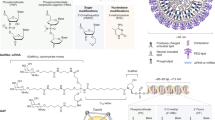
Key Points
-
Although conceptually elegant, the prospect of using nucleic-acid molecules for treating human diseases remains tantalizing, but uncertain. The main cause of this uncertainty is the apparent randomness with which these materials modulate the expression of their intended targets.
-
Strategies for modulating gene expression can be directed towards perturbing the process of transcription, or post-transcriptional events, including mRNA processing and translation. Conveniently, these approaches can be categorized as 'anti-gene' or 'anti-mRNA.'
-
Gene targeting can be accomplished by homologous recombination, triple-helix-forming oligodeoxynucleotides (TFOs) and decoy molecules.
-
Targeting mRNA can be accomplished by various strategies as well, including the use of antisense DNA, antisense RNA and RNA-decoy molecules.
-
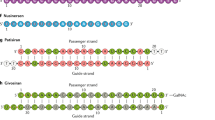
A new approach that has received a great deal of attention in the past year is called post-transcriptional gene silencing, or RNA interference (RNAi).
-
Molecule delivery to targeted cells specific compartments within cells, and identification of hybridization-accessible sequence within the genomic DNA or RNA remain core stumbling blocks that hold up progress in the field.
-
Nucleic acids that are used for experimental purposes and those designed for the clinic are now routinely modified to enhance their stability, as well as the strength of their hybridization with RNA.
-
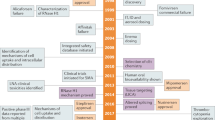
Many successful uses of this strategy in the laboratory have been reported. Despite the fact that the mechanism whereby these molecules modulate gene expression is not always certain, clinical development of nucleic-acid compounds has proceeded to the point at which a number of these drugs have entered Phase I/II, and in a few cases, Phase III trials.
-
RNA-encoding proteins that are involved in key signal-transduction pathways and transcription factors are the primary targets. Some encouraging reports of the clinical usefulness of these molecules, either alone, or predominantly in combination with other treatment modalities, have been reported.
Abstract
The sequencing of the human genome and the elucidation of many molecular pathways that are important in disease have provided unprecedented opportunities for the development of new therapeutics. The types of molecule in development are increasingly varied, and include antisense oligonucleotides and ribozymes. Antisense technology and catalytic nucleic-acid enzymes are important tools for blocking the expression of abnormal genes. One FDA-approved antisense drug is already in the clinic for the treatment of cytomegalovirus retinitis, and other nucleic-acid therapies are undergoing clinical trials. This article reviews different strategies for modulating gene expression, and discusses the successes and problems that are associated with this type of therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vile, R. G., Russell, S. J. & Lemoine, N. R. Cancer gene therapy: hard lessons and new courses. Gene Ther. 7, 2–8 (2000).
Gewirtz, A. M., Sokol, D. L. & Ratajczak, M. Z. Nucleic acid therapeutics: state of the art and future prospects. Blood 92, 712–736 (1998).
Mann, M. J. et al. Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet 354, 1493–1498 (1999).
Ehsan, A., Mann, M. J., Dell'Acqua, G. & Dzau, V. J. Long-term stabilization of vein graft wall architecture and prolonged resistance to experimental atherosclerosis after E2F decoy oligonucleotide gene therapy. J. Thorac. Cardiovasc. Surg. 121, 714–722 (2001).
Dean, N. M., McKay, R., Condon, T. P. & Bennett, C. F. Inhibition of protein kinase C-α expression in human A549 cells by antisense oligonucleotides inhibits induction of intercellular adhesion molecule 1 (ICAM-1) mRNA by phorbol esters. J. Biol. Chem. 269, 16416–16424 (1994).
Yacyshyn, B. R. et al. A placebo-controlled trial of ICAM-1 antisense oligonucleotide in the treatment of Crohn's disease. Gastroenterology 114, 1133–1142 (1998).
Macpherson, J. L., Ely, J. A., Sun, L. Q. & Symonds, G. P. Ribozymes in gene therapy of HIV-1. Front. Biosci. 4, D497–D505 (1999).
Welch, P. J., Yei, S. & Barber, J. R. Ribozyme gene therapy for hepatitis C virus infection. Clin. Diagn. Virol. 10, 163–171 (1998).
Zu Putlitz, J., Yu, Q., Burke, J. M. & Wands, J. R. Combinatorial screening and intracellular antiviral activity of hairpin ribozymes directed against hepatitis B virus. J. Virol. 73, 5381–5387 (1999).
Compagno, D. et al. Antisense oligonucleotides containing modified bases inhibit in vitro translation of Leishmania amazonensis mRNAs by invading the mini-exon hairpin. J. Biol. Chem. 274, 8191–8198 (1999).
Katz, S. M. et al. Effect of ICAM-1/LFA-1 blockade on pancreatic islet allograft survival, function, and early cytokine production. Transplant. Proc. 29, 748–749 (1997).
Gewirtz, A. M. Oligonucleotide therapeutics: a step forward. J. Clin. Oncol. 18, 1809–1811 (2000).
Paterson, B. M., Roberts, B. E. & Kuff, E. L. Structural gene identification and mapping by DNA–mRNA hybrid-arrested cell-free translation. Proc. Natl Acad. Sci. USA 74, 4370–4374 (1977).
Stephenson, M. L. & Zamecnik, P. C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc. Natl Acad. Sci. USA 75, 285–288 (1978).A classic reference that first suggested the possibility of using 'antisense' DNA for therapeutic purposes.
Simons, R. W. & Kleckner, N. Translational control of IS10 transposition. Cell 34, 683–691 (1983).
Mizuno, T., Chou, M. Y. & Inouye, M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc. Natl Acad. Sci. USA 81, 1966–1970 (1984).
Melton, D. W. Gene targeting in the mouse. Bioessays 16, 633–638 (1994).
Stasiak, A. Getting down to the core of homologous recombination. Science 272, 828–829 (1996).
Helene, C. Control of oncogene expression by antisense nucleic acids. Eur. J. Cancer 30A, 1721–1726 (1994).
Knauert, M. P. & Glazer, P. M. Triplex forming oligonucleotides: sequence-specific tools for gene targeting. Hum. Mol. Genet. 10, 2243–2251 (2001).
Luo, Z., Macris, M. A., Faruqi, A. F. & Glazer, P. M. High-frequency intrachromosomal gene conversion induced by triplex-forming oligonucleotides microinjected into mouse cells. Proc. Natl Acad. Sci. USA 97, 9003–9008 (2000).An important study that shows the use of triple-helix-forming oligonucleotides to affect target-gene modification at frequencies > 50-fold higher than are usually reported.
Gamper, H. B. et al. The DNA strand of chimeric RNA/DNA oligonucleotides can direct gene repair/conversion activity in mammalian and plant cell-free extracts. Nucleic Acids Res. 28, 4332–4339 (2000).
Sharma, H. W., Perez, J. R., Higgins-Sochaski, K., Hsiao, R. & Narayanan, R. Transcription factor decoy approach to decipher the role of NF-κB in oncogenesis. Anticancer Res. 16, 61–69 (1996).
Kielkopf, C. L., Baird, E. E., Dervan, P. B. & Rees, D. C. Structural basis for G·C recognition in the DNA minor groove. Nature Struct. Biol. 5, 104–109 (1998).
Kielkopf, C. L. et al. A structural basis for recognition of A·T and T·A base pairs in the minor groove of B-DNA. Science 282, 111–115 (1998).
Kielkopf, C. L. et al. Structural effects of DNA sequence on T·A recognition by hydroxypyrrole/pyrrole pairs in the minor groove. J. Mol. Biol. 295, 557–567 (2000).
Urbach, A. R. & Dervan, P. B. Toward rules for 1:1 polyamide:DNA recognition. Proc. Natl Acad. Sci. USA 98, 4343–4348 (2001).This paper discusses issues related to the development of polyamides for inhibiting transcription.
Beelman, C. A. & Parker, R. Degradation of mRNA in eukaryotes. Cell 81, 179–183 (1995).
Liebhaber, S. A. mRNA stability and the control of gene expression. Nucleic Acids Symp. Ser. 36, 29–32 (1997).
Weiss, I. M. & Liebhaber, S. A. Erythroid cell-specific mRNA stability elements in the α2-globin 3′ nontranslated region. Mol. Cell. Biol. 15, 2457–2465 (1995).
Chkheidze, A. N. et al. Assembly of the α-globin mRNA stability complex reflects binary interaction between the pyrimidine-rich 3′ untranslated region determinant and poly(C) binding protein αCP. Mol. Cell. Biol. 19, 4572–4581 (1999).
Scanlon, K. J. et al. Oligonucleotide-mediated modulation of mammalian gene expression. FASEB J. 9, 1288–1296 (1995).
Stein, C. A. How to design an antisense oligodeoxynucleotide experiment: a consensus approach. Antisense Nucleic Acid Drug Dev. 8, 129–132 (1998).
Kole, R. & Sazani, P. Antisense effects in the cell nucleus: modification of splicing. Curr. Opin. Mol. Ther. 3, 229–234 (2001).
Dominski, Z. & Kole, R. Identification and characterization by antisense oligonucleotides of exon and intron sequences required for splicing. Mol. Cell. Biol. 14, 7445–7454 (1994).
Summerton, J. & Weller, D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 7, 187–195 (1997).
Iversen, P. L. Phosphorodiamidate morpholino oligomers: favorable properties for sequence-specific gene inactivation. Curr. Opin. Mol. Ther. 3, 235–238 (2001).
Zamaratski, E., Pradeepkumar, P. I. & Chattopadhyaya, J. A critical survey of the structure–function of the antisense oligo/RNA heteroduplex as substrate for RNase H. J. Biochem. Biophys. Methods 48, 189–208 (2001).
Crooke, S. T. Molecular mechanisms of antisense drugs: RNase H. Antisense Nucleic Acid Drug Dev. 8, 133–134 (1998).
Castanotto, D., Scherr, M. & Rossi, J. J. Intracellular expression and function of antisense catalytic RNAs. Methods Enzymol. 313, 401–420 (2000).
Rossi, J. J. Ribozymes, genomics and therapeutics. Chem. Biol. 6, R33–R37 (1999).
Santoro, S. W. & Joyce, G. F. A general purpose RNA-cleaving DNA enzyme. Proc. Natl Acad. Sci. USA 94, 4262–4266 (1997).
Wu, Y. et al. Inhibition of BCR–ABL oncogene expression by novel deoxyribozymes (DNAzymes). Hum. Gene Ther. 10, 2847–2857 (1999).
Earnshaw, D. J. & Gait, M. J. Progress toward the structure and therapeutic use of the hairpin ribozyme. Antisense Nucleic Acid Drug Dev. 7, 403–411 (1997).
Hampel, A. The hairpin ribozyme: discovery, two-dimensional model, and development for gene therapy. Prog. Nucleic Acid Res. Mol. Biol. 58, 1–39 (1998).
Dahm, S. C. & Uhlenbeck, O. C. Role of divalent metal ions in the hammerhead RNA cleavage reaction. Biochemistry 30, 9464–9469 (1991).
Eckstein, F. The hammerhead ribozyme. Biochem. Soc. Trans. 24, 601–604 (1996).
Hegg, L. A. & Fedor, M. J. Kinetics and thermodynamics of intermolecular catalysis by hairpin ribozymes. Biochemistry 34, 15813–15828 (1995).
Hertel, K. J., Herschlag, D. & Uhlenbeck, O. C. A kinetic and thermodynamic framework for the hammerhead ribozyme reaction. Biochemistry 33, 3374–3385 (1994).A physical-chemical study of hammerhead-ribozyme binding and cleavage to an mRNA target.
Irie, A. et al. Anti-oncogene ribozymes for cancer gene therapy. Adv. Pharmacol. 40, 207–257 (1997).
Irie, A. et al. Therapeutic efficacy of an adenovirus-mediated anti-H-Ras ribozyme in experimental bladder cancer. Antisense Nucleic Acid Drug Dev. 9, 341–349 (1999).
Datta, H. J. & Glazer, P. M. Intracellular generation of single-stranded DNA for chromosomal triplex formation and induced recombination. Nucleic Acids Res. 29, 5140–5147 (2001).
Usman, N. & Blatt, L. M. Nuclease-resistant synthetic ribozymes: developing a new class of therapeutics. J. Clin. Invest. 106, 1197–1202 (2000).
Breaker, R. R. & Joyce, G. F. A DNA enzyme that cleaves RNA. Chem. Biol. 1, 223–229 (1994).The authors' hypothesis that DNA could have the same catalytic activity as RNA was shown in this interesting work, which used a novel in vitro selection method to identify a metal-dependent DNA enzyme.
Feldman, A. R. & Sen, D. A new and efficient DNA enzyme for the sequence-specific cleavage of RNA. J. Mol. Biol. 313, 283–294 (2001).
Sioud, M. Nucleic acid enzymes as a novel generation of anti-gene agents. Curr. Mol. Med. 1, 575–588 (2001).
Nishikura, K. A short primer on RNAi: RNA-directed RNA polymerase acts as a key catalyst. Cell 107, 415–418 (2001).
Tuschl, T. Expanding small RNA interference. Nature Biotechnol. 20, 446–448 (2002).
Elbashir, S. M., Lendeckel, W. & Tuschl, T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15, 188–200 (2001).
Bernstein, E., Caudy, A. A., Hammond, S. M. & Hannon, G. J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366 (2001).
Hammond, S. M., Boettcher, S., Caudy, A. A., Kobayashi, R. & Hannon, G. J. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 293, 1146–1150 (2001).
Ketting, R. F. et al. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 15, 2654–2659 (2001).
Yang, S., Tutton, S., Pierce, E. & Yoon, K. Specific double-stranded RNA interference in undifferentiated mouse embryonic stem cells. Mol. Cell. Biol. 21, 7807–7816 (2001).
Paddison, P. J., Caudy, A. A. & Hannon, G. J. Stable suppression of gene expression by RNAi in mammalian cells. Proc. Natl Acad. Sci. USA 99, 1443–1448 (2002).An important study that supports the possibility that RNAi might be achieved in mammalian cells.
Bernstein, E., Denli, A. M. & Hannon, G. J. The rest is silence. RNA 7, 1509–1521 (2001).
Yang, D., Lu, H. & Erickson, J. W. Evidence that processed small dsRNAs may mediate sequence-specific mRNA degradation during RNAi in Drosophila embryos. Curr. Biol. 10, 1191–1200 (2000).
Zamore, P. D., Tuschl, T., Sharp, P. A. & Bartel, D. P. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 101, 25–33 (2000).An important observation, which shows that mediators of RNAi are short, 21–23-nt fragments that are cleaved from longer dsRNA.
Elbashir, S. M., Martinez, J., Patkaniowska, A., Lendeckel, W. & Tuschl, T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 20, 6877–6888 (2001).
Sierakowska, H., Sambade, M. J., Agrawal, S. & Kole, R. Repair of thalassemic humanβ-globin mRNA in mammalian cells by antisense oligonucleotides. Proc. Natl Acad. Sci. USA 93, 12840–12844 (1996).The use of antisense DNA to regulate mRNA splicing as opposed to its more usual use as an RNA blocker or destroyer.
Sierakowska, H., Agrawal, S. & Kole, R. Antisense oligonucleotides as modulators of pre-mRNA splicing. Methods Mol. Biol. 133, 223–233 (2000).
Lacerra, G. et al. Restoration of hemoglobin A synthesis in erythroid cells from peripheral blood of thalassemic patients. Proc. Natl Acad. Sci. USA 97, 9591–9596 (2000).
Mercatante, D. R., Bortner, C. D., Cidlowski, J. A. & Kole, R. Modification of alternative splicing of Bcl-x pre-mRNA in prostate and breast cancer cells. analysis of apoptosis and cell death. J. Biol. Chem. 276, 16411–16417 (2001).
Agrawal, S. & Zhao, Q. Mixed backbone oligonucleotides: improvement in oligonucleotide-induced toxicity in vivo. Antisense Nucleic Acid Drug Dev. 8, 135–139 (1998).
Crooke, S. T. Molecular mechanisms of action of antisense drugs. Biochim. Biophys. Acta 1489, 31–44 (1999).
Stein, C. A. Is irrelevant cleavage the price of antisense efficacy? Pharmacol. Ther. 85, 231–236 (2000).
Nielsen, P. E. DNA analogues with nonphosphodiester backbones. Annu. Rev. Biophys. Biomol. Struct. 24, 167–183 (1995).
Wong-Staal, F., Poeschla, E. M. & Looney, D. J. A controlled, Phase I clinical trial to evaluate the safety and effects in HIV-1 infected humans of autologous lymphocytes transduced with a ribozyme that cleaves HIV-1 RNA. Hum. Gene Ther. 9, 2407–2425 (1998).
Amado, R. G. et al. A Phase I trial of autologous CD34+ hematopoietic progenitor cells transduced with an anti-HIV ribozyme. Hum. Gene Ther. 10, 2255–2270 (1999).
Sereni, D. et al. Pharmacokinetics and tolerability of intravenous trecovirsen (GEM 91), an antisense phosphorothioate oligonucleotide, in HIV positive subjects. J. Clin. Pharmacol. 39, 47–54 (1999).
Bishop, M. R. et al. Phase I trial of an antisense oligonucleotide OL(1)p53 in hematologic malignancies. J. Clin. Oncol. 14, 1320–1326 (1996).
Galbraith, W. M., Hobson, W. C., Giclas, P. C., Schechter, P. J. & Agrawal, S. Complement activation and hemodynamic changes following intravenous administration of phosphorothioate oligonucleotides in the monkey. Antisense Res. Dev. 4, 201–206 (1994).
Nemunaitis, J. et al. Phase I evaluation of ISIS 3521, an antisense oligodeoxynucleotide to protein kinase C-α, in patients with advanced cancer. J. Clin. Oncol. 17, 3586–3595 (1999).
Waters, J. S. et al. Phase I clinical and pharmacokinetic study of BCL2 antisense oligonucleotide therapy in patients with non-Hodgkin's lymphoma. J. Clin. Oncol. 18, 1812–1823 (2000).
Chi, K. N. et al. A Phase I dose-finding study of combined treatment with an antisense Bcl2 oligonucleotide (Genasense) and mitoxantrone in patients with metastatic hormone-refractory prostate cancer. Clin. Cancer Res. 7, 3920–3927 (2001).
Yuen, A. R. et al. Phase I study of an antisense oligonucleotide to protein kinase C-α (ISIS 3521/CGP 64128A) in patients with cancer. Clin. Cancer Res. 5, 3357–3363 (1999).
Yang, E. & Korsmeyer, S. J. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood 88, 386–401 (1996).
Reed, J. C. Bcl2 family proteins: regulators of chemoresistance in cancer. Toxicol. Lett. 82–83, 155–158 (1995).
Gazitt, Y. et al. Bcl-2 overexpression is associated with resistance to paclitaxel, but not gemcitabine, in multiple myeloma cells. Int. J. Oncol. 13, 839–848 (1998).
Reed, J. C. et al. Antisense-mediated inhibition of BCL2 protooncogene expression and leukemic cell growth and survival: comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res. 50, 6565–6570 (1990).
Webb, A. et al. BCL-2 antisense therapy in patients with non-Hodgkin lymphoma. Lancet 349, 1137–1141 (1997).
Jansen, B. et al. Chemosensitisation of malignant melanoma by BCL2 antisense therapy. Lancet 356, 1728–1733 (2000).
Tolcher, A. W. Preliminary phase I results of G3139 (Bcl2 antisense oligonucleotide) therapy in combination with docetaxel in hormone-refractory prostate cancer. Semin. Oncol. 28, 67–70 (2001).
Luger, S. M. et al. Oligodeoxynucleotide-mediated inhibition of c-myb gene expression in autografted bone marrow: a pilot study. Blood 99, 1150–1158 (2002).The clinical use of an antisense DNA with good activity against its mRNA target and pharmacodynamic correlates.
Dean, N. M. et al. Antisense oligonucleotides as inhibitors of signal transduction: development from research tools to therapeutic agents. Biochem. Soc. Trans. 24, 623–629 (1996).
Dean, N. et al. Inhibition of growth of human tumor cell lines in nude mice by an antisense of oligonucleotide inhibitor of protein kinase C-α expression. Cancer Res. 56, 3499–3507 (1996).
Cunningham, C. C. et al. A Phase I trial of H-ras antisense oligonucleotide ISIS 2503 administered as a continuous intravenous infusion in patients with advanced carcinoma. Cancer 92, 1265–1271 (2001).
Brennscheidt, U. et al. Raf-1 is a necessary component of the mitogenic response of the human megakaryoblastic leukemia cell line MO7 to human stem cell factor, granulocyte-macrophage colony-stimulating factor, interleukin 3, and interleukin 9. Cell Growth Differ. 5, 367–372 (1994).
Monia, B. P., Johnston, J. F., Geiger, T., Muller, M. & Fabbro, D. Antitumor activity of a phosphorothioate antisense oligodeoxynucleotide targeted against C-raf kinase. Nature Med. 2, 668–675 (1996).A useful mouse xenograft model for examining the usefulness of an oligodeoxynucleotide.
Rudin, C. M. et al. Phase I trial of ISIS 5132, an antisense oligonucleotide inhibitor of c-raf-1, administered by 24-hour weekly infusion to patients with advanced cancer. Clin. Cancer Res. 7, 1214–1220 (2001).
Eckstein, F. Exogenous application of ribozymes for inhibiting gene expression. Ciba Found. Symp. 209, 207–212 (1997).
Looney, D. & Yu, M. Clinical aspects of ribozymes as therapeutics in gene therapy. Methods Mol. Biol. 74, 469–486 (1997).
Brower, V. et al. All clear for HIV-targeting ribozyme in Phase II. Nature Biotechnol. 16, 123 (1998).
Bennett, C. F., Condon, T. P., Grimm, S., Chan, H. & Chiang, M. Y. Inhibition of endothelial cell adhesion molecule expression with antisense oligonucleotides. J. Immunol. 152, 3530–3540 (1994).
Nestle, F. O., Mitra, R. S., Bennett, C. F., Chan, H. & Nickoloff, B. J. Cationic lipid is not required for uptake and selective inhibitory activity of ICAM-1 phosphorothioate antisense oligonucleotides in keratinocytes. J. Invest. Dermatol. 103, 569–575 (1994).
Miele, M. E., Bennett, C. F., Miller, B. E. & Welch, D. R. Enhanced metastatic ability of TNF-α-treated malignant melanoma cells is reduced by intercellular adhesion molecule-1 (ICAM-1, CD54) antisense oligonucleotides. Exp. Cell Res. 214, 231–241 (1994).
Schreiber, S. et al. Absence of efficacy of subcutaneous antisense ICAM-1 treatment of chronic active Crohn's disease. Gastroenterology 120, 1339–1346 (2001).
Wraight, C. J. et al. Reversal of epidermal hyperproliferation in psoriasis by insulin-like growth factor I receptor antisense oligonucleotides. Nature Biotechnol. 18, 521–526 (2000).
Roque, F. et al. Safety of intracoronary administration of c-myc antisense oligomers after percutaneous transluminal coronary angioplasty (PTCA). Antisense Nucleic Acid Drug Dev. 11, 99–106 (2001).
Kutryk, M. J. et al. Local intracoronary administration of antisense oligonucleotide against c-myc for the prevention of in-stent restenosis: results of the randomized investigation by the Thoraxcenter of antisense DNA using local delivery and IVUS after coronary stenting (ITALICS) trial. J. Am. Coll. Cardiol. 39, 281–287 (2002).
Krieg, A. M. et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374, 546–549 (1995).A seminal report on the ability of vertebate immune cells to recognize unmethylated CpG dinucleotide motifs present in prokaryotes. These findings contribute to the hypothesis that synthetic ODN-containing CpG motifs might function as effective immunological adjuvants.
Krug, A. et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 31, 3026–3037 (2001).
Brazolot Millan, C. L., Weeratna, R., Krieg, A. M., Siegrist, C. A. & Davis, H. L. CpG DNA can induce strong TH1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc. Natl Acad. Sci. USA 95, 15553–15558 (1998).
Krieg, A. M., Yi, A. K., Schorr, J. & Davis, H. L. The role of CpG dinucleotides in DNA vaccines. Trends Microbiol. 6, 23–27 (1998).
Jahrsdorfer, B. et al. CpG DNA increases primary malignant B cell expression of costimulatory molecules and target antigens. J. Leukoc. Biol. 69, 81–88 (2001).
Methia, N., Louache, F., Vainchenker, W. & Wendling, F. Oligodeoxynucleotides antisense to the proto-oncogene c-mpl specifically inhibit in vitro megakaryocytopoiesis. Blood 82, 1395–1401 (1993).
Good, L., Awasthi, S. K., Dryselius, R., Larsson, O. & Nielsen, P. E. Bactericidal antisense effects of peptide-PNA conjugates. Nature Biotechnol. 19, 360–364 (2001).
Meshorer, E. et al. Alternative splicing and neuritic mRNA translocation under long-term neuronal hypersensitivity. Science 295, 508–512 (2002).
Andrews, D. W. et al. Results of a pilot study involving the use of an antisense oligodeoxynucleotide directed against the insulin-like growth factor type I receptor in malignant astrocytomas. J. Clin. Oncol. 19, 2189–2200 (2001).
Cunningham, C. C. et al. A Phase I trial of c-Raf kinase antisense oligonucleotide ISIS 5132 administered as a continuous intravenous infusion in patients with advanced cancer. Clin. Cancer Res. 6, 1626–1631 (2000).
Gewirtz, A. M., Stein, C. A. & Glazer, P. M. Facilitating oligonucleotide delivery: helping antisense deliver on its promise. Proc. Natl Acad. Sci. USA 93, 3161–3163 (1996).
Lebedeva, I. & Stein, C. A. Antisense oligonucleotides: promise and reality. Annu. Rev. Pharmacol. Toxicol. 41, 403–419 (2001).
Baskerville, S. & Ellington, A. D. RNA structure. Describing the elephant. Curr. Biol. 5, 120–123 (1995).
Monia, B. P. et al. Sequence-specific antitumor activity of a phosphorothioate oligodeoxyribonucleotide targeted to human C-raf kinase supports an antisense mechanism of action in vivo. Proc. Natl Acad. Sci. USA 93, 15481–15484 (1996).
Sczakiel, G., Homann, M. & Rittner, K. Computer-aided search for effective antisense RNA target sequences of the human immunodeficiency virus type 1. Antisense Res. Dev. 3, 45–52 (1993).
Milner, N., Mir, K. U. & Southern, E. M. Selecting effective antisense reagents on combinatorial oligonucleotide arrays. Nature Biotechnol. 15, 537–541 (1997).
Sohail, M. & Southern, E. M. Selecting optimal antisense reagents. Adv. Drug Deliv. Rev. 44, 23–34 (2000).
Ho, S. P. et al. Mapping of RNA accessible sites for antisense experiments with oligonucleotide libraries. Nature Biotechnol. 16, 59–63 (1998).
Scherr, M., Rossi, J. J., Sczakiel, G. & Patzel, V. RNA accessibility prediction: a theoretical approach is consistent with experimental studies in cell extracts. Nucleic Acids Res. 28, 2455–2461 (2000).An interesting strategy for mapping hybridization-accessible sites in mRNA.
Sokol, D. L., Zhang, X., Lu, P. & Gewirtz, A. M. Real time detection of DNA. RNA hybridization in living cells. Proc. Natl Acad. Sci. USA 95, 11538–11543 (1998).A new strategy for visualizing mRNA expression and hybridization-accessible sites in living cells.
Yakubov, L. A. et al. Mechanism of oligonucleotide uptake by cells: involvement of specific receptors? Proc. Natl Acad. Sci. USA 86, 6454–6458 (1989).
Beltinger, C. et al. Binding, uptake, and intracellular trafficking of phosphorothioate-modified oligodeoxynucleotides. J. Clin. Invest. 95, 1814–1823 (1995).A study that examines the mechanism of oligodeoxynucleotide uptake.
Laktionov, P. et al. Uptake of oligonucleotides by keratinocytes. Nucleosides Nucleotides 18, 1697–1699 (1999).
Mechti, N., Leonetti, J. P., Clarenc, J. P., Degols, G. & Lebleu, B. Nuclear location of synthetic oligonucleotides microinjected somatic cells: its implication in an antisense strategy. Nucleic Acids Symp. Ser. 147–150 (1991).
Clarenc, J. P., Lebleu, B. & Leonetti, J. P. Characterization of the nuclear binding sites of oligodeoxyribonucleotides and their analogs. J. Biol. Chem. 268, 5600–5604 (1993).
Juliano, R. L., Alahari, S., Yoo, H., Kole, R. & Cho, M. Antisense pharmacodynamics: critical issues in the transport and delivery of antisense oligonucleotides. Pharm. Res. 16, 494–502 (1999).A useful review of oligonucleotide uptake and distribution in cells and whole animals.
DeLong, R. K. et al. Novel cationic amphiphiles as delivery agents for antisense oligonucleotides. Nucleic Acids Res. 27, 3334–3341 (1999).
Baer, M. R., Augustinos, P. & Kinniburgh, A. J. Defective c-myc and c-myb RNA turnover in acute myeloid leukemia cells. Blood 79, 1319–1326 (1992).
Bies, J., Nazarov, V. & Wolff, L. Alteration of proteolytic processing of c-Myb as a consequence of its truncation in murine myeloid leukemia. Leukemia 13, S116–S117 (1999).
Kitada, S., Miyashita, T., Tanaka, S. & Reed, J. C. Investigations of antisense oligonucleotides targeted against Bcl2 RNAs. Antisense Res. Dev. 3, 157–169 (1993).
Mandiyan, S. et al. Molecular and cellular characterization of baboon c-Raf as a target for antiproliferative effects of antisense oligonucleotides. Antisense Nucleic Acid Drug Dev. 7, 539–548 (1997).
Haklai, R. et al. Dislodgment and accelerated degradation of Ras. Biochemistry 37, 1306–1314 (1998).
Basilion, J. P. et al. Selective killing of cancer cells based on loss of heterozygosity and normal variation in the human genome: a new paradigm for anticancer drug therapy. Mol. Pharmacol. 56, 359–369 (1999).
Stein, C. A. Does antisense exist? Nature Med. 1, 1119–1121 (1995).
De Smet, M. D., Meenken, C. J. & van den Horn, G. J. Fomivirsen — a phosphorothioate oligonucleotide for the treatment of CMV retinitis. Ocul. Immunol. Inflamm. 7, 189–198 (1999).
Mulamba, G. B., Hu, A., Azad, R. F., Anderson, K. P. & Coen, D. M. Human cytomegalovirus mutant with sequence-dependent resistance to the phosphorothioate oligonucleotide fomivirsen. Antimicrob. Agents Chemother. 42, 971–973 (1998).
Anderson, K. P., Fox, M. C., Brown-Driver, V., Martin, M. J. & Azad, R. F. Inhibition of human cytomegalovirus immediate-early gene expression by an antisense oligonucleotide complementary to immediate-early RNA. Antimicrob. Agents Chemother. 40, 2004–2011 (1996).
The Vitravene Study Group. A randomized controlled clinical trial of intravitreous Fomiversen for treatment of newly diagnosed peripheral cytomegalovirus retinitis in patients with AIDs. Am. J. Opthalmol. 133, 467–474 (2002).
Coudert, B. et al. Phase II with ISIS 5132 in patients with small-cell (SCLC) and non-small-cell (NSCLC) lung cancer. A European Organization for Research and Treatment of Cancer (EORTC) early clinical studies group report. Eur. J. Cancer 37, 2194–2198 (2001).
Acknowledgements
This work is supported by a grant from the National Institutes of Health. A.M.G. is a Distinguished Clinical Scientist of the Doris Duke Charitable Foundation. The editorial assistance of E. R. Bien and M. Goodrum is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Cancer.gov
LocusLink
2′,5′-oligodenylate synthetase
Medscape DrugInfo
OMIM
FURTHER INFORMATION
Encyclopedia of Life Sciences
antisense nucleic acids in biotechnology
Glossary
- EXOGENOUS NUCLEIC ACIDS
-
In this context, synthetic oligonucleotides of varying chemistry (typically 16–25 nucleotides), which are introduced into cells by various means, or simply (although inefficiently) by concentration-driven endocytosis.
- ANTISENSE
-
Reverse complement of any DNA or RNA sequence.
- TRIPLE-HELIX-FORMING OLIGODEOXYNUCLEOTIDE
-
(TFO). A synthetic, single-stranded oligodeoxynucleotide, which, through Hoogsten-bond formation, hybridizes to purine/pyrymidine-rich sequences in double-stranded DNA. Formation of stable triple helices can prevent the unwinding that is necessary for transcription of the targeted region or block the binding of transcription-factor complexes.
- MAJOR GROOVE AND MINOR GROOVE
-
Channels formed by the twisting of two complementary DNA strands around each other to form a double helix. The major groove is ∼22 Å wide and the minor groove is ∼12 Å wide.
- HOOGSTEEN BOND
-
Triple-helix-forming oligonucleotides hybridize with purine bases that comprise polypurine/polypyrimidine tracks in the DNA. The hydrogen bonds that are formed under these conditions are referred to as Hoogsteen bonds after the individual who first described them. They can form in parallel or antiparallel (reverse-Hoogsteen) orientations.
- NUCLEOSOME
-
A packing unit for DNA within the cell nucleus, which gives the chromatin a 'beads-on-a-string' structure. The 'beads' consist of complexes of nuclear proteins (histones) and DNA, and the 'string' consists of DNA only. A histone octamer forms a core around which the double-stranded DNA helix is wound twice.
- LEXITROPSIN
-
A molecule that extragenetically reads the base sequence of double-stranded DNA.
- RIBOZYME
-
RNA molecule that contains one of a variety of catalytic motifs that cleave RNA to which it hybridizes.
- DNAzyme
-
A DNA molecule that contains a catalytic motif that cleaves RNA to which it hybridizes.
- MORPHOLINO OLIGODEOXYNUCLEOTIDE
-
(PMO). The base is attached to a morpholino instead of a ribofuranosyl ring, and the backbone is composed of a phosphorodiamidate linkage.
- RESTENOSIS
-
A reduction in lumenal size after an inter-arterial coronary intervention.
Rights and permissions
About this article
Cite this article
Opalinska, J., Gewirtz, A. Nucleic-acid therapeutics: basic principles and recent applications. Nat Rev Drug Discov 1, 503–514 (2002). https://doi.org/10.1038/nrd837
Issue Date:
DOI: https://doi.org/10.1038/nrd837
This article is cited by
-
PTK2B promotes TBK1 and STING oligomerization and enhances the STING-TBK1 signaling
Nature Communications (2023)
-
The evolution of commercial drug delivery technologies
Nature Biomedical Engineering (2021)
-
LINC00673 is activated by YY1 and promotes the proliferation of breast cancer cells via the miR-515-5p/MARK4/Hippo signaling pathway
Journal of Experimental & Clinical Cancer Research (2019)
-
Enzymatic construction of shRNA library from oligonucleotide library
Genes & Genomics (2019)
-
Antisense oligonucleotides: the next frontier for treatment of neurological disorders
Nature Reviews Neurology (2018)