Key Points
-
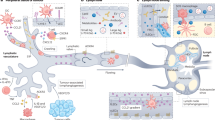
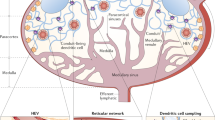
The lymphatic system serves an integral role in fluid homeostasis, lipid metabolism and immune defence, and influences a diverse range of diseases, including infection, inflammatory and metabolic diseases, and cancer.
-
Targeted delivery to the lymphatics and lymphoid tissues has the potential to improve oral bioavailability, enhance vaccination and tolerance induction, target delivery to lymph-resident cancer metastasis and infection, and promote the utility of treatments for diseases ranging from infections such as HIV to cancer and inflammatory and metabolic disease.
-
Selective delivery to the lymph is largely dictated by size, as macromolecules or particulate carriers are excluded from access to blood capillaries, whereas interstitial fluid flow sweeps larger constructs into the more permeable lymphatics.
-
Lymphatic targeting may be achieved via the delivery of macromolecular therapeutics (for example, proteins and peptides), small-molecule therapeutics in association with macromolecular carriers (for example, nanoparticles, polymers, liposomes and dendrimers) or small-molecule therapeutics that associate, in situ, with endogenous macromolecular constructs (for example, lipoproteins and proteins) or cells that are transported from interstitial tissues via lymphatic rather than blood capillaries.
-
The design of lymphatic delivery systems ranges from simple systems that rely on passive lymphatic access to more complex structures that integrate into endogenous lymph transport processes. Recent studies have suggested the presence of active transport processes that facilitate entry across the lymphatic endothelium, and delivery systems that harness these processes are emerging.
-
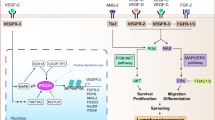
In many cases, disease progression results in lymphatic remodelling. Next-generation lymphatic targeting approaches will probably seek to harness a better understanding of changes to lymphatic structure and function in disease to promote targeting to the lymphatics and enhance therapeutic utility.
-
Future efforts in lymphatic drug delivery might usefully address barriers to the clinical translation of lymphotropic delivery vehicles, such as the lack of well-validated models to predict lymphatic uptake in humans.
Abstract
The lymphatic system serves an integral role in fluid homeostasis, lipid metabolism and immune control. In cancer, the lymph nodes that drain solid tumours are a primary site of metastasis, and recent studies have suggested intrinsic links between lymphatic function, lipid deposition, obesity and atherosclerosis. Advances in the current understanding of the role of the lymphatics in pathological change and immunity have driven the recognition that lymph-targeted delivery has the potential to transform disease treatment and vaccination. In addition, the design of lymphatic delivery systems has progressed from simple systems that rely on passive lymphatic access to sophisticated structures that use nanotechnology to mimic endogenous macromolecules and lipid conjugates that 'hitchhike' onto lipid transport processes. Here, we briefly summarize the lymphatic system in health and disease and the varying mechanisms of lymphatic entry and transport, as well as discussing examples of lymphatic delivery that have enhanced therapeutic utility. We also outline future challenges to effective lymph-directed therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Girard, J. P., Moussion, C. & Forster, R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 12, 762–773 (2012).
Randolph, G. J. & Miller, N. E. Lymphatic transport of high-density lipoproteins and chylomicrons. J. Clin. Invest. 124, 929–935 (2014).
Miller, N. E. et al. Secretion of adipokines by human adipose tissue in vivo: partitioning between capillary and lymphatic transport. Am. J. Physiol. Endocrinol. Metab. 301, E659–E667 (2011).
Wiig, H. & Swartz, M. A. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol. Rev. 92, 1005–1060 (2012).
Starling, E. H. On the absorption of fluids from the connective tissue spaces. J. Physiol. 19, 312–326 (1896).
Levick, J. R. & Michel, C. C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 87, 198–210 (2010).
Mortimer, P. S. & Rockson, S. G. New developments in clinical aspects of lymphatic disease. J. Clin. Invest. 124, 915–921 (2014).
Card, C. M., Yu, S. S. & Swartz, M. A. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J. Clin. Invest. 124, 943–952 (2014).
Pabst, O. & Mowat, A. M. Oral tolerance to food protein. Mucosal Immunol. 5, 232–239 (2012).
Lichtenstein, L. et al. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell. Metabolism 12, 580–592 (2010).
Macpherson, A. J. & Smith, K. Mesenteric lymph nodes at the center of immune anatomy. J. Exp. Med. 203, 497–500 (2006).
Dixon, J. B. Lymphatic lipid transport: sewer or subway? Trends Endocrinol. Metab. 21, 480–487 (2010).
Martel, C. et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J. Clin. Invest. 123, 1571–1579 (2013). A report on the important role of lymphatic vessels in facilitating HDL-mediated reverse cholesterol transport from tissues and atherosclerotic plaques to the systemic circulation, ultimately for excretion via the liver.
Lim, H. Y. et al. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell. Metabolism 17, 671–684 (2013). This article confirms the important role of lymphatic vessels in facilitating HDL-mediated reverse cholesterol transport from tissues, and provides evidence that HDL enters the lymphatics by active transcytosis across LECs via SRB1.
Harvey, N. L. The link between lymphatic function and adipose biology. Ann. NY Acad. Sci. 1131, 82–88 (2008).
Pond, C. M. Adipose tissue and the immune system. Prostaglandins Leukot. Essent. Fatty Acids 73, 17–30 (2005).
Harvey, N. L. et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 37, 1072–1081 (2005). This report highlights the links between lymphatics and adipose function and the development of obesity.
Sawane, M. et al. Apelin inhibits diet-induced obesity by enhancing lymphatic and blood vessel integrity. Diabetes 62, 1970–1980 (2013).
Blum, K. S. et al. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS ONE 9, e94713 (2014).
Arngrim, N., Simonsen, L., Holst, J. J. & Bulow, J. Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: a possible link between obesity and local tissue inflammation? Int. J. Obes. 37, 748–750 (2013).
Savetsky, I. L. et al. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am. J. Physiol. Heart Circ. Physiol. 307, H165–H172 (2014).
Weitman, E. S. et al. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS ONE 8, e70703 (2013).
Kim, C. S. et al. Visceral fat accumulation induced by a high-fat diet causes the atrophy of mesenteric lymph nodes in obese mice. Obesity 16, 1261–1269 (2008).
Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 17, 1371–1380 (2011). A review of advances in our current understanding of the role of lymphatics in pathological change and disease.
Kesler, C. T., Liao, S., Munn, L. L. & Padera, T. P. Lymphatic vessels in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 5, 111–124 (2013).
Wang, Y. & Oliver, G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 24, 2115–2126 (2010).
Swartz, M. A. & Lund, A. W. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat. Rev. Cancer 12, 210–219 (2012).
Dieterich, L. C., Seidel, C. D. & Detmar, M. Lymphatic vessels: new targets for the treatment of inflammatory diseases. Angiogenesis 17, 359–371 (2014).
Proulx, S. T. et al. Expansion of the lymphatic vasculature in cancer and inflammation: new opportunities for in vivo imaging and drug delivery. J. Control. Release 172, 550–557 (2013).
von der Weid, P. Y., Rehal, S. & Ferraz, J. G. Role of the lymphatic system in the pathogenesis of Crohn's disease. Curr. Opin. Gastroenterol. 27, 335–341 (2011).
Alessio, S. et al. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J. Clin. Invest. 124, 3863–3878 (2014).
Huggenberger, R. et al. An important role of lymphatic vessel activation in limiting acute inflammation. Blood 117, 4667–4678 (2011).
Zhang, Q. et al. Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthritis Res. Ther. 9, R118 (2007).
Baluk, P. et al. TNF-α drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J. Clin. Invest. 119, 2954–2964 (2009).
Machnik, A. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 15, 545–552 (2009).
Ribera, J. et al. Increased nitric oxide production in lymphatic endothelial cells causes impairment of lymphatic drainage in cirrhotic rats. Gut 62, 138–145 (2012).
Jones, D. & Min, W. An overview of lymphatic vessels and their emerging role in cardiovascular disease. J. Cardiovasc. Dis. Res. 2, 141–152 (2011).
Fletcher, C. V. et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl Acad. Sci. USA 111, 2307–2312 (2014). The data presented in this article links persistent HIV replication with low antiretroviral drug concentrations in lymphatic tissues in humans. Increasing drug distribution to lymph may therefore provide a treatment benefit.
Pantaleo, G. et al. Lymphoid organs function as major reservoirs for human-immunodeficiency-virus. Proc. Natl Acad. Sci. USA 88, 9838–9842 (1991).
Giannini, C. et al. Association between persistent lymphatic infection by hepatitis C virus after antiviral treatment and mixed cryoglobulinemia. Blood 111, 2943–2945 (2008).
Bennuru, S. & Nutman, T. B. Lymphangiogenesis and lymphatic remodeling induced by filarial parasites: implications for pathogenesis. PLoS Pathog. 5, e1000688 (2009).
Feldmann, H. & Geisbert, T. W. Ebola haemorrhagic fever. Lancet 377, 849–862 (2011).
Deitch, E. A. Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Ann. NY Acad. Sci. 1207, E103–E111 (2010).
Kerjaschki, D. et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 12, 230–234 (2006).
Wang, X. et al. Mechanism of oral tolerance induction to therapeutic proteins. Adv. Drug Deliv. Rev. 65, 759–773 (2013).
Swartz, M. A., Hirosue, S. & Hubbell, J. A. Engineering approaches to immunotherapy. Sci. Transl. Med. 4, 148rv9 (2012).
Trevaskis, N. L., Charman, W. N. & Porter, C. J. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv. Drug Deliv. Rev. 60, 702–716 (2008).
Ryan, G. M., Kaminskas, L. M. & Porter, C. J. Nano-chemotherapeutics: maximising lymphatic drug exposure to improve the treatment of lymph-metastatic cancers. J. Control. Release 193, 241–256 (2014).
Yáñez, J. A., Wang, S. W. J., Knemeyer, I. W., Wirth, M. A. & Alton, K. B. Intestinal lymphatic transport for drug delivery. Adv. Drug Deliv. Rev. 63, 923–942 (2011).
Supersaxo, A., Hein, W. R. & Steffen, H. Effect of molecular-weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm. Res. 7, 167–169 (1990). The first paper to describe the relationship between molecular mass of proteins and lymphatic uptake from interstitial injection sites in sheep.
Irvine, D. J., Swartz, M. A. & Szeto, G. L. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 12, 978–990 (2013).
Charman, S. A., McLennan, D. N., Edwards, G. A. & Porter, C. J. H. Lymphatic absorption is a significant contributor to the subcutaneous bioavailability of insulin in a sheep model. Pharm. Res. 18, 1620–1626 (2001).
Charman, S. A., Segrave, A. M., Edwards, G. A. & Porter, C. J. H. Systemic availability and lymphatic transport of human growth hormone administered by subcutaneous injection. J. Pharm. Sci. 89, 168–177 (2000).
Kota, J. et al. Lymphatic absorption of subcutaneously administered proteins: influence of different injection sites on the absorption of darbepoetin alfa using a sheep model. Drug Metab. Dispos. 35, 2211–2217 (2007).
McLennan, D. et al. Pharmacokinetic model to describe the lymphatic absorption of r-methu-leptin after subcutaneous injection to sheep. Pharm. Res. 20, 1156–1162 (2003).
McLennan, D. et al. The absorption of darbepoetin alfa occurs predominantly via the lymphatics following subcutaneous administration to sheep. Pharm. Res. 23, 2060–2066 (2006).
McLennan, D. N. et al. Lymphatic absorption is the primary contributor to the systemic availability of epoetin alfa following subcutaneous administration to sheep. J. Pharmacol. Exp. Ther. 313, 345–351 (2005).
Oussoren, C., Zuidema, J., Crommelin, D. J. & Storm, G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. II. Influence of liposomal size, lipid composition and lipid dose. Biochim. Biophys. Acta 1328, 261–272 (1997). The first paper to establish the influence of size and composition on lymphatic uptake and retention of model delivery systems (liposomes).
Reddy, S. T. et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotech. 25, 1159–1164 (2007).
Reed, A. L., Rowson, S. A. & Dixon, J. B. Demonstration of ATP-dependent, transcellular transport of lipid across the lymphatic endothelium using an in vitro model of the lacteal. Pharm. Res. 30, 3271–3280 (2013).
Laakkonen, P. et al. Antitumor activity of a homing peptide that targets tumor lymphatics and tumor cells. Proc. Natl Acad. Sci. USA 101, 9381–9386 (2004).
Laakkonen, P., Porkka, K., Hoffman, J. A. & Ruoslahti, E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat. Med. 8, 751–755 (2002).
Parker, J. C., Gilchrist, S. & Cartledge, J. T. Plasma–lymph exchange and interstitial distribution volumes of charged macromolecules in the lung. J. Appl. Physiol. 59, 1128–1136 (1985).
Stylianopoulos, T. et al. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophys. J. 99, 1342–1349 (2010).
Kaminskas, L. M. et al. PEGylation of polylysine dendrimers improves absorption and lymphatic targeting following SC administration in rats. J. Control. Release 140, 108–116 (2009). This article shows that PEGylation of the therapeutic protein interferon-α2 increases lymphatic distribution and ultimately increases therapeutic efficacy against a lymph-resident cancer.
Rao, D. A., Forrest, M. L., Alani, A. W., Kwon, G. S. & Robinson, J. R. Biodegradable PLGA based nanoparticles for sustained regional lymphatic drug delivery. J. Pharm. Sci. 99, 2018–2031 (2010).
Harvey, A. J. et al. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharm. Res. 28, 107–116 (2011).
Lambert, P. H. & Laurent, P. E. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine 26, 3197–3208 (2008).
Nicolas, J.-F. & Guy, B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev. Vaccines 7, 1201–1214 (2008).
Bocci, V., Pessina, G. P., Paulesu, L. & Nicoletti, C. The lymphatic route. VI. Distribution of recombinant interferon-α2 in rabbit and pig plasma and lymph. J. Biolog. Response Mod. 7, 390–400 (1988).
Feng, L. et al. Roles of dextrans on improving lymphatic drainage for liposomal drug delivery system. J. Drug Target. 18, 168–178 (2010).
Pessina, G. P., Bocci, V., Carraro, F., Naldini, A. & Paulesu, L. The lymphatic route. IX. Distribution of recombinant interferon-α 2 administered subcutaneously with oedematogenic drugs. Physiol. Res. 42, 243–250 (1993).
Liu, H. et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507, 519–522 (2014). A pioneering article that uses targeted delivery to the lymphatics to enhance vaccination. This was achieved by the conjugation of peptides to lipids that bind to albumin and 'hitchhike' onto transport pathways from the interstitium into the lymphatics.
Jiang, G. et al. Hyaluronic acid–polyethyleneimine conjugate for target specific intracellular delivery of siRNA. Biopolymers 89, 635–642 (2008).
Fogal, V., Zhang, L., Krajewski, S. & Ruoslahti, E. Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma. Cancer Res. 68, 7210–7218 (2008).
Karmali, P. P. et al. Targeting of albumin-embedded paclitaxel nanoparticles to tumors. Nanomedicine 5, 73–82 (2009).
Luo, G. et al. LyP-1-conjugated nanoparticles for targeting drug delivery to lymphatic metastatic tumors. Int. J. Pharm. 385, 150–156 (2010).
Yan, Z. et al. LyP-1-conjugated PEGylated liposomes: a carrier system for targeted therapy of lymphatic metastatic tumor. J. Control. Release 157, 118–125 (2012).
Desgrosellier, J. S. & Cheresh, D. A. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 10, 9–22 (2010).
Andorko, J., Hess, K. & Jewell, C. Harnessing biomaterials to engineer the lymph node microenvironment for immunity or tolerance. AAPS J. 17, 323–338 (2014). A summary of the mechanisms by which materials can be engineered to promote delivery to cells within the lymphatics to enhance vaccination and tolerance induction.
Zeng, Q. et al. Cationic micelle delivery of Trp2 peptide for efficient lymphatic draining and enhanced cytotoxic T-lymphocyte responses. J. Control. Release 200, 1–12 (2015).
Wang, C. et al. Lymphatic-targeted cationic liposomes: a robust vaccine adjuvant for promoting long-term immunological memory. Vaccine 32, 5475–5483 (2014).
Azad, A. K., Rajaram, M. V. & Schlesinger, L. S. Exploitation of the macrophage mannose receptor (CD206) in infectious disease diagnostics and therapeutics. J. Cytol. Mol. Biol. 1, 1000003 (2014).
Kwon, Y. J., James, E., Shastri, N. & Fréchet, J. M. J. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on pH-responsive microparticles. Proc. Natl Acad. Sci. USA 102, 18264–18268 (2005).
Dahlberg, A. M. et al. The lymphatic system plays a major role in the intravenous and subcutaneous pharmacokinetics of trastuzumab in rats. Mol. Pharm. 11, 496–504 (2014).
Ryan, G. M. et al. PEGylated polylysine dendrimers increase lymphatic exposure to doxorubicin when compared to PEGylated liposomal and solution formulations of doxorubicin. J. Control. Release 172, 128–136 (2013).
Tseng, Y. C., Xu, Z., Guley, K., Yuan, H. & Huang, L. Lipid–calcium phosphate nanoparticles for delivery to the lymphatic system and SPECT/CT imaging of lymph node metastases. Biomaterials 35, 4688–4698 (2014).
Iliff, J. J. et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Invest. 123, 1299–1309 (2013).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111 (2012). This article provides the first description of the glymphatic system — a brain-wide paravascular pathway for CSF and ISF exchange that facilitates the clearance of solutes and waste from the brain.
Iliff, J. J. et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199 (2013).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Yang, L. et al. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J. Transl. Med. 11, 107 (2013).
Aspelund, A., et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Louveau, A., et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Shackleford, D., Porter, C. H. & Charman, W. in Prodrugs Vol. 5 (eds Stella, V. et al.) 653–682 (Springer, 2007).
Lambert, D. M. Rationale and applications of lipids as prodrug carriers. Eur. J. Pharm. Sci. 11 (Suppl. 2), S15–S27 (2000).
Kunisawa, J., Kurashima, Y. & Kiyono, H. Gut-associated lymphoid tissues for the development of oral vaccines. Adv. Drug Deliv. Rev. 64, 523–530 (2012).
Bakhru, S. H., Furtado, S., Morello, A. P. & Mathiowitz, E. Oral delivery of proteins by biodegradable nanoparticles. Adv. Drug Deliv. Rev. 65, 811–821 (2013).
Florence, A. T. Nanoparticle uptake by the oral route: fulfilling its potential? Drug Discov. Today Technol. 2, 75–81 (2005).
Khoo, S. M., Shackleford, D. M., Porter, C. J., Edwards, G. A. & Charman, W. N. Intestinal lymphatic transport of halofantrine occurs after oral administration of a unit-dose lipid-based formulation to fasted dogs. Pharm. Res. 20, 1460–1465 (2003). This article uses a dog model to demonstrate the potential for even a single capsule of lipid to promote significant intestinal lymphatic drug transport.
Caliph, S. M., Charman, W. N. & Porter, C. J. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J. Pharm. Sci. 89, 1073–1084 (2000).
Trevaskis, N. L. et al. A mouse model to evaluate the impact of species, sex, and lipid load on lymphatic drug transport. Pharm. Res. 30, 3254–3270 (2013). An article describing a mesenteric lymph duct cannulated mouse model to evaluate intestinal lymphatic drug transport and provides a cross comparison of preclinical species.
Charman, W. N. & Stella, V. J. Estimating the maximum potential for intestinal lymphatic transport of lipophilic drug molecules. Int. J. Pharm. 34, 175–178 (1986). The first paper to suggest the importance of logP and lipid solubility in indicating the potential for drug absorption via the intestinal lymphatics.
Myers, R. A. & Stella, V. J. Factors affecting the lymphatic transport of penclomedine (NSC-338720), a lipophilic cytotoxic drug — comparison to DDT and hexachlorobenzene. Int. J. Pharm. 80, 51–62 (1992).
Trevaskis, N. L., Shanker, R. M., Charman, W. N. & Porter, C. J. The mechanism of lymphatic access of two cholesteryl ester transfer protein inhibitors (CP524,515 and CP532,623) and evaluation of their impact on lymph lipoprotein profiles. Pharm. Res. 27, 1949–1964 (2010).
Choo, E. F. et al. The role of lymphatic transport on the systemic bioavailability of the Bcl-2 protein family inhibitors navitoclax (ABT-263) and ABT-199. Drug Metab. Dispos. 42, 207–212 (2014). This article demonstrates significant intestinal lymphatic transport of a clinical drug candidate in dogs.
Gershkovich, P. et al. The role of molecular physicochemical properties and apolipoproteins in association of drugs with triglyceride-rich lipoproteins: in-silico prediction of uptake by chylomicrons. J. Pharm. Pharmacol. 61, 31–39 (2009).
Gershkovich, P. & Hoffman, A. Uptake of lipophilic drugs by plasma derived isolated chylomicrons: linear correlation with intestinal lymphatic bioavailability. Eur. J. Pharm. Sci. 26, 394–404 (2005).
Lawless, E., Griffin, B., O'Mahony, A. & O'Driscoll, C. Exploring the impact of drug properties on the extent of intestinal lymphatic transport — in vitro and in vivo studies. Pharm. Res. 32, 1817–1829 (2014).
Lu, Y. et al. Biomimetic reassembled chylomicrons as novel association model for the prediction of lymphatic transportation of highly lipophilic drugs via the oral route. Int. J. Pharm. 483, 69–76 (2015).
Holm, R. & Hoest, J. Successful in silico predicting of intestinal lymphatic transfer. Int. J. Pharm. 272, 189–193 (2004).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 (2001).
Hopkins, A. L., Keseru, G. M., Leeson, P. D., Rees, D. C. & Reynolds, C. H. The role of ligand efficiency metrics in drug discovery. Nat. Rev. Drug Discov. 13, 105–121 (2014).
Han, S. et al. Targeted delivery of a model immunomodulator to the lymphatic system: comparison of alkyl ester versus triglyceride mimetic lipid prodrug strategies. J. Control. Release 177, 1–10 (2014). This article reports glyceride mimetic prodrugs that are more efficiently transported into the intestinal lymph following oral delivery compared with alkyl ester or amide prodrugs, and that they enhance drug delivery to MLNs.
Sugihara, J., Furuuchi, S., Nakano, K. & Harigaya, S. Studies on intestinal lymphatic absorption of drugs. I. Lymphatic absorption of alkyl ester derivatives and alpha-monoglyceride derivatives of drugs. J. Pharmacobiodyn. 11, 369–376 (1988).
Sugihara, J., Furuuchi, S., Ando, H., Takashima, K. & Harigaya, S. Studies on intestinal lymphatic absorption of drugs. II. Glyceride prodrugs for improving lymphatic absorption of naproxen and nicotinic-acid. J. Pharmacobiodyn. 11, 555–562 (1988).
Dahan, A. et al. The oral absorption of phospholipid prodrugs: in vivo and in vitro mechanistic investigation of trafficking of a lecithin–valproic acid conjugate following oral administration. J. Control. Release 126, 1–9 (2008).
Sakai, A., Mori, N., Shuto, S. & Suzuki, T. Deacylation-reacylation cycle: a possible absorption mechanism for the novel lymphotropic antitumor agent dipalmitoylphosphatidylfluorouridine in rats. J. Pharm. Sci. 82, 575–578 (1993).
Hussain, N., Jaitley, V. & Florence, A. T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev. 50, 107–142 (2001).
Yun, Y., Cho, Y. W. & Park, K. Nanoparticles for oral delivery: targeted nanoparticles with peptidic ligands for oral protein delivery. Adv. Drug Deliv. Rev. 65, 822–832 (2013).
Pasetti, M. F., Simon, J. K., Sztein, M. B. & Levine, M. M. Immunology of gut mucosal vaccines. Immunol. Rev. 239, 125–148 (2011).
Attili-Qadri, S. et al. Oral delivery system prolongs blood circulation of docetaxel nanocapsules via lymphatic absorption. Proc. Natl Acad. Sci. USA 110, 17498–17503 (2013).
Pridgen, E. M. et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal Fc receptor for oral delivery. Sci. Transl. Med. 5, 213ra167 (2013).
Neutra, M. R. & Kozlowski, P. A. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 6, 148–158 (2006).
Schulz, O. et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J. Exp. Med. 206, 3101–3114 (2009).
Rescigno, M. Intestinal dendritic cells. Adv. Immunol. 107, 109–138 (2010).
Niess, J. H. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 (2005).
Clark, M. A., Hirst, B. H. & Jepson, M. A. Lectin-mediated mucosal delivery of drugs and microparticles. Adv. Drug Deliv. Rev. 43, 207–223 (2000).
Hussain, N. & Florence, A. Utilizing bacterial mechanisms of epithelial cell entry: invasin-induced oral uptake of latex nanoparticles. Pharm. Res. 15, 153–156 (1998).
Fievez, V. et al. Targeting nanoparticles to M cells with non-peptidic ligands for oral vaccination. Eur. J. Pharm. Biopharm. 73, 16–24 (2009).
Jin, Y. et al. Goblet cell-targeting nanoparticles for oral insulin delivery and the influence of mucus on insulin transport. Biomaterials 33, 1573–1582 (2012).
Reineke, J. J. et al. Unique insights into the intestinal absorption, transit, and subsequent biodistribution of polymer-derived microspheres. Proc. Natl Acad. Sci. USA 110, 13803–13808 (2013).
Desai, M. P., Labhasetwar, V., Amidon, G. L. & Levy, R. J. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm. Res. 13, 1838–1845 (1996).
Jani, P., Halbert, G. W., Langridge, J. & Florence, A. T. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J. Pharm. Pharmacol. 42, 821–826 (1990).
Ebel, J. A. Method for quantifying particle absorption from the small intestine of the mouse. Pharm. Res. 7, 848–851 (1990).
Jenkins, P. G. et al. The quantitation of the absorption of microparticles into the intestinal lymph of Wistar rats. Int. J. Pharm. 102, 261–266 (1994).
Lefevre, M. E., Joel, D. D. & Schidlovsky, G. Retention of ingested latex particles in Peyer's patches of germfree and conventional mice. Proc. Soc. Exp. Biol. Med. 179, 522–528 (1985).
Hussain, N., Jani, P. U. & Florence, A. T. Enhanced oral uptake of tomato lectin-conjugated nanoparticles in the rat. Pharm. Res. 14, 613–618 (1997).
Ralay-Ranaivo, B. et al. Novel self assembling nanoparticles for the oral administration of fondaparinux: synthesis, characterization and in vivo evaluation. J. Control. Release 194, 323–331 (2014).
Zhang, N. et al. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int. J. Pharm. 327, 153–159 (2006).
Florence, A. T., Sakthivel, T. & Toth, I. Oral uptake and translocation of a polylysine dendrimer with a lipid surface. J. Control. Release 65, 253–259 (2000).
Ryan, G. M. et al. Pulmonary administration of PEGylated polylysine dendrimers: absorption from the lung versus retention within the lung is highly size-dependent. Mol. Pharm. 10, 2986–2995 (2013).
Lycke, N. Recent progress in mucosal vaccine development: potential and limitations. Nat. Rev. Immunol. 12, 592–605 (2012).
Meeusen, E. N. Exploiting mucosal surfaces for the development of mucosal vaccines. Vaccine 29, 8506–8511 (2011).
Stano, A. et al. PPS nanoparticles as versatile delivery system to induce systemic and broad mucosal immunity after intranasal administration. Vaccine 29, 804–812 (2011).
Stano, A., Nembrini, C., Swartz, M. A., Hubbell, J. A. & Simeoni, E. Nanoparticle size influences the magnitude and quality of mucosal immune responses after intranasal immunization. Vaccine 30, 7541–7546 (2012).
Rytting, E., Nguyen, J., Wang, X. & Kissel, T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin. Drug Deliv. 5, 629–639 (2008).
Patton, J. S., Fishburn, C. S. & Weers, J. G. The lungs as a portal of entry for systemic drug delivery. Proc. Am. Thorac. Soc. 1, 338–344 (2004).
Schraufnagel, D. E. Lung lymphatic anatomy and correlates. Pathophysiology 17, 337–343 (2010).
Pabst, R. & Tschernig, T. Bronchus-associated lymphoid tissue. Am. J. Respir. Cell. Mol. Biol. 43, 137–141 (2010).
Geiser, M. Update on macrophage clearance of inhaled micro- and nanoparticles. J. Aerosol Med. Pulm. Drug Deliv. 23, 207–217 (2010).
Wanner, A., Salathe, M. & O'Riordan, T. G. Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med. 154, 1868–1902 (1996).
Kambouchner, M. & Bernaudin, J. F. Intralobular pulmonary lymphatic distribution in normal human lung using D2-40 antipodoplanin immunostaining. J. Histochem. Cytochem. 57, 643–648 (2009).
Botelho, M. F. et al. Visualization of deep lung lymphatic network using radioliposomes. Rev. Port. Pneumol. 17, 124–130 (in Portuguese) (2011).
Hanatani, K. et al. Molecular weight-dependent lymphatic transfer of fluorescein isothiocyanate-labeled dextrans after intrapulmonary administration and effects of various absorption enhancers on the lymphatic transfer of drugs in rats. J. Drug Target 3, 263–271 (1995).
Choi, H. S. et al. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat. Biotech. 28, 1300–1303 (2010).
Li, A. V. et al. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci. Transl. Med. 5, 204ra130 (2013).
Videira, M. A. et al. Lymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticles. J. Drug Target 10, 607–613 (2002).
Latimer, P. et al. Aerosol delivery of liposomal formulated paclitaxel and vitamin E analog reduces murine mammary tumor burden and metastases. Exp. Biol. Med. (Maywood) 234, 1244–1252 (2009).
Mohammad, A. K., Amayreh, L. K., Mazzara, J. M. & Reineke, J. J. Rapid lymph accumulation of polystyrene nanoparticles following pulmonary administration. Pharm. Res. 30, 424–434 (2013).
Mackay, C. R., Marston, W. L. & Dudler, L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 171, 801–817 (1990).
Braun, A. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat. Immunol. 12, 879–887 (2011).
Moghimi, S. M. & Bonnemain, B. Subcutaneous and intravenous delivery of diagnostic agents to the lymphatic system: applications in lymphoscintigraphy and indirect lymphography. Adv. Drug Deliv. Rev. 37, 295–312 (1999).
Sensken, S.-C., Bode, C. & Gräler, M. H. Accumulation of fingolimod (FTY720) in lymphoid tissues contributes to prolonged efficacy. J. Pharmacol. Exp. Ther. 328, 963–969 (2009).
Manolova, V. et al. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 38, 1404–1413 (2008). The first demonstration of the relative importance of direct drainage versus transport after cellular uptake compared to lymph node uptake of particles after interstitial injection.
Moghimi, S. M. et al. Surface engineered nanospheres with enhanced drainage into lymphatics and uptake by macrophages of the regional lymph nodes. FEBS Lett. 344, 25–30 (1994).
Oussoren, C. et al. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection: IV. Fate of liposomes in regional lymph nodes. Biochim. Biophys. Acta 1370, 259–272 (1998).
Reddy, S. T., Rehor, A., Schmoekel, H. G., Hubbell, J. A. & Swartz, M. A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release 112, 26–34 (2006). An article demonstrating the size dependency of lymphatic uptake, lymph node retention and lymph node dendritic cell uptake of nanoparticles.
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Caserta, S., Alessi, P., Guarnerio, J., Basso, V. & Mondino, A. Synthetic CD4+ T cell-targeted antigen-presenting cells elicit protective antitumor responses. Cancer Res. 68, 3010–3018 (2008).
Moon, J. J. et al. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl Acad. Sci. USA 109, 1080–1085 (2012). A key paper demonstrating that targeting delivery to lymph nodes enhances vaccination. See also references 207 and 209.
Oussoren, C. & Storm, G. Liposomes to target the lymphatics by subcutaneous administration. Adv. Drug Deliv. Rev. 50, 143–156 (2001).
Takakura, Y., Matsumoto, S., Hashida, M. & Sezaki, H. Enhanced lymphatic delivery of mitomycin C conjugated with dextran. Cancer Res. 44, 2505–2510 (1984).
Kim, C. K. & Han, J. H. Lymphatic delivery and pharmacokinetics of methotrexate after intramuscular injection of differently charged liposome-entrapped methotrexate to rats. J. Microencapsul. 12, 437–446 (1995).
Kaminskas, L. M. et al. Methotrexate-conjugated PEGylated dendrimers show differential patterns of deposition and activity in tumor-burdened lymph nodes after intravenous and subcutaneous administration in rats. Mol. Pharm. 12, 432–443 (2015).
Nune, S. K., Gunda, P., Majeti, B. K., Thallapally, P. K. & Forrest, M. L. Advances in lymphatic imaging and drug delivery. Adv. Drug Deliv. Rev. 63, 876–885 (2011).
Shackleford, D. M. et al. Contribution of lymphatically transported testosterone undecanoate to the systemic exposure of testosterone after oral administration of two andriol formulations in conscious lymph duct-cannulated dogs. J. Pharmacol. Exp. Ther. 306, 925–933 (2003).
White, K. L. et al. Lymphatic transport of methylnortestosterone undecanoate (MU) and the bioavailability of methylnortestosterone are highly sensitive to the mass of coadministered lipid after oral administration of MU. J. Pharmacol. Exp. Ther. 331, 700–709 (2009).
Surampudi, P. et al. Single, escalating dose pharmacokinetics, safety and food effects of a new oral androgen dimethandrolone undecanoate in man: a prototype oral male hormonal contraceptive. Andrology 2, 579–587 (2014).
Khoo, S. M., Edwards, G. A., Porter, C. J. H. & Charman, W. N. A conscious dog model for assessing the absorption, enterocyte-based metabolism, and intestinal lymphatic transport of halofantrine. J. Pharm. Sci. 90, 1599–1607 (2001).
Trevaskis, N. L. et al. Intestinal lymphatic transport enhances the post-prandial oral bioavailability of a novel cannabinoid receptor agonist via avoidance of first-pass metabolism. Pharm. Res. 26, 1486–1495 (2009).
Trevaskis, N. L., Porter, C. J. & Charman, W. N. An examination of the interplay between enterocyte-based metabolism and lymphatic drug transport in the rat. Drug Metab. Dispos. 34, 729–733 (2006).
Zhang, Z. et al. A self-assembled nanocarrier loading teniposide improves the oral delivery and drug concentration in tumor. J. Control. Release 166, 30–37 (2013).
Garzonaburbeh, A., Poupaert, J. H., Claesen, M., Dumont, P. & Atassi, G. 1,3-dipalmitoylglycerol ester of chlorambucil as a lymphotropic, orally administrable anti-neoplastic agent. J. Med. Chem. 26, 1200–1203 (1983).
Kaminskas, L. M. et al. PEGylation of interferon α2 improves lymphatic exposure after subcutaneous and intravenous administration and improves antitumour efficacy against lymphatic breast cancer metastases. J. Control. Release 168, 200–208 (2013).
Li, S., Goins, B., Hrycushko, B. A., Phillips, W. T. & Bao, A. Feasibility of eradication of breast cancer cells remaining in postlumpectomy cavity and draining lymph nodes following intracavitary injection of radioactive immunoliposomes. Mol. Pharm. 9, 2513–2522 (2012).
Cai, S., Xie, Y., Davies, N. M., Cohen, M. S. & Forrest, M. L. Carrier-based intralymphatic cisplatin chemotherapy for the treatment of metastatic squamous cell carcinoma of the head and neck. Ther. Delivery 1, 237–245 (2010).
Qin, L. et al. Polymeric micelles for enhanced lymphatic drug delivery to treat metastatic tumors. J. Control. Release 171, 133–142 (2013).
Rafi, M. et al. Polymeric micelles incorporating (1,2-diaminocyclohexane)platinum (II) suppress the growth of orthotopic scirrhous gastric tumors and their lymph node metastasis. J. Control. Release 159, 189–196 (2012).
Kourtis, I. C. et al. Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PLoS ONE 8, e61646 (2013).
Liu, R. et al. Prevention of nodal metastases in breast cancer following the lymphatic migration of paclitaxel-loaded expansile nanoparticles. Biomaterials 34, 1810–1819 (2013).
Cai, S., Xie, Y., Bagby, T. R., Cohen, M. S. & Forrest, M. L. Intralymphatic chemotherapy using a hyaluronan–cisplatin conjugate. J. Surg. Res. 147, 247–252 (2008).
Akamo, Y. et al. Chemotherapy targeting regional lymph nodes by gastric submucosal injection of liposomal adriamycin in patients with gastric carcinoma. Jpn J. Cancer Res. 85, 652–658 (1994).
Khullar, O. V. et al. Nanoparticle migration and delivery of paclitaxel to regional lymph nodes in a large animal model. J. Am. Coll. Surg. 214, 328–337 (2012).
Ling, R. et al. Lymphatic chemotherapy induces apoptosis in lymph node metastases in a rabbit breast carcinoma model. J. Drug Target. 13, 137–142 (2005).
Yang, F. et al. Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur. J. Cancer 47, 1873–1882 (2011).
Zhao, C. et al. Local targeted therapy of liver metastasis from colon cancer by galactosylated liposome encapsulated with doxorubicin. PLoS ONE 8, e73860 (2013).
Dane, K. Y. et al. Nano-sized drug-loaded micelles deliver payload to lymph node immune cells and prolong allograft survival. J. Control. Release 156, 154–160 (2011).
Trevaskis, N. L., Charman, W. N. & Porter, C. J. Targeted drug delivery to lymphocytes: a route to site-specific immunomodulation? Mol. Pharm. 7, 2297–2309 (2010).
Okanobo, A., Chauhan, S. K., Dastjerdi, M. H., Kodati, S. & Dana, R. Efficacy of topical blockade of interleukin-1 in experimental dry eye disease. Am. J. Ophthalmol. 154, 63–71 (2012).
Shinriki, S. et al. Interleukin-6 signalling regulates vascular endothelial growth factor-C synthesis and lymphangiogenesis in human oral squamous cell carcinoma. J. Pathol. 225, 142–150 (2011).
Polzer, K. et al. Tumour necrosis factor blockade increases lymphangiogenesis in murine and human arthritic joints. Ann. Rheum. Dis. 67, 1610–1616 (2008).
Pal, I. & Ramsey, J. D. The role of the lymphatic system in vaccine trafficking and immune response. Adv. Drug Deliv. Rev. 63, 909–922 (2011).
Woodruff, M. C. et al. Trans-nodal migration of resident dendritic cells into medullary interfollicular regions initiates immunity to influenza vaccine. J. Exp. Med. 211, 1611–1621 (2014).
Senti, G., Johansen, P. & Kundig, T. M. Intralymphatic immunotherapy. Curr. Opin. Allergy Clin. Immunol. 9, 537–543 (2009).
Senti, G. et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc. Natl Acad. Sci. USA 105, 17908–17912 (2008). A clinical trial demonstrating the benefit of intralymphatic administration to induce allergen tolerance and reduce allergen-induced rhinoconjunctivitis.
Jewell, C. M., Bustamante López, S. C. & Irvine, D. J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl Acad. Sci. USA 108, 15745–15750 (2011).
Maloy, K. J. et al. Intralymphatic immunization enhances DNA vaccination. Proc. Natl Acad. Sci. USA 98, 3299–3303 (2001).
De Titta, A. et al. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl Acad. Sci. USA 110, 19902–19907 (2013).
Xu, Z. et al. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. J. Control. Release 172, 259–265 (2013).
Moon, J. J. et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 10, 243–251 (2011).
St John, A. L., Chan, C. Y., Staats, H. F., Leong, K. W. & Abraham, S. N. Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat. Mater. 11, 250–257 (2012).
Jeanbart, L. et al. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol. Res. 2, 436–447 (2014).
Thomas, S. N., Vokali, E., Lund, A. W., Hubbell, J. A. & Swartz, M. A. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 35, 814–824 (2014).
Kim, S. H., Lee, K. Y. & Jang, Y. S. Mucosal immune system and M cell-targeting strategies for oral mucosal vaccination. Immune Netw. 12, 165–175 (2012).
Zhu, Q. et al. Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat. Med. 18, 1291–1296 (2012).
Ballester, M. et al. Nanoparticle conjugation and pulmonary delivery enhance the protective efficacy of Ag85B and CpG against tuberculosis. Vaccine 29, 6959–6966 (2011).
Nembrini, C. et al. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proc. Natl Acad. Sci. USA 108, E989–E997 (2011). This article demonstrates enhanced immunization and protection against influenza-ova infection via pulmonary administration of lymph node-targeted antigens in nanoparticles with CpG relative to administration of soluble antigens with CpG.
Faria, A. M. C. & Weiner, H. L. Oral tolerance: therapeutic implications for autoimmune diseases. Clin. Dev. Immunol. 13, 143–157 (2006).
Miller, S. D., Turley, D. M. & Podojil, J. R. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat. Rev. Immunol. 7, 665–677 (2007).
Weiner, H. L., da Cunha, A. P., Quintana, F. & Wu, H. Oral tolerance. Immunol. Rev. 241, 241–259 (2011).
Scandling, J. D., Busque, S., Shizuru, J. A., Engleman, E. G. & Strober, S. Induced immune tolerance for kidney transplantation. N. Engl. J. Med. 365, 1359–1360 (2011).
Faria, A. M. C. & Weiner, H. L. Oral tolerance. Immunol. Rev. 206, 232–259 (2005).
Burks, A. W., Laubach, S. & Jones, S. M. Oral tolerance, food allergy, and immunotherapy: implications for future treatment. J. Allergy Clin. Immunol. 121, 1344–1350 (2008).
Worbs, T. et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 203, 519–527 (2006).
Spahn, T. W. et al. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches. Eur. J. Immunol. 32, 1109–1113 (2002).
Spahn, T. W. et al. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur. J. Immunol. 31, 1278–1287 (2001).
Kraus, T. A. et al. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J. Clin. Invest. 115, 2234–2243 (2005).
Fujihashi, K. et al. Peyer's patches are required for oral tolerance to proteins. Proc. Natl Acad. Sci. USA 98, 3310–3315 (2001).
Suzuki, H. et al. Ovalbumin-protein sigma 1 M-cell targeting facilitates oral tolerance with reduction of antigen-specific CD4+ T cells. Gastroenterology 135, 917–925 (2008).
Masuda, K., Horie, K., Suzuki, R., Yoshikawa, T. & Hirano, K. Oral delivery of antigens in liposomes with some lipid compositions modulates oral tolerance to the antigens. Microbiol. Immunol. 46, 55–58 (2002).
Kim, W. et al. Suppression of collagen-induced arthritis by single feeding of poilylactic-poilyglycolic acid entrapping immunodominant peptide of type II collagen: involvement of CD4+ IL-10+ T cells in Peyer's pathces. Ann. Rheum. Dis. 62, 168–168 (2003).
Goldmann, K., Hoffmann, J., Eckl, S., Spriewald, B. M. & Ensminger, S. M. Attenuation of transplant arteriosclerosis by oral feeding of major histocompatibility complex encoding chitosan-DNA nanoparticles. Transplant Immunol. 28, 9–13 (2013).
Pecquet, S. et al. Oral tolerance elicited in mice by β-lactoglobulin entrapped in biodegradable microspheres. Vaccine 18, 1196–1202 (2000).
Shirali, A. C. et al. Nanoparticle delivery of mycophenolic acid upregulates PD-L1 on dendritic cells to prolong murine allograft survival. Am. J. Transplant. 11, 2582–2592 (2011).
Maldonado, R. A. et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl Acad. Sci. USA 112, E156–E165 (2015). Provides an innovative approach to enhance and prolong tolerance induction via the administration of 'tolerogenic' nanoparticles loaded with antigens and the tolerogenic immunomodulator rapamycin that are efficiently transport to lymphoid organs and captured by resident APCs.
Capini, C. et al. Antigen-specific suppression of inflammatory arthritis using liposomes. J. Immunol. 182, 3556–3565 (2009).
Getts, D. R. et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotech. 30, 1217–1224 (2012).
Kinman, L. et al. Lipid-drug association enhanced HIV-1 protease inhibitor indinavir localization in lymphoid tissues and viral load reduction: a proof of concept study in HIV-2287-infected macaques. J. Acquir. Immune Defic. Syndr. 34, 387–397 (2003).
Freeling, J. P., Koehn, J., Shu, C., Sun, J. & Ho, R. J. Y. Long-acting three-drug combination anti-HIV nanoparticles enhance drug exposure in primate plasma and cells within lymph nodes and blood. AIDS 28, 2625–2627 (2014).
Freeling, J. P. & Ho, R. J. Y. Anti-HIV drug particles may overcome lymphatic drug insufficiency and associated HIV persistence. Proc. Natl Acad. Sci. USA 111, E2512–E2513 (2014).
das Neves, J., Amiji, M. M., Bahia, M. F. & Sarmento, B. Nanotechnology-based systems for the treatment and prevention of HIV/AIDS. Adv. Drug Deliv. Rev. 62, 458–477 (2010).
Edagwa, B. J., Zhou, T., McMillan, J. M., Liu, X. M. & Gendelman, H. E. Development of HIV reservoir targeted long acting nanoformulated antiretroviral therapies. Curr. Med. Chem. 21, 4186–4198 (2014).
Sosnik, A., Chiappetta, D. A. & Carcaboso, Á. M. Drug delivery systems in HIV pharmacotherapy: what has been done and the challenges standing ahead. J. Control. Release 138, 2–15 (2009).
Lalanne, M. et al. Synthesis and biological evaluation of two glycerolipidic prodrugs of didanosine for direct lymphatic delivery against HIV. Bioorg. Med. Chem. Lett. 17, 2237–2240 (2007).
Skanji, R. et al. A new nanomedicine based on didanosine glycerolipidic prodrug enhances the long term accumulation of drug in a HIV sanctuary. Int. J. Pharm. 414, 285–297 (2011).
Puligujja, P. et al. Pharmacodynamics of long-acting folic acid-receptor targeted ritonavir-boosted atazanavir nanoformulations. Biomaterials 41, 141–150 (2015).
Horst, H. J. et al. Lymphatic absorption and metabolism of orally administered testosterone undecanoate in man. Klin. Wochenschr. 54, 875–879 (1976). One of the very few studies to have quantified drug uptake into the lymph in humans.
Edwards, G. A., Porter, C. J., Caliph, S. M., Khoo, S. M. & Charman, W. N. Animal models for the study of intestinal lymphatic drug transport. Adv. Drug Deliv. Rev. 50, 45–60 (2001).
Seeger, M. & Bewig, B. Ultrasound imaging of the thoracic duct. N. Engl. J. Med. 359, e28 (2008).
Nadolski, G. & Itkin, M. Thoracic duct embolization for the management of chylothoraces. Curr. Opin. Pulm. Med. 19, 380–386 (2013).
Thomas, S. N. & Schudel, A. Overcoming transport barriers for interstitial-, lymphatic-, and lymph node-targeted drug delivery. Curr. Opin. Chem. Engineer. 7, 65–74 (2015).
Miteva, D. O. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ. Res. 106, 920–931 (2010).
Banerji, S. et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 144, 789–801 (1999).
Dixon, J. B., Raghunathan, S. & Swartz, M. A. A tissue-engineered model of the intestinal lacteal for evaluating lipid transport by lymphatics. Biotechnol. Bioengineer. 103, 1224–1235 (2009).
John, T. A., Vogel, S. M., Tiruppathi, C., Malik, A. B. & Minshall, R. D. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L187–L196 (2003).
Schubert, W. et al. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J. Biol. Chem. 276, 48619–48622 (2001).
Mendelsohn, A. R. & Larrick, J. W. Sleep facilitates clearance of metabolites from the brain: glymphatic function in aging and neurodegenerative diseases. Rejuven. Res. 16, 518–523 (2013).
Thrane, V. R. et al. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci. Rep. 3, 2582 (2013).
Florence, A. T. & Hussain, N. Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv. Drug Deliv. Rev. 50 (Suppl. 1), 69–89 (2001).
des Rieux, A., Fievez, V., Garinot, M., Schneider, Y. J. & Preat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 116, 1–27 (2006).
Hunter, A. C., Elsom, J., Wibroe, P. P. & Moghimi, S. M. Polymeric particulate technologies for oral drug delivery and targeting: a pathophysiological perspective. Nanomedicine 8, S5–S20 (2012).
Ravi, P. R., Aditya, N., Kathuria, H., Malekar, S. & Vats, R. Lipid nanoparticles for oral delivery of raloxifene: optimization, stability, in vivo evaluation and uptake mechanism. Eur. J. Pharm. Biopharm. 87, 114–124 (2014).
Sun, M. et al. Intestinal absorption and intestinal lymphatic transport of sirolimus from self-microemulsifying drug delivery systems assessed using the single-pass intestinal perfusion (SPIP) technique and a chylomicron flow blocking approach: Linear correlation with oral bioavailabilities in rats. Eur. J. Pharm. Sci. 43, 132–140 (2011).
Zhang, Z. et al. Bile salts enhance the intestinal absorption of lipophilic drug loaded lipid nanocarriers: mechanism and effect in rats. Int. J. Pharm. 452, 374–381 (2013).
Fu, C. et al. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 34, 2565–2575 (2013).
Dahan, A. & Hoffman, A. Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur. J. Pharm. Sci. 24, 381–388 (2005).
Bernard, A., Carlier, H. & Caselli, C. Biochemical and ultrastructural study of actidione-cycloheximide effect on fat intestinal absorption in the rat (author's transl). J. Physiol. (Paris). 76, 147–157 (1980) (in French).
Alitalo, A. & Detmar, M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene 31, 4499–4508 (2012).
Hwee, Y. L. et al. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am. J. Pathol. 175, 1328–1337 (2009).
Liao, S. et al. Impaired lymphatic contraction associated with immunosuppression. Proc. Natl Acad. Sci. USA 108, 18784–18789 (2011).
Bagby, T. R. et al. Lymphatic trafficking kinetics and near-infrared imaging using star polymer architectures with controlled anionic character. Eur. J. Pharm. Sci. 47, 287–294 (2012).
Karlsson, M. et al. “Tolerosomes” are produced by intestinal epithelial cells. Eur. J. Immunol. 31, 2892–2900 (2001).
Menard, S., Cerf-Bensussan, N. & Heyman, M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 3, 247–259 (2010).
Wang, Y. H. et al. Chylomicrons promote intestinal absorption and systemic dissemination of dietary antigen (ovalbumin) in mice. PLoS ONE 4, e8442 (2009).
Jang, M. H. et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl Acad. Sci. USA 101, 6110–6115 (2004).
Neutra, M. R. & Kraehenbuhl, J. P. in Mucosal Immunology 3rd edn (eds Mestecky, J. et al.) 111–130 (Elsevier, 2005).
Caliph, S. M. et al. The impact of lymphatic transport on the systemic disposition of lipophilic drugs. J. Pharm. Sci. 102, 2395–2408 (2013).
Carrasco, Y. R. & Batista, F. D. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 27, 160–171 (2007).
Junt, T. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007).
Acknowledgements
The title of this article was inspired by, and modified from, an excellent review of lymphatic lipid transport by J. B. Dixon (cited as reference 12 in this article). The authors gratefully acknowledge the reviewers of this article and the insightful comments provided by M. Swartz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C.J.H.P. and N.L.T. are named inventors on a patent application in the area of lymph-targeted prodrugs.
Glossary
- Lipoproteins
-
Biochemical complexes of lipids and soluble apolipoproteins that transport lipids in lymph fluid and blood to tissues throughout the body. The largest and least dense lipoproteins, chylomicrons, are assembled in the small intestine. Very low-density lipoproteins (VLDLs) and the smallest and most dense lipoproteins, high-density lipoproteins (HDLs), are assembled in both the liver and the intestine. Low-density lipoproteins (LDLs) are formed following removal of lipids from VLDL by tissues.
- Initial lymphatic capillaries
-
Small blind-ended lymphatic vessels in the tissue periphery that have a discontinuous basement membrane, lack smooth muscle and are characterized by button-like interendothelial junctions and short anchoring filaments that are tethered to elastin fibres in the surrounding tissue. Initial lymphatics are adapted for the uptake of fluid and cells.
- Collecting lymphatic vessels
-
These lymphatic vessels are characterized by a continuous smooth muscle cell layer and the presence of semilunar valves that facilitate the unidirectional transport of lymph and associated components. Afferent collecting lymphatics carry lymph into lymph nodes whereas efferent collecting lymphatics carry lymph from lymph nodes.
- Thoracic lymph duct
-
The largest lymphatic vessel, sometimes called the left thoracic lymph duct, that collects most of the lymph in the body apart from the lymph draining the right thorax, arm, head and neck. The latter drain instead into the right lymphatic duct. Lymph empties from the thoracic lymph duct into the systemic circulation at the junction of the left subclavian and left internal jugular veins.
- Antigen presenting cells
-
(APCs). A heterogeneous group of immune cells that initiate the cellular immune response by processing and presenting antigens for recognition by lymphocytes such as T cells. Classical APCs include dendritic cells, macrophages, Langerhans cells and B cells.
- Tolerance
-
A state of immune unresponsiveness to an antigen that results from the suppression of immune responses to antigens that have been administered or encountered previously.
- Lymphangiogenesis
-
The formation of new lymphatic vessels from pre-existing lymphatic vessels.
- Glymphatic system
-
A recently identified paravascular pathway that enables the exchange of cerebrospinal fluid with interstitial fluid in the brain and provides a function similar to the lymphatic system elsewhere in the body. In this way, the glymphatics facilitate the clearance of solutes and waste products from the brain.
- LogP
-
The logarithm of the ratio of the concentrations of un-ionized solute (drug) in two immiscible liquid phases (usually octanol and water) at equilibrium. LogP provides one measure of drug lipophilicity, with high logP values indicating higher lipophilicity.
- Lipophilicity
-
The affinity of a molecule for a lipophilic environment (lipid or lipid-like). Lipophilic literally means 'fat loving'.
- Rule of 5
-
Limits to a series of molecular properties of drugs (logP, molecular weight, hydrogen bond donors and acceptors), suggested by Lipinski to increase the likelihood of good oral absorption.
- Mesenteric lymphatic vessels
-
The lymphatic vessels that collect lymph from the intestine. This includes the initial lymphatic capillaries ('lacteals'), pre-nodal (afferent) collecting lymphatic vessels and the post-nodal (efferent) mesenteric lymph duct. The mesenteric lymph duct collects almost all lymph from the small intestine.
- High endothelial venules
-
(HEVs). Specialized post-capillary venules that are characterized by plump, as opposed to thin, endothelial cells. HEVs are found in lymph nodes and other lymphoid tissues and support high levels of lymphocyte extravasation from the blood into these tissues.
Rights and permissions
About this article
Cite this article
Trevaskis, N., Kaminskas, L. & Porter, C. From sewer to saviour — targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov 14, 781–803 (2015). https://doi.org/10.1038/nrd4608
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd4608
This article is cited by
-
Evidence for an accumulation of mineral oil saturated hydrocarbons (MOSH) in human tissues: a re-evaluation of biopsy and autopsy data
Journal of Consumer Protection and Food Safety (2024)
-
Effective treatment of metastatic sentinel lymph nodes by dual-targeting melittin nanoparticles
Journal of Nanobiotechnology (2023)
-
Cracking the intestinal lymphatic system window utilizing oral delivery vehicles for precise therapy
Journal of Nanobiotechnology (2023)
-
Lymphatics drain nanoparticles from tumours
Nature Materials (2023)
-
Lymph node targeted multi-epitope subunit vaccine promotes effective immunity to EBV in HLA-expressing mice
Nature Communications (2023)