paediatric to adult health care. J. Paediatr. Child Health 34, 414–417 (1998).
Bellin, M. H. et al. Correlates of depressive and anxiety symptoms in young adults with spina bifida. J. Pediatr. Psychol. 35, 778–789 (2010).
Murray, C. B. et al. The influence of social adjustment on normative and risky health behaviors in emerging adults with spina bifida. Health Psychol. 33, 1153–1163 (2014).
Cohen, P., Kasen, S., Chen, H., Hartmark, C. & Gordon, K. Variations in patterns of developmental transitions in the emerging adulthood period. Dev. Psychol. 39, 657–669 (2003).
Zukerman, J. M., Devine, K. A. & Holmbeck, G. N. Adolescent predictors of emerging adulthood milestones in youth with spina bifida. J. Pediatr. Psychol. 36, 265–276 (2011).
McDonnell, G. V. & McCann, J. P. Link between the CSF shunt and achievement in adults with spina bifida. J. Neurol. Neurosurg. Psychiatry 68, 800 (2000).
Roach, J. W., Short, B. F. & Saltzman, H. M. Adult consequences of spina bifida: a cohort study. Clin. Orthop. Relat. Res. 469, 1246–1252 (2011).
Hamilton, S. & Hamilton, M. in Emerging Adults in America: Coming of Age in the 21st Century (eds Arnett, J. & Tanner, J. ) 257–277 (American Psychological Association, 2006).
Gerhardt, C. A. et al. Educational and occupational outcomes among survivors of childhood cancer during the transition to emerging adulthood. J. Dev. Behav. Pediatr. 28, 448–455 (2007).
Verhoef, M. et al. Sex education, relationships, and sexuality in young adults with spina bifida. Arch. Phys. Med. Rehabil. 86, 979–987 (2005).
Sawin, K. J., Buran, C. F., Brei, T. J. & Fastenau, P. S. Sexuality issues in adolescents with a chronic neurological condition. J. Perinat. Educ. 11, 22–34 (2002).
Sawyer, S. M. & Roberts, K. V. Sexual and reproductive health in young people with spina bifida. Dev. Med. Child Neurol. 41, 671–675 (1999).
Shurtleff, D. B., Walker, W. O., Duguay, S., Peterson, D. & Cardenas, D. Obesity and myelomeningocele: anthropometric measures. J. Spinal Cord. Med. 33, 410–419 (2010).
Dosa, N. P., Foley, J. T., Eckrich, M., Woodall-Ruff, D. & Liptak, G. S. Obesity across the lifespan among persons with spina bifida. Disabil. Rehabil. 31, 914–920 (2009).
Boudos, R. M. & Mukherjee, S. Barriers to community participation: teens and young adults with spina bifida. J. Pediatr. Rehabil. Med. 1, 303–310 (2008).
Kelly, E. H., Altiok, H., Gorzkowski, J. A., Abrams, J. R. & Vogel, L. C. How does participation of youth with spina bifida vary by age? Clin. Orthop. Relat. Res. 469, 1236–1245 (2011).
Barf, H. A. et al. Restrictions in social participation of young adults with spina bifida. Disabil. Rehabil. 31, 921–927 (2009).
Park, M., Mulye, T., Adams, S., Brindis, C. & Irwin, C. E. Jr. The health status of young adults in the United States. J. Adolesc. Health 39, 305–317 (2006).
Dicianno, B. E. et al. Rehabilitation and medical management of the adult with spina bifida. Am. J. Phys. Med. Rehabil. 87, 1027–1050 (2008).
Schriner, K. F., Roessler, R. T. & Johnson, P. Identifying the employment concerns of people with spina bifida. J. Appl. Rehabil. Counsel. 24, 32 (1993).
Krupp, D. R. et al. Missing genetic risk in neural tube defects: can exome sequencing yield an insight?. Birth Defects Res. A Clin. Mol. Teratol. 100, 642–646 (2014).
Greene, N. D., Stanier, P. & Moore, G. E. The emerging role of epigenetic mechanisms in the aetiology of neural tube defects. Epigenetics. 6, 875–883 (2011).
Wilde, J. J., Petersen, J. R. & Niswander, L. Genetic, epigenetic, and environmental contributions to neural tube closure. Annu. Rev. Genet. 48, 583–611 (2014).
Chen, R. et al. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell 148, 1293–1307 (2012). This paper provides the first description of a method for providing a personal health profile based on a variety of computational and omics techniques, including genomic, gene expression, protein, metabolic and autoantibody data.
Copp, A. J., Greene, N. D. E. & Murdoch, J. N. The genetic basis of mammalian neurulation. Nat. Rev. Genet. 4, 784–793 (2003).
Hook, E. B. & Czeizel, A. E. Can terathanasia explain the protective effect of folic-acid supplementation on birth defects? Lancet 350, 513–515 (1997).
Marean, A., Graf, A., Zhang, Y. & Niswander, L. Folic acid supplementation can adversely affect murine neural tube closure and embryonic survival. Hum. Mol. Genet. 20, 3678–3683 (2011).
Burren, K. A. et al. Gene–environment interactions in the causation of neural tube defects: folate deficiency increases susceptibility conferred by loss of Pax3 function. Hum. Mol. Genet. 17, 3675–3685 (2008). This study in a well-defined mouse model of NTDs confirms that severe folate deficiency during pregnancy is not sufficient to cause neural tube defects, but that it is a risk factor in the presence of a genetic predisposition.
Molloy, A. M. et al. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics 123, 917–923 (2009).
Czeizel, A. E., Dudas, I., Paput, L. & Banhidy, F. Prevention of neural-tube defects with periconceptional folic acid, methylfolate, or multivitamins? Ann. Nutr. Metab. 58, 263–271 (2011).
Leung, K. Y., de Castro, S. C., Savery, D., Copp, A. J. & Greene, N. D. Nucleotide precursors prevent folic acid-resistant neural tube defects in the mouse. Brain 136, 2836–2841 (2013).
Greene, N. D. E. & Copp, A. J. Inositol prevents folate-resistant neural tube defects in the mouse. Nat. Med. 3, 60–66 (1997).
Cavalli, P., Tonni, G., Grosso, E. & Poggiani, C. Effects of inositol supplementation in a cohort of mothers at risk of producing an NTD pregnancy. Birth Defects Res. A Clin. Mol. Teratol. 91, 962–965 (2011). This is the first study showing that inositol supplementation is safe in mothers at high risk of pregnancy complicated by NTDs.
Copp, A. J., Greene, N. D. & Chitty, L. S. Prevention of Neural Tube Defects by Inositol in Conjunction With Folic Acid (PONTI Study). [online], (2015).
Fauza, D. O., Jennings, R. W., Teng, Y. D. & Snyder, E. Y. Neural stem cell delivery to the spinal cord in an ovine model of fetal surgery for spina bifida. Surgery 144, 367–373 (2008).
Li, H. et al. Therapeutic potential of in utero mesenchymal stem cell (MSCs) transplantation in rat foetuses with spina bifida aperta. J. Cell. Mol. Med. 16, 1606–1617 (2012).
Saadai, P. et al. Human induced pluripotent stem cell-derived neural crest stem cells integrate into the injured spinal cord in the fetal lamb model of myelomeningocele. J. Pediatr. Surg. 48, 158–163 (2013).
Hosper, N. A., Bank, R. A. & van den Berg, P. P. Human amniotic fluid-derived mesenchymal cells from fetuses with a neural tube defect do not deposit collagen type I protein after TGF-ß1 stimulation in vitro. Stem Cells Dev. 23, 555–562 (2013).
Stubberud, J., Langenbahn, D., Levine, B., Stanghelle, J. & Schanke, A. K. Emotional health and coping in spina bifida after goal management training: a randomized controlled trial. Rehabil. Psychol. 60, 1–16 (2014).
O'Mahar, K., Holmbeck, G. N., Jandasek, B. & Zukerman, J. A camp-based intervention targeting independence among individuals with spina bifida. J. Pediatr. Psychol. 35, 848–856 (2010).
Holbein, C. E. et al. A camp-based psychosocial intervention to promote independence and social function in individuals with spina bifida: moderators of treatment effectiveness. J. Pediatr. Psychol. 38, 412–424 (2013).
Grewal, J., Carmichael, S. L., Ma, C., Lammer, E. J. & Shaw, G. M. Maternal periconceptional smoking and alcohol consumption and risk for select congenital anomalies. Birth Defects Res. A Clin. Mol. Teratol. 82, 519–526 (2008).
Schmidt, R. J. et al. Maternal caffeine consumption and risk of neural tube defects. Birth Defects Res. A Clin. Mol. Teratol. 85, 879–889 (2009).
Kirke, P. N. et al. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q. J. Med. 86, 703–708 (1993).
Carmichael, S. L. et al. Reduced risks of neural tube defects and orofacial clefts with higher diet quality. Arch. Pediatr. Adolesc. Med. 166, 121–126 (2012).
Yazdy, M. M., Mitchell, A. A., Liu, S. & Werler, M. M. Maternal dietary glycaemic intake during pregnancy and the risk of birth defects. Paediatr. Perinat. Epidemiol. 25, 340–346 (2011).
Shaw, G. M., Velie, E. M. & Schaffer, D. M. Is dietary intake of methionine associated with a reduction in risk for neural tube defect-affected pregnancies. Teratology. 56, 295–299 (1997).
Shaw, G. M. et al. Choline and risk of neural tube defects in a folate-fortified population. Epidemiology 20, 714–719 (2009).
Ray, J. G. & Blom, H. J. Vitamin B12 insufficiency and the risk of fetal neural tube defects. Q. J. Med. 96, 289–295 (2003).
Schorah, C. J., Wild, J., Hartley, R., Sheppard, S. & Smithells, R. W. The effect of periconceptional supplementation on blood vitamin concentrations in women at recurrence risk for neural tube defect. Br. J. Nutr. 49, 203–211 (1983).
Velie, E. M. et al. Maternal supplemental and dietary zinc intake and the occurrence of neural tube defects in California. Am. J. Epidemiol. 150, 605–616 (1999).
Moretti, M. E., Bar-Oz, B., Fried, S. & Koren, G. Maternal hyperthermia and the risk for neural tube defects in offspring: systematic review and meta-analysis. Epidemiology 16, 216–219 (2005).
Wasserman, C. R., Shaw, G. M., Selvin, S., Gould, J. B. & Syme, S. L. Socioeconomic status, neighborhood social conditions, and neural tube defects. Am. J. Publ. Health 88, 1674–1680 (1998).
Shaw, G. M., Todoroff, K., Velie, E. M. & Lammer, E. J. Maternal illness, including fever, and medication use as risk factors for neural tube defects. Teratology. 57, 1–7 (1998).
Becerra, J. E., Khoury, M. J., Cordero, J. F. & Erickson, J. D. Diabetes mellitus during pregnancy and the risks for specific birth defects: a population-based case-control study. Pediatrics 85, 1–9 (1990).
Carmichael, S. L. & Shaw, G. M. Maternal life event stress and congenital anomalies. Epidemiology 11, 30–35 (2000).
Suarez, L., Cardarelli, K. & Hendricks, K. Maternal stress, social support, and risk of neural tube defects among Mexican Americans. Epidemiology 14, 612–616 (2003).
Vajda, F. J., O'Brien, T. J., Graham, J. E., Lander, C. M. & Eadie, M. J. Dose dependence of fetal malformations associated with valproate. Neurology 81, 999–1003 (2013).
Lupo, P. J. et al. Maternal exposure to ambient levels of benzene and neural tube defects among offspring: Texas, 1999–2004. Environ. Health Perspect. 119, 397–402 (2011).
Padula, A. M. et al. The association of ambient air pollution and traffic exposures with selected congenital anomalies in the San Joaquin Valley of California. Am. J. Epidemiol. 177, 1074–1085 (2013).
Righi, E. et al. Trihalomethanes, chlorite, chlorate in drinking water and risk of congenital anomalies: a population-based case-control study in Northern Italy. Environ. Res. 116, 66–73 (2012).
Li, Z. et al. Indoor air pollution from coal combustion and the risk of neural tube defects in a rural population in Shanxi Province, China. Am. J. Epidemiol. 174, 451–458 (2011).
Brender, J. D. et al. Prenatal nitrate intake from drinking water and selected birth defects in offspring of participants in the national birth defects prevention study. Environ. Health Perspect. 121, 1083–1089 (2013).
Cordier, S. et al. Congenital malformations and maternal occupational exposure to glycol ethers. Epidemiology 8, 355–363 (1997).
Brender, J. D., Felkner, M., Suarez, L., Canfield, M. A. & Henry, J. P. Maternal pesticide exposure and neural tube defects in Mexican Americans. Ann. Epidemiol. 20, 16–22 (2010).
Yang, W. et al. Residential agricultural pesticide exposures and risk of neural tube defects and orofacial clefts among offspring in the San Joaquin Valley of California. Am. J. Epidemiol. 179, 740–748 (2014).
Ren, A. et al. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc. Natl Acad. Sci. USA 138, 12770–12775 (2011).
Hutchins, G. M. et al. Acquired spinal cord injury in human fetuses with myelomeningocele. Pediatr. Pathol. Lab. Med. 16, 701–712 (1996).
Korenromp, M. J., Van Gool, J. D., Bruinese, H. W. & Kriek, R. Early fetal leg movements in myelomeningocele. Lancet 327, 917–918 (1986).
Sival, D. A. et al. Perinatal motor behaviour and neurological outcome in spina bifida aperta. Early Hum. Dev. 50, 27–37 (1997).
Duckworth, T., Sharrard, W. J., Lister, J. & Seymour, N. Hemimyelocele. Dev. Med. Child Neurol. 10, (Suppl. 16), 69–75 (1968).
Meuli, M. et al. In utero surgery rescues neurological function at birth in sheep with spina bifida. Nat. Med. 1, 342–347 (1995). This investigation in sheep fetuses shows that amniotic fluid exposure accounts for the neural tissue destruction in the sheep model of spina bifida and that timely in utero repair of myelomeningocele lesions might rescue neurological function in humans.
Drewek, M. J., Bruner, J. P., Whetsell, W. O. & Tulipan, N. Quantitative analysis of the toxicity of human amniotic fluid to cultured rat spinal cord. Pediatr. Neurosurg. 27, 190–193 (1997).
Campbell, J., Gilbert, W. M., Nicolaides, K. H. & Campbell, S. Ultrasound screening for spina bifida: cranial and cerebellar signs in a high-risk population. Obstet. Gynecol. 70, 247–250 (1987).
Smith, N. C. & Hau, C. A six year study of the antenatal detection of fetal abnormality in six Scottish health boards. Br. J. Obstet. Gynaecol. 106, 206–212 (1999).
Boyd, P. A., Chamberlain, P. & Hicks, N. R. 6-year experience of prenatal diagnosis in an unselected population in Oxford, UK. Lancet 352, 1577–1581 (1998).
Shirley, I. M., Bottomley, F. & Robinson, V. P. Routine radiographer screening for fetal abnormalities by ultrasound in an unselected low risk population. Br. J. Radiol. 65, 564–569 (1992).
Chitty, L. S., Hunt, G. H., Moore, J. & Lobb, M. O. Effectiveness of routine ultrasonography in detecting fetal structural abnormalities in a low risk population. Br. Med. J. 303, 1165–1169 (1991).
Luck, C. A. Value of routine ultrasound scanning at 19 weeks: a four year study of 8849 deliveries. Br. Med. J. 304, 1474–1478 (1992).
Papp, Z. et al. Impact of prenatal mid-trimester screening on the prevalence of fetal structural anomalies: a prospective epidemiological study. Ultrasound Obstet. Gynecol. 6, 320–326 (1995).
Acknowledgements
The authors acknowledge grants from The Wellcome Trust (grant 087525 to A.J.C.), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, US National Institutes of Health (grants U10 HD041666 to N.S.A., P01 HD35946 to J.M.F. and R01-HD048629 to G.N.H.) and the March of Dimes Foundation (grant 12-FY13-271 to G.N.H.). Images of human embryonic material are provided by the Joint Medical Research Council–Wellcome Trust Human Developmental Biology Resource (www.hdbr.org; grant 099175).
Author information
Authors and Affiliations
Contributions
Introduction (A.J.C.); Epidemiology (G.M.S.); Mechanisms/pathophysiology (A.J.C. and J.M.F.); Diagnosis, screening and prevention (G.M.S. and L.S.C.); Management (N.S.A.); Quality of life (G.N.H.); Outlook (A.J.C. and G.N.H.); overview of Primer (A.J.C.).
Corresponding author
Ethics declarations
Competing interests
G.M.S. has received consulting fees from: Advanced Micro Devices and NXP Semiconductors for semiconductor employment and birth defects; GlaxoSmithKline for paroxetine use and birth defects; and Vivus, Inc. for topiramate use and oral clefts. A.J.C., N.S.A., L.S.C., J.M.F. and G.N.H. declare no competing interests.
Rights and permissions
About this article
Cite this article
Copp, A., Adzick, N., Chitty, L. et al. Spina bifida. Nat Rev Dis Primers 1, 15007 (2015). https://doi.org/10.1038/nrdp.2015.7
Published:
DOI: https://doi.org/10.1038/nrdp.2015.7
This article is cited by
-
GPR161 structure uncovers the redundant role of sterol-regulated ciliary cAMP signaling in the Hedgehog pathway
Nature Structural & Molecular Biology (2024)
-
Spina bifida and cardiorespiratory profile: the impact of leisure sport activities on physical fitness
Child's Nervous System (2024)
-
Multidisciplinary management of people with spina bifida across the lifespan
Pediatric Nephrology (2024)
-
Identification and functional analysis of rare HECTD1 missense variants in human neural tube defects
Human Genetics (2024)
-
A qualitative analysis of patient and caregiver experiences with myelomeningocele through online discussion boards
Child's Nervous System (2024)