Key Points
-
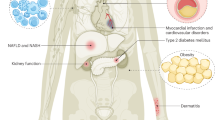
Obesity and type 2 diabetes have become epidemic medical problems and will cause significant morbidity and mortality if new therapeutic agents cannot be brought forward.
-
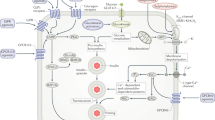
Protein tyrosine phosphatase 1B (PTP1B) has been shown to be involved in the negative regulation of both insulin and leptin action at the in vitro, ex vivo and in vivo levels. A growing body of human genetic data also support the hypothesis that PTP1B has an important role in insulin signalling and possibly in obesity in humans.
-
PTP1B has been implicated in diabetes and obesity in knockout-mouse studies on a normal background. When crossed onto ob/ob mice, improvement in blood glucose and obesity is seen. When Ptp1b antisense oligonucleotides are administered in vivo to ob/ob mice, normalized blood glucose levels are obtained. Insulin sensitivity is improved at the cellular level.
-
PTP1B is tractable to structure-based drug design, and the crystal structure is well known. Recently, the crystal structure of the closely related T-cell protein tyrosine phosphatase (TCPTP) has become available, raising the possibility for designing selective inhibitors.
-
Potent (Ki values in the nM range) inhibitors of PTP1B are known, and oxamic acids are a common structural motif found in these compounds. The high polar surface area (PSA) and charge of this functionality make further optimization difficult, and an intense search to uncover phosphotyrosine mimetics with improved physico-chemical properties is in progress.
-
Pro-drugs of the oxamic acids and replacement of the acid functionality with isosteric functional groups might provide compounds with improved physico-chemical properties.
-
Lipophilic compounds have been reported — one of the two reported structures seems to be a competitive inhibitor of PTP1B and the other does not. This highlights the need for a clear understanding of the mechanism of inhibition and an understanding of the kinetics of compounds.
Abstract
Increased incidence of type 2 diabetes mellitus and obesity has elevated the medical need for new agents to treat these disease states. Resistance to the hormones insulin and leptin are hallmarks of both type 2 diabetes and obesity. Drugs that can ameliorate this resistance should be effective in treating type 2 diabetes and possibly obesity. Protein tyrosine phosphatase 1B (PTP1B) is thought to function as a negative regulator of insulin and leptin signal transduction. This article reviews PTP1B as a novel target for type 2 diabetes, and looks at the challenges in developing small-molecule inhibitors of this phosphatase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Zimmet, P., Alberti, K. G. M. M. & Shaw, J. Global and societal implications of the diabetes epidemic. Nature 414, 782–787 (2001).An excellent overview of the epidemiology of diabetes.
Sinha, R. et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N. Engl. J. Med. 346, 802–810 (2002).
Rocchini, A. P. Childhood obesity and a diabetes epidemic. N. Engl. J. Med. 346, 854–855 (2002).
Dunstan, D. W. et al. The rising prevalence of diabetes and impaired glucose tolerance. The Australian diabetes, obesity and lifestyle study. Diabetes Care 25, 829–834 (2002).
Groop, L. & Orho-Melander, M. The dysmetabolic syndrome. J. Int. Med. 250, 105–120 (2001).
Poitout, V. & Robertson, P. R. Minireview: secondary β-cell failure in type 2 diabetes – a convergence of glucotoxity and lipotoxicity. Endocrinology 143, 339–342 (2002).
Weyer, C., Tartanni, P. A., Bogardus, C. & Pratley, R. E. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care 24, 89–94 (2001).
Stumvoll, M., Fritsche, A. & Haring, H. U. Clinical characterization of insulin secretion as the basis for genetic analyses. Diabetes 51 (Suppl. 1), S122–S129 (2002).
Uusitupa, M. et al. The Finnish Diabetes Prevention Study. Br. J. Nutr. 83 (Suppl. 1), S137–S142 (2000).
The Diabetes Prevention Study Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346, 393–403 (2002).
Heine, R. J. & Dekker, J. M. Beyond postprandial hyperglycaemia: metabolic factors associated with cardiovascular disease. Diabetologia 45, 461–475 (2002).
Marx, J. Unraveling the causes of diabetes. Science 296, 686–689 (2002).
Saltiel, A. R. & Kahn, C. R. Insulin signaling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (2001).An excellent overview of metabolic insulin signal transduction.
Obici, S., Feng, Z., Karkanias, G., Baskin, D. G. & Rossetti, L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nature Neurosci. 5, 566–572 (2002).This article shows the role of the insulin receptor at the level of the CNS.
Saltiel, A. R. & Pessin, J. E. Insulin signaling pathways in time and space. Trends Cell Biol. 12, 65–71 (2002).
Bryant, N. J., Govers, R. & James, D. E. Regulated transport of the glucose transporter GLUT4. Nature Rev. Mol. Cell Biol. 3, 267–277 (2002).
Smith, U. Impaired ('diabetic') insulin signaling and action occur in fat cells long before glucose intolerance – is insulin resistance initiated in the adipose tissue? Int. J. Obes. Relat. Metab. Disord. 26, 897–904 (2002).
Ostman, A. & Bohmer, F. D. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases. Trends Cell Biol. 11, 258–266 (2001).
Cheng, A., Dube, N., Gu, F. & Tremblay, M. L. Coordinated action of protein tyrosine phosphatases in insulin signal transduction. Eur. J. Biochem. 269, 1050–1059 (2002).
Goldstein, B. J., Bittner-Kowalczyk, A., White, M. F. & Harbeck, M. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the GRB2 adaptor protein. J. Biol. Chem. 275, 4283–4289 (2000).
Wu, X. et al. Depot-specific variation in protein-tyrosine phosphatase activities in human omental and subcutaneous adipose tissue: a potential contribution to differential insulin sensitivity. J. Clin. Endocrinol. Metab. 86, 5973–5980 (2001).
Cheung, A. et al. Marked impairment of protein tyrosine phosphatase 1B activity in adipose tissue of obese subjects with and without type 2 diabetes mellitus. J. Lab. Clin. Med. 134, 115–123 (1999).
Forsell, P. K. A. L., Boie, Y., Montalibet, J., Collins, S. & Kennedy, B. P. Genomic characterization of the human and mouse protein tyrosine phosphatase-1B genes. Gene 260, 145–153 (2000).
Mok, A. et al. A single nucleotide polymorphism in protein tyrosine phosphatase PTP1B is associated with protection from diabetes or impaired glucose tolerance in Oji–Cree. J. Clin. Endocrinol. Metab. 87, 724–727 (2002).
Di Paola, R. et al. A variation in 3′UTR of hPTP1B increases specific gene expression and associates with insulin resistance. Am. J. Hum. Genet. 70, 806–812 (2002).
Echwald, S. M. et al. A P387L variant in protein tyrosine phosphatase-1B (PTP1B) is associated with type 2 diabetes and impaired serine phosphorylation of PTP1B in vitro. Diabetes 51, 1–6 (2002).
Goldstein, B. J. Protein tyrosine phosphatase 1B (PTP1B): a novel therapeutic target for type 2 diabetes mellitus, obesity and related states of insulin resistance. Curr. Drug Targets Immune Endocr. Metab. Disord. 1, 265–275 (2001).
Ukkola, O. & Santaniemi, M. Protein tyrosine phosphatase 1B: a new target for the treatment of obesity and associated co-morbidities. J. Int. Med. 251, 467–475 (2002).References 27 and 28 review the importance of PTP1B in insulin signalling.
Egawa, K. et al. Protein-tyrosine phosphatase-1B negatively regulates insulin signaling in l6 myocytes and Fao hepatoma cells. J. Biol. Chem. 276, 10207–10211 (2001).
Chen, H. et al. Protein-tyrosine phosphatases PTP1B and SYP are modulators of insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. J. Biol. Chem. 272, 8026–8031 (1997).
Venable, C. L. et al. Overexpression of protein-tyrosine phosphatase-1B in adipocytes inhibits insulin-stimulated phosphoinositide 3-kinase activity without altering glucose transport or Akt/Protein kinase B activation. J. Biol. Chem. 275, 18318–18326 (2000).
Mahadev, K. et al. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J. Biol. Chem. 276, 48662–48669 (2001).
Tao, J., Malbon, C. C. & Wang, H. Insulin stimulates tyrosine phosphorylation and inactivation of protein tyrosine phosphatase 1B in vivo. J. Biol. Chem. 276, 29520–29525 (2001).
Salmeen, A., Andersen, J. N., Myers, M. P., Tonks, N. K. & Barford, D. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol. Cell 7, 615–621 (2001).
Barford, D. The mechanism of protein kinase regulation by protein phosphatases. Biochem. Soc. Trans. 29, 385–391 (2001)
Xie, L., Zhang, Y. L. & Zhang, Z. Y. Design and characterization of an improved protein tyrosine phosphatase substrate-trapping mutant. Biochemistry 41, 4032–4039 (2002).
Myers, M. P. et al. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 276, 47771–47774 (2001).
Haj, F. G., Verveer, P. J., Squire, A., Neel, B. G. & Bastiaens, P. I. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science 295, 1708–1711 (2002).
Gill, G. N. A pit stop at the ER. Science 295, 1654–1655 (2002).Reference 39 is a commentary about reference 38 that brings into perspective how PTP1B might have roles in regulating various RTKs.
Dadke, S. & Chernoff, J. Interaction of protein tyrosine phosphatase (PTP) 1B with its substrates is influenced by two distinct binding domains. Biochem. J. 364, 377–383 (2002).
Elchebly, M. et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283, 1544–1548 (1999).
Klaman, L. D. et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 20, 5479–5489 (2000).
Kaszubska, W. et al. The role of PTP1B in leptin signalling. Keystone Symposia: Diabetes Mellitus: Molecular Mechanisms, Genetics and New Therapies Jan 10–16 226, 81 (2002).PTP1B has been implicated in negatively regulating insulin signal transduction, and this role is extended to include negative regulation of leptin signal transduction.
Zabolotny, J. M. et al. PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2, 489–495 (2002).
Cook, W. S. & Unger, R. H. Protein tyrosine phosphatase 1B: a potential leptin resistance factor of obesity. Dev. Cell 2, 385–387 (2002).
Cheng, A. et al. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell 2, 497–503 (2002).
Zinker, B. A. et al. Reduction of protein tyrosine phosphatase 1B normalizes glucose and improves insulin sensitivity in diabetic mice. Proc. Natl Acad. Sci. USA 99, 11357–11362 (2002).
Tonks, N. K., Diltz, C. D. & Fischer, E. H. Purification of the major protein-tyrosine phosphatases of human placenta. J. Biol. Chem. 263, 6722–6730 (1988).
Barford, D., Flint, A. J. & Tonks, N. K. Crystal structure of human protein tyrosine phosphatase 1B. Science 263, 1397–1404 (1994).
Jia, Z., Barford, D., Flint, A. J. & Tonks, N. K. Structural basis for phosphotyrosine peptide recognition by protein tyrosine phosphatase 1B. Science 268, 1754–1758 (1998).
Groves, M. R., Yao, Z. J., Roller, P. P., Burke, T. R. Jr & Barford, D. Structural basis for inhibition of the protein tyrosine phosphatase 1B by phosphotyrosine peptide mimetics. Biochemistry 37, 17773–17783 (1998).
Pannifer, A. D. B., Flint, A. J., Tonks, N. K. & Barford, D. Visualization of the cysteinyl-phosphate intermediate of a protein-tyrosine phosphatase by X-ray crystallography. J. Biol. Chem. 273, 10454–10462 (1998).
Puius, Y. A. et al. Identification of a second aryl phosphate binding site in protein-tyrosine phosphatase 1B: a paradigm for inhibitor design. Proc. Natl Acad. Sci. USA 94, 13420–13425 (1997).
Hadjuk, P. J., Meadows, R. P. & Fesik, S. W. Discovering high affinity ligands for proteins. Science 278, 497–499 (1997).
Iversen, L. F. et al. Structure based design of a low molecular weight, non-phosphorous, non-peptide, and highly selective inhibitor of protein-tyrosine phosphatase 1B. J. Biol. Chem. 275, 10300–10307 (2000).This paper describes the structure-based design of PTP1B inhibitors.
Asante-Appiah, E. et al. The YRD motif is a major determinant of substrate and inhibitor specificity in T-cell protein-tyrosine phosphatase. J. Biol. Chem. 276, 26036–26043 (2001).
Peters, G. H. et al. Residue 259 is a key determinant of substrate specificity of protein-tyrosine phosphatases 1B and α. J. Biol. Chem. 275, 18201–18209 (2000).
Iversen, L. F. et al. Steric hindrance as a basis for structure-based design of selective inhibitors of protein tyrosine phosphatases. Biochemistry 40, 14812–14820 (2001).
Iversen, L. F. et al. Structure determination of T cell protein-tyrosine phosphatase. J. Biol. Chem. 277, 19982–19990 (2002).
Ibarra-Sanchez, M. D. et al. Murine embryonic fibroblasts lacking TC-PTP display delayed G1 phase through defective NF-κB activation. Oncogene 20, 4728–4739 (2001).
You-Ten, K. E. et al. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J. Exp. Med. 18, 683–693 (1997).A review of PTP inhibitors from a medicinal chemistry perspective that provides a detailed analysis of the inhibitors identified so far.
Blaskovitch, M. A. & Kim, H.-O. Recent advances in discovery and development of protein tyrosine phosphatase inhibitors. Expert Opin. Ther. Patents 12, 871–905 (2002).
Burke, T. R., Kole, H. K. & Roller, P. P. Potent inhibition of insulin receptor dephosphorylation by a hexamer peptide containing the phosphotyrosyl mimetic F2Pmp. Biochem. Biophys. Res. Commun. 204, 129–134 (1994).
Burke, T. R. Jr et al. Small molecule interactions with protein-tyrosine phosphatase PTP1B and their use in inhibitor design. Biochemistry 35, 15989–15996 (1996).
Taing, M. et al. Potent and highly selective inhibitors of the protein tyrosine phosphatase 1B. Biochemistry 38, 3793–3803 (1999).
Jia, Z. et al. Structure of protein tyrosine phosphatase 1B in complex with inhibitors bearing two phosphotyrosine mimetics. J. Med. Chem. 44, 4584–4594 (2001).
Bayly, C. & Ohkubo, M. Sulfur substituted aryldifluoromethylphosphonic acids as PTP1B inhibitors. Patent WO 01/70754 (2001).
Leblanc, Y., Dufresne, C., Gauthier, J. Y. & Young, R. Aromatic phosphonates as protein tyrosine phosphatase 1B (PTP1B) inhibitors. Patent WO 01/46204 (2001).
Shen, K. et al. Acquisition of a specific and potent PTP1B inhibitor from a novel combinatorial library and screening procedure. J. Biol. Chem. 276, 47311–47319 (2001).
Leblanc, Y., Dufresne, C., Roy, P. & Wang, Z. Phosphonic and carboxylic acid derivatives as inhibitors of protein tyrosine phosphatase-1B. Patent WO 00/69889 (2000).
Burke T. R. Jr et al. Enantioselective synthesis of nonphosphorous-containing phosphotyrosyl mimetics and their use in the preparation of tyrosine phophatase inhibitory peptides. Tetrahedron 54, 9981–9994 (1998).
Bleasdale, J. E. et al. Small molecule peptidomimetics containing a novel phosphotyrosine bioisostere inhibit protein tyrosine phosphatase 1B and augment insulin action. Biochemistry 40, 5642–5654 (2001).
Larsen, S. D. et al. Synthesis and biological activity of a novel class of small molecular weight peptidomimetic competitive inhibitors of protein tyrosine phosphatase 1B. J. Med. Chem. 45, 598–622 (2002).
Liljebris, C., Larsen, S. D., Ogg, D., Palazuk, B. J. & Bleasedale, J. E. Investigation of potential bioisosteric replacements for the carboxyl groups of peptidomimetic inhibitors of protein tyrosine phosphatase 1B: identification of a tetrazole-containing inhibitor with cellular activity. J. Med. Chem. 45, 1785–1798 (2002).This article describes the production of inhibitors with improved physico-chemical properties.
Andersen, et al. 2-(Oxalylamino)benzoic acid is a general, competitive inhibitor of protein tyrosine phosphatases. J. Biol. Chem. 275, 7101–7108 (2000).
Iversen, L. F. et al. Structure based design of a low molecular weight, non-phosphorous, non-peptide, and highly selective inhibitor of protein-tyrosine phosphatase 1B. J. Biol. Chem. 275, 10300–10307 (2000).
Andersen, H. S. Method of inhibiting protein tyrosine phosphatase 1B and/or T-cell protein tyrosine phosphatase and/or other PTPases with Asp residue at position 48. Patent WO 01/17516 (2001).
Lui, G. et al. Discovery of competitive, potent, and selective protein tyrosine phosphatase 1B inhibitors. 223rd American Chemical Society National Meeting, Orlando, Florida, April 7–11, 2002 MEDI–002 (2002).
Wrobel, J. et al. PTP1B inhibition and antihyperglycemic activity in the ob/ob mouse model of novel 11-arylbenzo[b]naphtha[2,3-d]furans and 11-arylbenzo[b]naphto[2,3-d]thiophenes. J. Med. Chem. 42, 3199–3202 (1999).
Reuters Business Report. (10 Jun 2002).
McGovern, S. L., Caselli, E., Grigorieff, N. & Shoichet, B. K. A common mechanism underlying promiscuous inhibitors from virtual and high throughput screening. J. Med. Chem. 45, 1712–1722 (2002).
Doman, T. N. et al. Molecular docking and high throughput screening for novel inhibitors of protein tyrosine phosphatase-1B. J. Med. Chem. 45, 2213–2221 (2002).The establishment of robust assay systems to determine activity and mechanism.
Lubben, T., Clampit, J., Stashko, M., Trevillyan, J. & Jirousek, M. R. in Current Protocols in Pharmacology Vol. 8 Ch. 3 (Emma, S. I. et al.) 1–18 (John Wiley, New York, 2001).
Cornish-Bowden, A. Fundamentals of Enzyme Kinetics (Portland Press, London, 1995).
Copeland, R. A. Enzymes: a Practical Introduction to Structure, Mechanism, and Data Analysis 2nd edn (Wiley–VCH, New York, 2000).
Bieth, J. G. Theoretical and practical aspects of proteinase inhibition kinetics. Methods Enzymol. 248, 59–84 (1995).
Zhang, Y. L., Keng, Y. F., Zhao, Y., Wu, L. & Zhang, Z. Y. Suramin is an active site-directed, reversible, and tight-binding inhibitor of protein-tyrosine phosphatases. J. Biol. Chem. 273, 12281–12287 (1998).
Lu, B. et al. Enhanced sensitivity of insulin-resistant adipocytes to vanadate is associated oxidative stress and decreased reduction of vanadate (+5) to vanadyl (+4). J. Biol. Chem. 276, 35589–35598 (2001).
Huyer, G. et al. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J. Biol. Chem. 272, 843–851 (1997).
Crans, D., Bunch, R. L. & Theisen, L. A. Interaction of trace levels of vanadium (IV) and vanadium (V) in biological systems. J. Am. Chem. Soc. 111, 7597–7607 (1989).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23, 3–25 (1997).
Palm, K., Stenberg, P., Luthman, K. & Artursson, P. Polar molecular surface properties predict the intestinal absorption of drugs in humans. Pharm. Res. 14, 568–571 (1997).
Veber, D. F. et al. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 45, 2615–2623 (2002).
Pei, Z. et al. (2R)-2-(2′,6′-dichloro-4′-dibenzo(b,d)furan-4″-ylphenoxy)-3-phenylpropanoic acid (A-321842) as a protein tyrosine phosphatase 1B (PTP1B) inhibitor with anti-diabetic effects in ob/ob mice. Diabetes 50 (Suppl. 1), 1524-P (2001).
Burkey, B. F. et al. Pharmacogenomic evaluation of a putative orally active PTP1B inhibitor using ob/ob mice reveals an alternative mechanism of action. Diabetes 51 (Suppl. 2), 138-OR (2002).
Acknowledgements
We apologize to colleagues for the many references we could not site due to space limitations. We thank A. Castro for collecting some of the data and J. Thomson, D. Vanderpool and J. Nelson for reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Glossary
- OBESITY
-
In the United States, obesity is defined as having a body-mass index (BMI; the weight in kg divided by the height (in m) squared) ≥ 30 or BMI ≥ 27 with diabetes, hypertension or dyslipidaemia.
- PANCREATIC ISLET
-
Islets of Langerhans, which are found in the pancreas, consist of insulin-producing β-cells, somatostatin-producing δ-cells, glucagon-producing α-cells and polypeptide-producing pp cells.
- POSTPRANDIAL
-
The period of macronutrient absorption after a meal, usually considered to be two hours in length.
- METABOLIC SYNDROME
-
The appearance of several metabolic disorders together. The World Health Organisation defines this as one of type 2 diabetes, impaired glucose tolerance or insulin resistance, in combination with two of the following presentations — hypertension, obesity, hypertriglyceridaemia or low HDL, or microalbuminuria.
- GLUCOSE HOMEOSTASIS
-
The maintenance of glucose levels during fluctuating physiological pressures, such as eating, exercising, resting or disease.
- PROTEIN TYROSINE PHOSPHATASE
-
An enzyme that removes phosphate from tyrosine residues in proteins.
- OMENTAL FAT
-
Adipose tissue located in the omental, such as the viscera below the subcutaneous space.
- OJI–CREE INDIANS
-
A Native North American tribe that lives in the Sandy Lake area of Canada. This population has an incidence of obesity-related type 2 diabetes or impaired glucose tolerance of ∼40%.
- SINGLE-NUCLEOTIDE POLYMORPHISM
-
(SNP). A variation in a nucleotide in a gene that could result in altered gene function or altered protein function if it affects amino-acid coding.
- LINKAGE DISEQUILIBRIUM
-
The association between a pair of alleles at closely linked loci that induces haplotype frequencies different than the product of the individual allele frequencies.
- HDL CHOLESTEROL
-
High-density lipoprotein (HDL) that is associated with a high proportion of protein (48%) and low triglyceride (3%) and cholesterol (19%) — HDL density is 1.06–1.21 g ml−1.
- ob/ob MOUSE
-
A homozygous mutation in the leptin gene located on chromosome 6 that causes the absence of functional leptin in the mouse strain C57BL/6J, producing a model of severe obesity with hyperphagia and diabetes.
- ARYL PHOSPHATE
-
The phosphate group attached at the 4-position of the phenyl group in tyrosine.
- NUCLEOPHILE
-
An atom that is attracted to the nucleus of another — in this case, the cysteine sulphur acts as a nucleophile and attacks the phosphotyrosine phosphorous atom.
- HYDROPHOBIC
-
A molecular interaction that occurs in the absence of aqueous solvent or water.
- SALT BRIDGE
-
The formation of an ionic-charge interaction between two molecules or between two regions of a molecule.
- PEPTIDOMIMETIC
-
Mimicking a peptide–peptide interaction through a non-peptide bond or structure.
Rights and permissions
About this article
Cite this article
Johnson, T., Ermolieff, J. & Jirousek, M. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat Rev Drug Discov 1, 696–709 (2002). https://doi.org/10.1038/nrd895
Issue Date:
DOI: https://doi.org/10.1038/nrd895
This article is cited by
-
Effects of the tetravanadate [V4O12]4− anion on the structural, magnetic, and biological properties of copper/phenanthroline complexes
JBIC Journal of Biological Inorganic Chemistry (2024)
-
Integrated use of ligand and structure-based virtual screening, molecular dynamics, free energy calculation and ADME prediction for the identification of potential PTP1B inhibitors
Molecular Diversity (2023)
-
Differences in ligand-induced protein dynamics extracted from an unsupervised deep learning approach correlate with protein–ligand binding affinities
Communications Biology (2022)
-
Synthesis and biological evaluation of (20S,24R)-epoxy-dammarane-3β,12β,25-triol derivatives as α-glucosidase and PTP1B inhibitors
Medicinal Chemistry Research (2022)
-
Mulberry Diels–Alder-type adducts: isolation, structure, bioactivity, and synthesis
Natural Products and Bioprospecting (2022)