Key Points
-
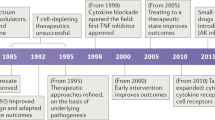
Despite the existence of effective therapies, allergic asthma and related atopic syndromes have recently emerged as epidemic diseases and important public health concerns. A remarkable collaboration has since ensued between basic researchers, clinicians and the pharmaceutical industry in an effort to develop more effective therapeutic and preventative strategies.
-
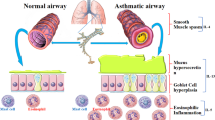
Studies from human and rodent models provide evidence that allergic asthma has an immune basis, involving antibodies such as immunoglobulin E and its receptor, FcɛRI, and T-helper type 2 (TH2)-cell-derived cytokines such as interleukin-4 (IL-4), IL-5 and IL-13.
-
Therapeutic strategies now being developed focus on interrupting IgE and TH2 effector pathways. These include anti-IgE antibodies, molecules directly associated with TH2 cells (co-stimulatory molecules, homing molecules, tyrosine kinases, transcription factors and CD4) or secreted TH2 cytokines, chemokines and intermediates in their signalling pathways. Other molecules that might suppress IgE and TH2 function include Toll-like receptors (TLRs), IL-12 and other cytokines.
-
Several potential immune targets seem to have limited therapeutic potential in allergic asthma either because they are relatively ineffective or because their general importance in immunity and other functions precludes safe targeting. These molecules include IL-5, the more widely shared cytokine-receptor signalling moieties, many transcription factors, including nuclear factor-κB (NF-κB) and most of the signal transducer and activator of transcription (STAT) factors, and the Janus-associated kinase (JAK) family of tyrosine kinases.
-
Molecules with more potential as therapeutic targets include: IgE and its receptor, FcɛRI; molecules that control TH2 homing, such as putative TH2-specific homing integrins, chemokines and chemokine receptors; and intermediates in the signalling pathways that involve IL-4, IL-13, the α-subunit of the IL-4 receptor (IL-4Rα) and TLR9. Although likely to be effective, approaches that target these molecules could be plagued by rapid disease recurrence with cessation of therapy.
-
The most promising approaches might be those that selectively remove or inactivate TH2 cells through the targeting of cell surface molecules such as T1, chemokine (C-C) motif receptor 8 (CCR8) and CCR4. Alternatively, molecules that prevent TH2 activation could be targeted. For example: IL-2-inducible tyrosine kinase (ITK); the co-stimulatory molecules CD28 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4); the transcription factor GATA-binding protein 3 (GATA3); or CD4.
-
In addition to these new molecules, new approaches to blocking them will be developed, including soluble receptors, immunotoxin antibodies and gene therapy.
Abstract
Recent discoveries on the molecular and cellular basis of asthma have markedly altered our understanding of this common respiratory disorder. These insights have come during an unexplained period of rising disease incidence and severity and are now being applied to develop improved therapies. This review explores the latest advances in our understanding of the pathogenesis of allergic asthma, and provides insight into the expanding collaborations between research scientists, clinicians and the pharmaceutical industry in the race to control the asthma epidemic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hartert, T. V. & Peebles, R. S. Jr. Epidemiology of asthma: the year in review. Curr. Opin. Pulm. Med. 6, 4–9 (2000).
Holgate, S. T. The epidemic of allergy and asthma. Nature 402, B2–B4 (1999).
Bousquet, J. Global Initiative for Asthma (GINA) and its objectives. Clin. Exp. Allergy 30, 2–5 (2000).
Lemanske, R. F. Jr & Busse, W. W. Asthma. J. Am. Med. Assoc. 278, 1855–1873 (1997).An outstanding general review of asthma.
Anonymous. Drugs for asthma. Med. Lett. Drugs Ther. 42, 19–24 (2000).
Wills-Karp, M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 17, 255–281 (1999).
Corry, D. B. et al. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol. Med. 4, 344–355 (1998).
Wills-Karp, M. et al. Interleukin-13: central mediator of allergic asthma. Science 282, 2258–2261 (1998).
Grunig, G. et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282, 2261–2263 (1998).References 7–9 established the existence of an antibody-independent mechanism underlying airway obstruction in experimental asthma and the crucial roles that IL-4, IL-13 and IL-4Rα have.
Obiri, N. I., Debinski, W., Leonard, W. J. & Puri, R. K. Receptor for interleukin 13. Interaction with interleukin 4 by a mechanism that does not involve the common γ-chain shared by receptors for interleukins 2, 4, 7, 9, and 15. J. Biol. Chem. 270, 8797–8804 (1995).
Lefort, S., Vita, N., Reeb, R., Caput, D. & Ferrara, P. IL-13 and IL-4 share signal transduction elements as well as receptor components in TF-1 cells. FEBS Lett. 366, 122–126 (1995).
Smerz-Bertling, C. & Duschl, A. Both interleukin 4 and interleukin 13 induce tyrosine phosphorylation of the 140-kDa subunit of the interleukin 4 receptor. J. Biol. Chem. 270, 966–970 (1995).
Murata, T. & Puri, R. K. Comparison of IL-13- and IL-4-induced signaling in EBV-immortalized human B cells. Cell. Immunol. 175, 33–40 (1997).
Orchansky, P. L., Ayres, S. D., Hilton, D. J. & Schrader, J. W. An interleukin (IL)-13 receptor lacking the cytoplasmic domain fails to transduce IL-13-induced signals and inhibits responses to IL-4. J. Biol. Chem. 272, 22940–22947 (1997).
Palmer-Crocker, R. L., Hughes, C. C. & Pober, J. S. IL-4 and IL-13 activate the JAK2 tyrosine kinase and STAT6 in cultured human vascular endothelial cells through a common pathway that does not involve the γc chain. J. Clin. Invest. 98, 604–609 (1996).
Madden, K. B. et al. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J. Immunol. 147, 1387–1391 (1991).
Finkelman, F. D. et al. IL-4 is required to generate and sustain in vivo IgE responses. J. Immunol. 141, 2335–2341 (1988).
Snapper, C. M., Finkelman, F. D. & Paul, W. E. Differential regulation of IgG1 and IgE synthesis by interleukin 4. J. Exp. Med. 167, 183–196 (1988).
Finkelman, F. D., Katona, I. M., Urban, J. F. Jr & Paul, W. E. Control of in vivo IgE production in the mouse by interleukin 4. Ciba Found. Symp. 147, 3–17; discussion 17–22 (1989).
Coffman, R. L., Seymour, B. W., Hudak, S., Jackson, J. & Rennick, D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science 245, 308–310 (1989).
Abbas, A. K., Murphy, K. M. & Sher, A. Functional diversity of helper T lymphocytes. Nature 383, 787–793 (1996).
Lohning, M. et al. T1/ST2 is preferentially expressed on murine TH2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for TH2 effector function. Proc. Natl Acad. Sci. USA 95, 6930–6935 (1998).This study established the selective expression of T1 on T H 2 cells and provided preliminary evidence that it is a T H 2-selective co-stimulatory molecule.
Mitcham, J. L. et al. T1/ST2 signaling establishes it as a member of an expanding interleukin-1 receptor family. J. Biol. Chem. 271, 5777–5783 (1996).
Meisel, C. et al. Regulation and function of T1/ST2 expression on CD4+ T cells: induction of type 2 cytokine production by T1/ST2 cross-linking. J. Immunol. 166, 3143–3150 (2001).
Lambrecht, B. N. et al. Myeloid dendritic cells induce TH2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J. Clin. Invest. 106, 551–559 (2000).
Coyle, A. J. et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J. Exp. Med. 190, 895–902 (1999).
Hoshino, K., et al. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J. Exp. Med. 190, 1541–1548 (1999).
Townsend, M. J., Fallon, P. G., Matthews, D. J., Jolin, H. E. & McKenzie, A. N. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 191, 1069–1076 (2000).References 27 and 28 established that although lack of T1 can result in diminished T H 2 cytokine production in vivo , the effect is small and insufficient to abrogate T H 2 effector phenotypes, including experimental asthma.
Corry, D. B., Reiner, S. L., Linsley, P. S. & Locksley, R. M. Differential effects of blockade of CD28–B7 in the development of TH1 or TH2 effector cells in experimental leishmaniasis. J. Immunol. 153, 4142–4148 (1994).
McArthur, J. G. & Raulet, D. H. CD28-induced costimulation of T helper type 2 cells mediated by induction of responsiveness to interleukin 4. J. Exp. Med. 178, 1645–1653 (1993).
Howland, K. C., Ausubel, L. J., London, C. A. & Abbas, A. K. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J. Immunol. 164, 4465–4470 (2000).
Padrid, P. A. et al. CTLA4Ig inhibits airway eosinophilia and hyperresponsiveness by regulating the development of TH1/TH2 subsets in a murine model of asthma. Am. J. Respir. Cell Mol. Biol. 18, 453–462 (1998).
Keane-Myers, A., Gause, W. C., Linsley, P. S., Chen, S. J. & Wills-Karp, M. B7–CD28/CTLA-4 costimulatory pathways are required for the development of T helper cell 2-mediated allergic airway responses to inhaled antigens. J. Immunol. 158, 2042–2049 (1997).
Van Oosterhout, A. J. et al. Murine CTLA4-IgG treatment inhibits airway eosinophilia and hyperresponsiveness and attenuates IgE upregulation in a murine model of allergic asthma. Am. J. Respir. Cell Mol. Biol. 17, 386–392 (1997).
Krinzman, S. J. et al. Inhibition of T cell costimulation abrogates airway hyperresponsiveness in a murine model. J. Clin. Invest. 98, 2693–2699 (1996).
Oosterwegel, M. A. et al. The role of CTLA-4 in regulating TH2 differentiation. J. Immunol. 163, 2634–2639 (1999).Compares CD28 and CTLA-4 co-stimulation with respect to T H 2 differentiation, finding CD28 to be a positive and CTLA-4 to be a negative regulator.
Butcher, E. C. & Picker, L. J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Sozzani, S. et al. The viral chemokine macrophage inflammatory protein-II is a selective TH2 chemoattractant. Blood 92, 4036–4039 (1998).
Zingoni, A. et al. The chemokine receptor CCR8 is preferentially expressed in TH2 but not TH1 cells. J. Immunol. 161, 547–551 (1998).
Andrew, D. P. et al. STCP-1 (MDC) CC chemokine acts specifically on chronically activated TH2 lymphocytes and is produced by monocytes on stimulation with TH2 cytokines IL-4 and IL-13. J. Immunol. 161, 5027–5038 (1998).
Panina-Bordignon, P. et al. The CC chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J. Clin. Invest. 107, 1357–1364 (2001).These investigators examined expression of the two chemokine receptors previously thought to be unique to mouse T H 2 cells, CCR4 and CCR8. Both receptors were expressed on human T H 2 cells obtained from asthmatic patients.
Cosmi, L. et al. CRTH2 is the most reliable marker for the detection of circulating human type 2 TH and type 2 T cytotoxic cells in health and disease. Eur. J. Immunol. 30, 2972–2979 (2000).
Hirai, H. et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 193, 255–261 (2001).
Gu, L. et al. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 404, 407–411 (2000).
Huang, D. R., Wang, J., Kivisakk, P., Rollins, B. J. & Ransohoff, R. M. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J. Exp. Med. 193, 713–726 (2001).
Boring, L. et al. Impaired monocyte migration and reduced type 1 (TH1) cytokine responses in CC chemokine receptor 2 knockout mice. J. Clin. Invest. 100, 2552–2561 (1997).
Sato, N. et al. CC chemokine receptor (CCR)2 is required for langerhans cell migration and localization of T helper cell type 1 (TH1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by TH2 cytokines, B cell outgrowth, and sustained neutrophilic inflammation. J. Exp. Med. 192, 205–218 (2000).The first report of a tyrosine kinase (IL-2-inducible tyrosine kinase; ITK) required for activation and development of mouse T H 2 cells.
MacLean, J. A. et al. CC chemokine receptor-2 is not essential for the development of antigen-induced pulmonary eosinophilia and airway hyperresponsiveness. J. Immunol. 165, 6568–6575 (2000).
Fowell, D. J. et al. Impaired NFATc translocation and failure of TH2 development in Itk-deficient CD4+ T cells. Immunity 11, 399–409 (1999).References 48 and 49 established tyrosine-kinase blockade as a feasible approach to inhibiting the activity of entire classes of cells.
Druker, B. J. et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 344, 1038–1042 (2001).
Druker, B. J. et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344, 1031–1037 (2001).
Henderson, W. R. Jr, Chi, E. Y. & Maliszewski, C. R. Soluble IL-4 receptor inhibits airway inflammation following allergen challenge in a mouse model of asthma. J. Immunol. 164, 1086–1095 (2000).
Borish, L. C. et al. Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 160, 1816–1823 (1999).
Borish, L. C. et al. Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. J. Allergy Clin. Immunol. 107, 963–970 (2001).
Webb, D. C. et al. Integrated signals between IL-13, IL-4, and IL-5 regulate airways hyperreactivity. J. Immunol. 165, 108–113 (2000).
Foster, P. S., Hogan, S. P., Ramsay, A. J., Matthaei, K. I. & Young, I. G. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J. Exp. Med. 183, 195–201 (1996).
Leckie, M. J. et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 356, 2144–2148 (2000).This surprising study found humanized anti-IL-5 antibodies to be highly effective in clearing eosinophils from blood and bronchoalveolar lavage of asthmatic patients, but ineffective in changing respiratory physiologic parameters associated with airway obstruction.
Townsend, J. M. et al. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 13, 573–583 (2000).Despite the apparently important role of IL-9 in experimental asthma as indicated by prior reports, this study, the first with IL-9-deficient mice, showed no absolute requirement but rather a contributory role to mast-cell development and goblet-cell metaplasia.
Bauer, J. H., Liu, K. D., You, Y., Lai, S. Y. & Goldsmith, M. A. Heteromerization of the γc chain with the interleukin-9 receptor A-subunit leads to STAT activation and prevention of apoptosis. J. Biol. Chem. 273, 9255–9260 (1998).
Dirksen, U. et al. Human pulmonary alveolar proteinosis associated with a defect in GM-CSF/IL-3/IL-5 receptor common β-chain expression. J. Clin. Invest. 100, 2211–2217 (1997).
Izuhara, K. et al. Recent advances in understanding how interleukin 13 signals are involved in the pathogenesis of bronchial asthma. Arch. Immunol. Ther. Exp. (Warsz) Exp. 48, 505–512 (2000).
Grunewald, S. M. et al. An antagonistic IL-4 mutant prevents type I allergy in the mouse: inhibition of the IL-4/IL-13 receptor system completely abrogates humoral immune response to allergen and development of allergic symptoms in vivo. J. Immunol. 160, 4004–4009 (1998).
Dent, A. L., Hu-Li, J., Paul, W. E. & Staudt, L. M. T helper type 2 inflammatory disease in the absence of interleukin 4 and transcription factor STAT6. Proc. Natl Acad. Sci. USA 95, 13823–13828 (1998).
Ye, B. H. et al. The BCL-6 proto-oncogene controls germinal-centre formation and TH2-type inflammation. Nature Genet. 16, 161–170 (1997).References 63 and 64 show that the two transcription factors STAT6 and BCL6 interact in a complex way, yet to be fully understood, to regulate IL-4 production and T H 2 development. Another unexpected finding is that STAT6 is not absolutely required for T H 2 responses.
Zheng, W. & Flavell, R. A. The transcription factor GATA-3 is necessary and sufficient for TH2 cytokine gene expression in CD4 T cells. Cell 89, 587–596 (1997).
Ouyang, W. et al. Stat6-independent GATA-3 autoactivation directs IL-4-independent TH2 development and commitment. Immunity 12, 27–37 (2000).
Zhang, D. H. et al. Inhibition of allergic inflammation in a murine model of asthma by expression of a dominant-negative mutant of GATA-3. Immunity 11, 473–482 (1999).
Das, J. et al. A critical role for NF-κB in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nature Immunol. 2, 45–50 (2001).The important role of GATA3 in experimental asthma was confirmed in references 67 and 68 . An interesting question raised by these reports is whether GATA3 inhibition in the target tissues of the lung might have similar efficacy.
Pandolfi, P. P. et al. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nature Genet. 11, 40–44 (1995).
Russell, S. M. et al. Interaction of IL-2Rβ and γc chains with Jak1 and Jak3: implications for XSCID and XCID. Science 266, 1042–1045 (1994).
Thomis, D. C. & Berg, L. J. Peripheral expression of Jak3 is required to maintain T lymphocyte function. J. Exp. Med. 185, 197–206 (1997).
Oakes, S. A. et al. Signaling via IL-2 and IL-4 in JAK3-deficient severe combined immunodeficiency lymphocytes: JAK3-dependent and independent pathways. Immunity 5, 605–615 (1996).
Wang, L. H. et al. Selective disruption of interleukin 4 autocrine-regulated loop by a tyrosine kinase inhibitor restricts activity of T-helper 2 cells. Blood 95, 3816–3822 (2000).One of the first reports to document the feasibility of tyrosine-kinase blockade using small molecules to inhibit T H 2 cells. Unfortunately, unlike ITK, the tyrosine kinases targeted here, JAK2 and JAK3, are so widely used in other signalling pathways that they are impractical targets.
Malabarba, M. G. et al. Interleukin-13 is a potent activator of JAK3 and STAT6 in cells expressing interleukin-2 receptor-γ and interleukin-4 receptor-α. Biochem. J. 319, 865–872 (1996).
Oshiba, A. et al. Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig)E and mice. J. Clin. Invest. 97, 1398–1408 (1996).
MacLean, J. A., Sauty, A., Luster, A. D., Drazen, J. M. & De Sanctis, G. T. Antigen-induced airway hyperresponsiveness, pulmonary eosinophilia, and chemokine expression in B cell-deficient mice. Am. J. Respir. Cell Mol. Biol. 20, 379–387 (1999).
Milgrom, H. et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb–E25 Study Group. N. Engl. J. Med. 341, 1966–1973 (1999).
Busse, W. et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 108, 184–190 (2001).
Fowell, D. J., Magram, J., Turck, C. W., Killeen, N. & Locksley, R. M. Impaired TH2 subset development in the absence of CD4. Immunity 6, 559–569 (1997).
Locksley, R. M., Reiner, S. L., Hatam, F., Littman, D. R. & Killeen, N. Helper T cells without CD4: control of leishman-iasis in CD4-deficient mice. Science 261, 1448–1451 (1993).Although never actually examined in asthma models, references 79 and 80 established an important functional role for CD4 in controlling T H 2 effector responses, thereby identifying CD4 as a potential target in asthma therapy.
Feito, M. J. et al. CD4 dependence of activation threshold and TCR signalling in mouse T lymphocytes. Scand. J. Immunol. 45, 166–174 (1997).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Kips, J. C. et al. Interleukin-12 inhibits antigen-induced airway hyperresponsiveness in mice. Am. J. Respir. Crit. Care Med. 153, 535–539 (1996).
Hofstra, C. L. et al. Differential effects of endogenous and exogenous interferon-γ on immunoglobulin E, cellular infiltration, and airway responsiveness in a murine model of allergic asthma. Am. J. Respir. Cell Mol. Biol. 19, 826–835 (1998).
Shirota, H., Sano, K., Kikuchi, T., Tamura, G. & Shirato, K. Regulation of murine airway eosinophilia and TH2 cells by antigen-conjugated CpG oligodeoxynucleotides as a novel antigen-specific immunomodulator. J. Immunol. 164, 5575–5582 (2000).
Kline, J. N. et al. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J. Immunol. 160, 2555–2559 (1998).
Bliss, J. et al. IL-12, as an adjuvant, promotes a T helper 1 cell, but does not suppress a T helper 2 cell recall response. J. Immunol. 156, 887–894 (1996).
Bryan, S. A. et al. Effects of recombinant human interleukin-12 on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 356, 2149–2153 (2000).
Bix, M. & Locksley, R. M. Independent and epigenetic regulation of the interleukin-4 alleles in CD4+ T cells. Science 281, 1352–1354 (1998).
Bix, M., Wang, Z. E., Thiel, B., Schork, N. J. & Locksley, R. M. Genetic regulation of commitment to interleukin 4 production by a CD4+ T cell-intrinsic mechanism. J. Exp. Med. 188, 2289–2299 (1998).
Medzhitov, R. CpG DNA: security code for host defense. Nature Immunol. 2, 15–16 (2001).
Kreitman, R. J. et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N. Engl. J. Med. 345, 241–247 (2001).One of the first reports in humans to show the efficacy of targeting potent toxins to discrete cell populations for their destruction. Such strategies could be applied to remove T H 2 cells in asthma.
Varga, L. V., Toth, S., Novak, I. & Falus, A. Antisense strategies: functions and applications in immunology. Immunol. Lett. 69, 217–224 (1999).
Nielsen, P. E. Peptide nucleic acids: on the road to new gene therapeutic drugs. Pharmacol. Toxicol. 86, 3–7 (2000).
Elbashir, S. M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).This newly-defined mechanism for RNA degradation could be used as an antisense-based strategy to block expression of RNA species relevant to the expression of asthma.
Lehrman, S. Virus treatment questioned after gene therapy death. Nature 401, 517–518 (1999).
Acknowledgements
I thank F. Kheradmand for assistance with the figures and F. Kheradmand, B. Dickey and D. Huston for careful review of the manuscript.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
FURTHER INFORMATION
Glossary
- ATOPY
-
The propensity to develop hypersensitivity reactions, such as asthma, which is strongly linked to T-helper type 2 cells and immunoglobulin E.
- TH2 CELL
-
A terminally differentiated subclass of T-helper cells that secretes a restricted repertoire of cytokines, including IL-4, IL-5, IL-9 and IL-13.
- CYTOKINE
-
Secreted products derived from many cells that regulate immune responses.
- AIRWAY HYPER-RESPONSIVENESS
-
An abnormal physiological alteration of the airways in which exaggerated, reversible bronchoconstriction occurs in response to various provocative stimuli.
- TYPE I HYPERSENSITIVITY
-
One of four types of immune mechanism in which immunoglobulin E has an essential pathogenic role. Cells activated by IgE to release inflammatory products contribute to allergic diseases such as asthma, allergic rhinitis and anaphylaxis.
- MAST CELL
-
A type of leukocyte with large secretory granules containing histamine and various protein mediators.
- EOSINOPHIL
-
An allergic effector cell strongly associated with allergic inflammation of many kinds. Secreted products of eosinophils might contribute to airway obstruction and other pathology in allergic asthma.
- TYPE IV HYPERSENSITIVITY
-
An immune hypersensitivity syndrome in which T-cell products, especially cytokines, contribute directly to the manifestations of diseases such as asthma.
- GOBLET CELL
-
A type of cell found in the epithelium of many organs, especially in the intestinal and respiratory tracts. They secrete mucus, a viscous fluid composed primarily of highly glycosylated proteins called mucins.
- JAK
-
Abbreviation for Janus-associated kinase, a subclass of tyrosine kinase involved in signal transduction through cytokine receptors.
- CO-STIMULATORY MOLECULE
-
A molecule that activates accessory signalling pathways that are essential for the activation of T cells.
- HOMING
-
The process of travelling from one organ or tissue to another in response to discrete signals.
- STAT
-
Abbreviation for signal transducer and activator of transcription. This family of transcription factors is crucial for activating the transcription of hormone- and cytokine-dependent genes.
- MAJOR HISTOCOMPATIBILITY COMPLEX
-
A complex of genetic loci, occurring in higher vertebrates, encoding a family of cellular antigens that help the immune system to recognize self from non-self.
- INNATE IMMUNE RECEPTORS
-
The receptors involved in mediating the crucial immune response during the early phase of host defence against infection by pathogens (such as bacteria and viruses), before the antigen-specific, adaptive immune response is induced.
- IMMUNOTOXIN
-
Any toxic agent coupled to an immune molecule, often an antibody. Typically used to kill cells expressing antibody-specific molecules.
Rights and permissions
About this article
Cite this article
Corry, D. Emerging immune targets for the therapy of allergic asthma. Nat Rev Drug Discov 1, 55–64 (2002). https://doi.org/10.1038/nrd702
Issue Date:
DOI: https://doi.org/10.1038/nrd702
This article is cited by
-
The role of miR-29c/B7-H3 axis in children with allergic asthma
Journal of Translational Medicine (2018)
-
The Role of Thymic Stromal Lymphopoietin in Allergic Inflammation and Chronic Obstructive Pulmonary Disease
Archivum Immunologiae et Therapiae Experimentalis (2010)
-
Hitting the MARCKS
Nature Reviews Drug Discovery (2004)
-
Combichem leads for asthma
Nature Reviews Drug Discovery (2003)