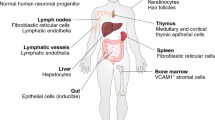
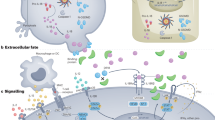
Key Points
-
Interleukin-21 (IL-21) is a pleiotropic cytokine with actions on a broad range of lymphoid, myeloid and epithelial cells. These actions include effects on proliferation, survival, differentiation and function.
-
IL-21 has a key role in B cell differentiation to plasma cells and in the development of T follicular helper cells, promoting functional germinal centres and immunoglobulin production.
-
IL-21 induces a functional programme in CD8+ T cells that leads to enhanced survival, antiviral activity and antitumour activity.
-
IL-21 has a key role in the development of T helper 17 (TH17) cells, which contribute to pathogenesis in a range of inflammatory diseases.
-
Clinical trials with IL-21 alone or in combination with other agents have yielded favourable results in the treatment of solid tumours.
-
IL-21 promotes a range of autoimmune diseases, including systemic lupus erythematosus, type 1 diabetes, multiple sclerosis, inflammatory bowel disease and psoriasis. Clinical trials using IL-21 inhibitors are in progress.
Abstract
Interleukin-21 is a cytokine with broad pleiotropic actions that affect the differentiation and function of lymphoid and myeloid cells. Since its discovery in 2000, a tremendous amount has been learned about its biological actions and the molecular mechanisms controlling IL-21-mediated cellular responses. IL-21 regulates both innate and adaptive immune responses, and it not only has key roles in antitumour and antiviral responses but also exerts major effects on inflammatory responses that promote the development of autoimmune diseases and inflammatory disorders. Numerous studies have shown that enhancing or inhibiting the action of IL-21 has therapeutic effects in animal models of a wide range of diseases, and various clinical trials are underway. The current challenge is to understand how to specifically modulate the actions of IL-21 in the context of each specific immune response or pathological situation. In this Review, we provide an overview of the basic biology of IL-21 and discuss how this information has been — and can be — exploited therapeutically.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Spolski, R. & Leonard, W. J. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 26, 57–79 (2008).
Parrish-Novak, J. et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408, 57–63 (2000).
Ozaki, K., Kikly, K., Michalovich, D., Young, P. R. & Leonard, W. J. Cloning of a type I cytokine receptor most related to the IL-2 receptor β chain. Proc. Natl Acad. Sci. USA 97, 11439–11444 (2000). References 2 and 3 are the first papers to describe IL-21 and its receptor.
Asao, H. et al. Cutting edge: the common γ-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 167, 1–5 (2001).
Noguchi, M. et al. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 73, 147–157 (1993). This is the first paper to describe the defect in patients with X-SCID.
Leonard, W. J. Cytokines and immunodeficiency diseases. Nature Rev. Immunol. 1, 200–208 (2001).
Kang, L., Bondensgaard, K., Li, T., Hartmann, R. & Hjorth, S. A. Rational design of interleukin-21 antagonist through selective elimination of the γC binding epitope. J. Biol. Chem. 285, 12223–12231 (2010).
Zeng, R. et al. The molecular basis of IL-21-mediated proliferation. Blood 109, 4135–4142 (2007).
Kwon, H. et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity 31, 941–952 (2009).
Li, P. et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature 490, 543–546 (2012).
Ciofani, M. et al. A validated regulatory network for Th17 cell specification. Cell 151, 289–303 (2012).
Glasmacher, E. et al. A genomic regulatory element that directs assembly and function of immune-specific AP-1-IRF complexes. Science 338, 975–980 (2012).
Yamaoka, K. & Tanaka, Y. Targeting the Janus kinases in rheumatoid arthritis: focus on tofacitinib. Expert Opin. Pharmacother. 15, 103–113 (2014).
Sandborn, W. J. et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N. Engl. J. Med. 367, 616–624 (2012).
Ozaki, K. et al. A critical role for IL-21 in regulating immunoglobulin production. Science 298, 1630–1634 (2002). This is the first paper to describe a role for IL-21 in immunoglobulin production.
Recher, M. et al. IL-21 is the primary common γ chain-binding cytokine required for human B-cell differentiation in vivo. Blood 118, 6824–6835 (2011).
Pene, J. et al. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J. Immunol. 172, 5154–5157 (2004).
Ettinger, R. et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 175, 7867–7879 (2005).
Avery, D. T., Bryant, V. L., Ma, C. S., de Waal Malefyt, R. & Tangye, S. G. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J. Immunol. 181, 1767–1779 (2008).
Bryant, V. L. et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J. Immunol. 179, 8180–8190 (2007).
Dullaers, M. et al. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity 30, 120–129 (2009).
Jin, H., Carrio, R., Yu, A. & Malek, T. R. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J. Immunol. 173, 657–665 (2004).
Mehta, D. S. et al. IL-21 induces the apoptosis of resting and activated primary B cells. J. Immunol. 170, 4111–4118 (2003).
Ozaki, K. et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J. Immunol. 173, 5361–5371 (2004).
Calame, K. L., Lin, K. I. & Tunyaplin, C. Regulatory mechanisms that determine the development and function of plasma cells. Annu. Rev. Immunol. 21, 205–230 (2003).
Diehl, S. A. et al. STAT3-mediated up-regulation of BLIMP1 is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J. Immunol. 180, 4805–4815 (2008).
Avery, D. T. et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 207, 155–171 (2010).
Rodriguez-Bayona, B., Ramos-Amaya, A., Bernal, J., Campos-Caro, A. & Brieva, J. A. Cutting edge: IL-21 derived from human follicular helper T cells acts as a survival factor for secondary lymphoid organ, but not for bone marrow, plasma cells. J. Immunol. 188, 1578–1581 (2012).
Hagn, M. et al. Activated mouse B cells lack expression of granzyme B. J. Immunol. 188, 3886–3892 (2012).
Hagn, M. et al. Human B cells differentiate into granzyme B-secreting cytotoxic B lymphocytes upon incomplete T-cell help. Immunol. Cell Biol. 90, 457–467 (2012).
Xu, W. et al. Human plasma cells express granzyme B. Eur. J. Immunol. 44, 275–284 (2014).
Brandt, K., Bulfone-Paus, S., Foster, D. C. & Ruckert, R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood 102, 4090–4098 (2003).
Wan, C. K. et al. The cytokines IL-21 and GM-CSF have opposing regulatory roles in the apoptosis of conventional dendritic cells. Immunity 38, 514–527 (2013).
Miossec, P. & Kolls, J. K. Targeting IL-17 and TH17 cells in chronic inflammation. Nature Rev. Drug Discov. 11, 763–776 (2012).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007).
Nurieva, R. et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007).
Zhou, L. et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature Immunol. 8, 967–974 (2007). References 35, 36 and 37 are the first to describe the role of IL-21 in the differentiation of the pro-inflammatory T H 17 population of cells.
Ivanov, I. I., Zhou, L. & Littman, D. R. Transcriptional regulation of Th17 cell differentiation. Semin. Immunol. 19, 409–417 (2007).
Coquet, J. M., Chakravarti, S., Smyth, M. J. & Godfrey, D. I. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J. Immunol. 180, 7097–7101 (2008).
Sonderegger, I., Kisielow, J., Meier, R., King, C. & Kopf, M. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur. J. Immunol. 38, 1833–1838 (2008).
Tangye, S. G., Ma, C. S., Brink, R. & Deenick, E. K. The good, the bad and the ugly — TFH cells in human health and disease. Nature Rev. Immunol. 13, 412–426 (2013).
King, C., Tangye, S. G. & Mackay, C. R. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu. Rev. Immunol. 26, 741–766 (2008).
Linterman, M. A. et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207, 353–363 (2010).
Zotos, D. et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207, 365–378 (2010).
Bauquet, A. T. et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature Immunol. 10, 167–175 (2009).
Nurieva, R. I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009).
Yu, D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009).
Eto, D. et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE 6, e17739 (2011).
Dienz, O. et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 206, 69–78 (2009).
King, I. L., Mohrs, K. & Mohrs, M. A nonredundant role for IL-21 receptor signaling in plasma cell differentiation and protective type 2 immunity against gastrointestinal helminth infection. J. Immunol. 185, 6138–6145 (2010).
Rasheed, M. A. et al. Interleukin-21 is a critical cytokine for the generation of virus-specific long-lived plasma cells. J. Virol. 87, 7737–7746 (2013).
Luthje, K. et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nature Immunol. 13, 491–498 (2012).
Reinhardt, R. L., Liang, H. E. & Locksley, R. M. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature Immunol. 10, 385–393 (2009).
Morita, R. et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121 (2011).
McGuire, H. M. et al. A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity 34, 602–615 (2011).
Johnston, R. J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
Pelletier, N. et al. Plasma cells negatively regulate the follicular helper T cell program. Nature Immunol. 11, 1110–1118 (2010).
Chung, Y. et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nature Med. 17, 983–988 (2011).
Linterman, M. A. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nature Med. 17, 975–982 (2011).
Pallikkuth, S. et al. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood 120, 985–993 (2012).
Attridge, K. et al. IL-21 inhibits T cell IL-2 production and impairs Treg homeostasis. Blood 119, 4656–4664 (2012).
Schmitz, I. et al. IL-21 restricts virus-driven Treg cell expansion in chronic LCMV infection. PLoS Pathog. 9, e1003362 (2013).
Vogelzang, A. et al. IL-21 contributes to fatal inflammatory disease in the absence of Foxp3+ T regulatory cells. J. Immunol. 192, 1404–1414 (2014).
Spolski, R., Kim, H. P., Zhu, W., Levy, D. E. & Leonard, W. J. IL-21 mediates suppressive effects via its induction of IL-10. J. Immunol. 182, 2859–2867 (2009).
Pot, C. et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 183, 797–801 (2009).
Zeng, R. et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 201, 139–148 (2005).
Hinrichs, C. S. et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 111, 5326–5333 (2008).
Mittal, A., Murugaiyan, G., Beynon, V., Hu, D. & Weiner, H. L. IL-27 induction of IL-21 from human CD8+ T cells induces granzyme B in an autocrine manner. Immunol. Cell Biol. 90, 831–835 (2012).
Williams, L. D. et al. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J. Virol. 85, 2316–2324 (2011).
Casanova, J. L., Holland, S. M. & Notarangelo, L. D. Inborn errors of human JAKs and STATs. Immunity 36, 515–528 (2012).
Kotlarz, D. et al. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J. Exp. Med. 210, 433–443 (2013).
Holland, S. M. et al. STAT3 mutations in the hyper-IgE syndrome. N. Engl. J. Med. 357, 1608–1619 (2007).
Minegishi, Y. et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 448, 1058–1062 (2007).
Ives, M. L. et al. Signal transducer and activator of transcription 3 (STAT3) mutations underlying autosomal dominant hyper-IgE syndrome impair human CD8+ T-cell memory formation and function. J. Allergy Clin. Immunol. 132, 400–411.e9 (2013). Along with reference 71, this paper first describes the immune phenotype of immunodeficient patients with mutations in the IL21R gene.
Ma, C. S. et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood 119, 3997–4008 (2012).
Milner, J. D. et al. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452, 773–776 (2008).
Siegel, A. M. et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity 35, 806–818 (2011).
Pesce, J. et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J. Clin. Invest. 116, 2044–2055 (2006).
Skak, K., Kragh, M., Hausman, D., Smyth, M. J. & Sivakumar, P. V. Interleukin 21: combination strategies for cancer therapy. Nature Rev. Drug Discov. 7, 231–240 (2008).
Wang, G. et al. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 63, 9016–9022 (2003).
Takaki, R. et al. IL-21 enhances tumor rejection through a NKG2D-dependent mechanism. J. Immunol. 175, 2167–2173 (2005).
Sivakumar, P. V. et al. Comparison of vascular leak syndrome in mice treated with IL21 or IL2. Comparative Med. 63, 13–21 (2013).
Moroz, A. et al. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J. Immunol. 173, 900–909 (2004).
Rosenberg, S. A. et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17, 4550–4557 (2011).
Gattinoni, L. et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 115, 1616–1626 (2005).
Ahmadzadeh, M. & Rosenberg, S. A. IL-2 administration increases CD4+ CD25hi Foxp3+ regulatory T cells in cancer patients. Blood 107, 2409–2414 (2006).
Santegoets, S. J. et al. IL-21 promotes the expansion of CD27+CD28+ tumor infiltrating lymphocytes with high cytotoxic potential and low collateral expansion of regulatory T cells. J. Transl. Med. 11, 37 (2013).
Gattinoni, L., Klebanoff, C. A. & Restifo, N. P. Paths to stemness: building the ultimate antitumour T cell. Nature Rev. Cancer 12, 671–684 (2012).
Roda, J. M. et al. Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J. Immunol. 177, 120–129 (2006).
Takeda, K. et al. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J. Exp. Med. 199, 437–448 (2004).
Smyth, M. J. et al. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J. Exp. Med. 201, 1973–1985 (2005).
He, H. et al. Combined IL-21 and low-dose IL-2 therapy induces anti-tumor immunity and long-term curative effects in a murine melanoma tumor model. J. Transl. Med. 4, 24 (2006).
Kishida, T. et al. Interleukin (IL)-21 and IL-15 genetic transfer synergistically augments therapeutic antitumor immunity and promotes regression of metastatic lymphoma. Mol. Ther. 8, 552–558 (2003).
Davis, I. D. et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin. Cancer Res. 15, 2123–2129 (2009).
Thompson, J. A. et al. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J. Clin. Oncol. 26, 2034–2039 (2008).
Steele, N. et al. A phase 1 trial of recombinant human IL-21 in combination with cetuximab in patients with metastatic colorectal cancer. Br. J. Cancer 106, 793–798 (2012).
Stolfi, C. et al. Involvement of interleukin-21 in the regulation of colitis-associated colon cancer. J. Exp. Med. 208, 2279–2290 (2011).
Gowda, A. et al. IL-21 mediates apoptosis through up-regulation of the BH3 family member BIM and enhances both direct and antibody-dependent cellular cytotoxicity in primary chronic lymphocytic leukemia cells in vitro. Blood 111, 4723–4730 (2008).
Ghalamfarsa, G. et al. Differential regulation of B-cell proliferation by IL21 in different subsets of chronic lymphocytic leukemia. Cytokine 62, 439–445 (2013).
Jahrsdorfer, B. et al. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood 108, 2712–2719 (2006).
Ahearne, M. J. et al. Enhancement of CD154/IL4 proliferation by the T follicular helper (Tfh) cytokine, IL21 and increased numbers of circulating cells resembling Tfh cells in chronic lymphocytic leukaemia. Br. J. Haematol. 162, 360–370 (2013).
Pascutti, M. F. et al. IL-21 and CD40L signals from autologous T cells can induce antigen-independent proliferation of CLL cells. Blood 122, 3010–3019 (2013).
Sarosiek, K. A. et al. Novel IL-21 signaling pathway up-regulates c-Myc and induces apoptosis of diffuse large B-cell lymphomas. Blood 115, 570–580 (2010).
Wood, B. et al. Abundant expression of interleukin-21 receptor in follicular lymphoma cells is associated with more aggressive disease. Leukemia Lymphoma 54, 1212–1220 (2013).
Akamatsu, N. et al. High IL-21 receptor expression and apoptosis induction by IL-21 in follicular lymphoma. Cancer Lett. 256, 196–206 (2007).
de Totero, D. et al. Heterogeneous expression and function of IL-21R and susceptibility to IL-21-mediated apoptosis in follicular lymphoma cells. Exp. Hematol. 38, 373–383 (2010).
Wahlin, B. E. et al. A unifying microenvironment model in follicular lymphoma: outcome is predicted by programmed death-1—positive, regulatory, cytotoxic, and helper T cells and macrophages. Clin. Cancer Res. 16, 637–650 (2010).
Brenne, A. T. et al. Interleukin-21 is a growth and survival factor for human myeloma cells. Blood 99, 3756–3762 (2002).
Menoret, E. et al. IL-21 stimulates human myeloma cell growth through an autocrine IGF-1 loop. J. Immunol. 181, 6837–6842 (2008).
Scheeren, F. A. et al. IL-21 is expressed in Hodgkin lymphoma and activates STAT5: evidence that activated STAT5 is required for Hodgkin lymphomagenesis. Blood 111, 4706–4715 (2008).
Iannitto, E., Ferreri, A. J., Minardi, V., Tripodo, C. & Kreipe, H. H. Angioimmunoblastic T-cell lymphoma. Crit. Rev. Oncol. Hematol. 68, 264–271 (2008).
Miyoshi, H. et al. Clinicopathologic analysis of peripheral T-cell lymphoma, follicular variant, and comparison with angioimmunoblastic T-cell lymphoma: Bcl-6 expression might affect progression between these disorders. Am. J. Clin. Pathol. 137, 879–889 (2012).
de Leval, L. et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood 109, 4952–4963 (2007).
Dorfman, D. M., Brown, J. A., Shahsafaei, A. & Freeman, G. J. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 30, 802–810 (2006).
Grogg, K. L. et al. Angioimmunoblastic T-cell lymphoma: a neoplasm of germinal-center T-helper cells? Blood 106, 1501–1502 (2005).
Marafioti, T. et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica 95, 432–439 (2010).
Morito, N. et al. Overexpression of c-Maf contributes to T-cell lymphoma in both mice and human. Cancer Res. 66, 812–819 (2006).
Iqbal, J. et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood 115, 1026–1036 (2010).
Timmerman, J. M. et al. A phase I dose-finding trial of recombinant interleukin-21 and rituximab in relapsed and refractory low grade B-cell lymphoproliferative disorders. Clin. Cancer Res. 18, 5752–5760 (2012).
Novy, P., Huang, X., Leonard, W. J. & Yang, Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J. Immunol. 186, 2729–2738 (2011).
Frohlich, A. et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324, 1576–1580 (2009).
Elsaesser, H., Sauer, K. & Brooks, D. G. IL-21 is required to control chronic viral infection. Science 324, 1569–1572 (2009).
Yi, J. S., Du, M. & Zajac, A. J. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324, 1572–1576 (2009). References 121, 122 and 123 are the first papers to determine that IL-21 has a role in chronic viral infections.
Yi, J. S., Ingram, J. T. & Zajac, A. J. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J. Immunol. 185, 4835–4845 (2010).
Cui, W., Liu, Y., Weinstein, J. S., Craft, J. & Kaech, S. M. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35, 792–805 (2011).
Hu, X. et al. Interleukin-21 is upregulated in hepatitis B-related acute-on-chronic liver failure and associated with severity of liver disease. J. Viral Hepat. 18, 458–467 (2011).
Li, L. et al. HBcAg-specific IL-21-producing CD4+ T cells are associated with relative viral control in patients with chronic hepatitis B. Scand. J. Immunol. 78, 439–446 (2013).
Ma, S. W. et al. High serum IL-21 levels after 12 weeks of antiviral therapy predict HBeAg seroconversion in chronic hepatitis B. J. Hepatol. 56, 775–781 (2012).
Publicover, J. et al. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J. Clin. Invest. 121, 1154–1162 (2011). This paper describes the role of IL-21 related to the age-related response to human hepatitis B infection.
Publicover, J. et al. Age-dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J. Clin. Invest. 123, 3728–3739 (2013).
Feng, G. et al. Interleukin-21 mediates hepatitis B virus-associated liver cirrhosis by activating hepatic stellate cells. Hepatol. Res. http://dx.doi.org/10.1111/hepr.12215 (2013).
Kared, H., Fabre, T., Bedard, N., Bruneau, J. & Shoukry, N. H. Galectin-9 and IL-21 mediate cross-regulation between Th17 and Treg cells during acute hepatitis C. PLoS Pathog. 9, e1003422 (2013).
Iannello, A. et al. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J. Immunol. 184, 114–126 (2010).
Yue, F. Y. et al. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J. Immunol. 185, 498–506 (2010).
Chevalier, M. F. et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J. Virol. 85, 733–741 (2011).
White, L. et al. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV). Blood 109, 3873–3880 (2007).
Lindqvist, M. et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J. Clin. Invest. 122, 3271–3280 (2012).
Cubas, R. A. et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nature Med. 19, 494–499 (2013).
Pallikkuth, S. et al. Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine 29, 9229–9238 (2011).
Pallikkuth, S. et al. Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)-21. PLoS Pathog. 9, e1003471 (2013).
Spolski, R. et al. IL-21 promotes the pathologic immune response to pneumovirus infection. J. Immunol. 188, 1924–1932 (2012).
Hughes, T. et al. Fine-mapping and transethnic genotyping establish IL2/IL21 genetic association with lupus and localize this genetic effect to IL21. Arthritis Rheum. 63, 1689–1697 (2011).
Cooper, J. D. et al. Meta-analysis of genome-wide association study data identifies additional type 1 diabetes risk loci. Nature Genet. 40, 1399–1401 (2008).
Marquez, A. et al. Novel association of the interleukin 2-interleukin 21 region with inflammatory bowel disease. Am. J. Gastroenterol. 104, 1968–1975 (2009).
van Heel, D. A. et al. A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nature Genet. 39, 827–829 (2007).
Liu, Y. et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 4, e1000041 (2008).
McPhee, C. G. et al. IL-21 is a double-edged sword in the systemic lupus erythematosus-like disease of BXSB.Yaa mice. J. Immunol. 191, 4581–4588 (2013).
Bubier, J. A. et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc. Natl Acad. Sci. USA 106, 1518–1523 (2009).
Herber, D. et al. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J. Immunol. 178, 3822–3830 (2007).
Terrier, B. et al. Interleukin 21 correlates with T cell and B cell subset alterations in systemic lupus erythematosus. J. Rheumatol. 39, 1819–1828 (2012).
Jang, E. et al. A positive feedback loop of IL-21 signaling provoked by homeostatic CD4+CD25− T cell expansion is essential for the development of arthritis in autoimmune K/BxN mice. J. Immunol. 182, 4649–4656 (2009).
Niu, X. et al. IL-21 regulates Th17 cells in rheumatoid arthritis. Hum. Immunol. 71, 334–341 (2010).
Ma, J. et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin. Dev. Immunol. 2012, 827480 (2012).
Block, K. E. & Huang, H. The cellular source and target of IL-21 in K/BxN autoimmune arthritis. J. Immunol. 191, 2948–2955 (2013).
Gabay, C. et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 381, 1541–1550 (2013).
Carbone, G. et al. Interleukin-6 receptor blockade selectively reduces IL-21 production by CD4 T cells and IgG4 autoantibodies in rheumatoid arthritis. Int. J. Biol. Sci. 9, 279–288 (2013).
Kwok, S. K. et al. Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis. Arthritis Rheum. 64, 740–751 (2012).
King, C., Ilic, A., Koelsch, K. & Sarvetnick, N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 117, 265–277 (2004).
Spolski, R., Kashyap, M., Robinson, C., Yu, Z. & Leonard, W. J. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc. Natl Acad. Sci. USA 105, 14028–14033 (2008).
Sutherland, A. P. et al. Interleukin-21 is required for the development of type 1 diabetes in NOD mice. Diabetes 58, 1144–1155 (2009). References 158, 159 and 160 describe the crucial role of IL-21 in the development of type 1 diabetes.
Liu, S. M. et al. Differential IL-21 signaling in APCs leads to disparate Th17 differentiation in diabetes-susceptible NOD and diabetes-resistant NOD.Idd3 mice. J. Clin. Invest. 121, 4303–4310 (2011).
Van Belle, T. L., Nierkens, S., Arens, R. & von Herrath, M. G. Interleukin-21 receptor-mediated signals control autoreactive T cell infiltration in pancreatic islets. Immunity 36, 1060–1072 (2012).
Ramanathan, S. et al. Exposure to IL-15 and IL-21 enables autoreactive CD8 T cells to respond to weak antigens and cause disease in a mouse model of autoimmune diabetes. J. Immunol. 186, 5131–5141 (2011).
McGuire, H. M. et al. Interleukin-21 is critically required in autoimmune and allogeneic responses to islet tissue in murine models. Diabetes 60, 867–875 (2011).
Vollmer, T. L. et al. Differential effects of IL-21 during initiation and progression of autoimmunity against neuroantigen. J. Immunol. 174, 2696–2701 (2005).
Nohra, R. et al. RGMA and IL21R show association with experimental inflammation and multiple sclerosis. Genes Immun. 11, 279–293 (2010).
Tzartos, J. S. et al. IL-21 and IL-21 receptor expression in lymphocytes and neurons in multiple sclerosis brain. Am. J. Pathol. 178, 794–802 (2011).
Romme Christensen, J. et al. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS ONE 8, e57820 (2013).
Jones, J. L. et al. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J. Clin. Invest. 119, 2052–2061 (2009).
Yoshizaki, A. et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 491, 264–268 (2012). This paper describes the crucial role of IL-21 in the control of T cell autoimmunity by regulatory B cells.
Wang, L. et al. Key role for IL-21 in experimental autoimmune uveitis. Proc. Natl Acad. Sci. USA 108, 9542–9547 (2011).
Bouma, G. & Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nature Rev. Immunol. 3, 521–533 (2003).
Fantini, M. C., Monteleone, G. & MacDonald, T. T. IL-21 comes of age as a regulator of effector T cells in the gut. Mucosal Immunol. 1, 110–115 (2008).
Monteleone, G. et al. Interleukin-21 enhances T-helper cell type I signaling and interferon-γ production in Crohn's disease. Gastroenterology 128, 687–694 (2005).
Fina, D. et al. Regulation of gut inflammation and Th17 cell response by interleukin-21. Gastroenterology 134, 1038–1048 (2008).
van Leeuwen, M. A. et al. Increased production of interleukin-21, but not interleukin-17A, in the small intestine characterizes pediatric celiac disease. Mucosal Immunol. 6, 1202–1213 (2013).
Caruso, R. et al. A functional role for interleukin-21 in promoting the synthesis of the T-cell chemoattractant, MIP-3α, by gut epithelial cells. Gastroenterology 132, 166–175 (2007).
Monteleone, G. et al. Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21. Gut 55, 1774–1780 (2006).
Jauch, D. et al. Interleukin 21 controls tumour growth and tumour immunosurveillance in colitis-associated tumorigenesis in mice. Gut 60, 1678–1686 (2011).
Suto, A. et al. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line Cɛ transcription of IL-4-stimulated B cells. Blood 100, 4565–4573 (2002).
Frohlich, A. et al. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood 109, 2023–2031 (2007).
Hiromura, Y. et al. IL-21 administration into the nostril alleviates murine allergic rhinitis. J. Immunol. 179, 7157–7165 (2007).
Tamagawa-Mineoka, R., Kishida, T., Mazda, O. & Katoh, N. IL-21 reduces immediate hypersensitivity reactions in mouse skin by suppressing mast cell activation or IgE production. J. Invest. Dermatol. 131, 1513–1520 (2011).
Jin, H. et al. IL-21R is essential for epicutaneous sensitization and allergic skin inflammation in humans and mice. J. Clin. Invest. 119, 47–60 (2009).
Caruso, R. et al. Involvement of interleukin-21 in the epidermal hyperplasia of psoriasis. Nature Med. 15, 1013–1015 (2009).
Davis, I. D. et al. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin. Cancer Res. 13, 3630–3636 (2007).
Petrella, T. M. et al. Interleukin-21 has activity in patients with metastatic melanoma: a phase II study. J. Clin. Oncol. 30, 3396–3401 (2012).
Grunwald, V. et al. A phase I study of recombinant human interleukin-21 (rIL-21) in combination with sunitinib in patients with metastatic renal cell carcinoma (RCC). Acta Oncol. 50, 121–126 (2011).
Bhatia, S. et al. Recombinant interleukin-21 plus sorafenib for metastatic renal cell carcinoma: a phase 1/2 study. J. Immunother. Cancer 2, 2 (2014).
Hua, F. et al. Anti-IL21 receptor monoclonal antibody (ATR-107): Safety, pharmacokinetics, and pharmacodynamic evaluation in healthy volunteers: a phase I, first-in-human study. J. Clin. Pharmacol. 54, 14–22 (2014).
Bubier, J. A. et al. Treatment of BXSB-Yaa mice with IL-21R-Fc fusion protein minimally attenuates systemic lupus erythematosus. Ann. NY Acad. Sci. 1110, 590–601 (2007).
Acknowledgements
This work was supported by the Division of Intramural Research at the National Heart, Lung, and Blood Institute, US National Institutes of Health (NIH). We thank Drs E. E. West and J.-X. Lin for their critical comments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- HIV long-term non-progressors
-
Individuals who have been infected with HIV for long periods of time but can control the infection without the need for antiretroviral therapy. Their viral loads are under 10,000 copies per ml of blood and their CD4+ T cell counts are normal, although they may undergo a slow progression to lower CD4 counts.
- Fratricide
-
The induction of apoptotic death in nearby cells, which normally occurs via a death receptor and its ligand. This occurs naturally in the immune system and other systems, and it can also be induced by chimeric ligands.
- Elite controllers
-
Individuals who are infected with HIV but have extremely low viral loads (<50 copies of RNA per ml). These individuals are believed to have a strong and persistant anti-HIV immune response.
- MRL–Faslpr mice
-
A strain of mice that are deficient for the FAS receptor, which is involved in the induction of cell death. This strain spontaneously develops an autoimmune disease that resembles the human disease systemic lupus erythematosus.
- Tape-stripping epicutaneous sensitization
-
A method for inducing allergic skin inflammation that involves the application of antigen combined with tape stripping, which mimics scratching, leading to skin injury and a heightened allergic response.
Rights and permissions
About this article
Cite this article
Spolski, R., Leonard, W. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov 13, 379–395 (2014). https://doi.org/10.1038/nrd4296
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd4296
This article is cited by
-
Enhanced attenuation of chikungunya vaccines expressing antiviral cytokines
npj Vaccines (2024)
-
Interleukin-21 modulates balance between regulatory T cells and T-helper 17 cells in chronic hepatitis B virus infection
BMC Infectious Diseases (2023)
-
Phytogalactolipids activate humoral immunity against colorectal cancer
Journal of Experimental & Clinical Cancer Research (2023)
-
Tasmanian devil cathelicidins exhibit anticancer activity against Devil Facial Tumour Disease (DFTD) cells
Scientific Reports (2023)
-
CXCL13 expressed on inflamed cerebral blood vessels recruit IL-21 producing TFH cells to damage neurons following stroke
Journal of Neuroinflammation (2022)