Key Points
-
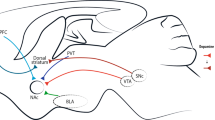
Glycine is released from presynaptic terminals as a classical inhibitory neurotransmitter that hyperpolarizes the postsynaptic membrane through the activation of glycine receptors, which have an integral anion channel. Glycine also modulates the activity of excitatory NMDA (N-methyl-D-aspartate)-selective glutamate receptors at excitatory synapses by acting as a co-agonist.
-
The glycine transporters GlyT1 and GlyT2 are expressed in neurons and in astrocytes that ensheath synapses. At inhibitory glycinergic synapses, GlyT1 terminates neurotransmission, whereas GlyT2 regulates the recycling of glycine into presynaptic terminals. At excitatory synapses, GlyT1 also controls the extracellular concentration of glycine near NMDA receptors.
-
Constitutive disruption of the genes encoding GlyT1 or GlyT2 leads to early postnatal fatality in mouse models, whereas mutations in the GLYT2 gene have been linked to startle disease in humans and other species. In the adult central nervous system, the balance between inhibitory and excitatory signalling in multiple neural pathways remains susceptible to modulation by GlyT1 and GlyT2, and therefore glycine transporters are potential new targets for the treatment of several neuropsychiatric conditions.
-
GlyT1 inhibition can facilitate NMDA receptor activity and is therefore primarily conceived as a potential remedy against the cognitive and affective deficiencies in schizophrenia, which are due to an underlying impairment in NMDA receptor signalling. The clinical efficacy of GlyT1 inhibitors as an adjunct therapy to improve global functioning in patients with schizophrenia is supported by the Phase II trials of bitopertin, which is currently under Phase III evaluation.
-
Preclinical models have shown that inhibition of GlyT1 can suppress alcohol intake and relapses in rats, and suggest a new application for GlyT1 inhibitors in the treatment of alcohol dependence. The anti-alcohol effect is attributed to the potentiation of glycinergic inhibition via the activation of glycine receptors in central reward pathways, which may be relevant to addiction in general.
-
The transmission of pain signals from the spinal cord to the brain is regulated by glycinergic inhibition in the dorsal horn mediated by signalling pathways converging on glycine receptors containing the α3 subunit. The local inhibition of GlyT1 or GlyT2 can effectively suppress pain transmission through the enhancement of local neuronal inhibition mediated by glycine receptors.
-
Further applications of glycine reuptake inhibition therapy to other diseases, such as obsessive-compulsive disorder, depression and epilepsy, have been proposed but the precise brain mechanisms involved in these disorders are less clear. In this Review, we consider potential factors that may determine the clinical efficacy of glycine reuptake inhibition therapy in these conditions, such as pharmacokinetics, brain circuitry and the localization of the relevant glycine transporters.
Abstract
Glycine transporters are endogenous regulators of the dual functions of glycine, which acts as a classical inhibitory neurotransmitter at glycinergic synapses and as a modulator of neuronal excitation mediated by NMDA (N-methyl-D-aspartate) receptors at glutamatergic synapses. The two major subtypes of glycine transporters, GlyT1 and GlyT2, have been linked to the pathogenesis and/or treatment of central and peripheral nervous system disorders, including schizophrenia and related affective and cognitive disturbances, alcohol dependence, pain, epilepsy, breathing disorders and startle disease (also known as hyperekplexia). This Review examines the rationale for the therapeutic potential of GlyT1 and GlyT2 inhibition, and surveys the latest advances in the biology of glycine reuptake and transport as well as the drug discovery and clinical development of compounds that block glycine transporters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Avila, A. et al. Glycine receptor α2 subunit activation promotes cortical interneuron migration. Cell Rep. 4, 738–750 (2013). This paper reveals a novel biological role for GlyRα2 in cortical interneuron migration during embryonic development.
Wolosker, H. NMDA receptor regulation by D-serine: new findings and perspectives. Mol. Neurobiol. 36, 152–164 (2007).
Eulenburg, V., Armsen, W., Betz, H. & Gomeza, J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem. Sci. 30, 325–333 (2005).
Harvey, R. J., Topf, M., Harvey, K. & Rees, M. I. The genetics of hyperekplexia: more than startle! Trends Genet. 24, 439–447 (2008).
Supplisson, S. & Roux, M. J. Why glycine transporters have different stoichiometries. FEBS Lett. 529, 93–101 (2002).
Aubrey, K. R. et al. The transporters GlyT2 and VIAAT cooperate to determine the vesicular glycinergic phenotype. J. Neurosci. 27, 6273–6281 (2007). This elegant study demonstrates why the functional properties of GlyT2 are suitable for mediating the high cytosolic glycine concentration that is required for efficient vesicular loading by the vesicular inhibitory amino acid transporter at inhibitory synapses.
Gomeza, J. et al. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron 40, 785–796 (2003). This is a groundbreaking study demonstrating that a severe phenotype in GlyT1-knockout mice, resembling glycine encephalopathy, is linked to the overactivation of glycine receptors at inhibitory glycinergic synapses.
Gomeza, J. et al. Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron 40, 797–806 (2003). This important paper demonstrates that GlyT2 is vital for efficient transmitter loading of synaptic vesicles in glycinergic nerve terminals, and that GlyT2-knockout mice are a model for human startle disease.
Eulenburg, V., Retiounskaia, M., Papadopoulos, T., Gomeza, J. & Betz, H. Glial glycine transporter 1 function is essential for early postnatal survival but dispensable in adult mice. Glia 58, 1066–1073 (2010).
Rousseau, F., Aubrey, K. R. & Supplisson, S. The glycine transporter GlyT2 controls the dynamics of synaptic vesicle refilling in inhibitory spinal cord neurons. J. Neurosci. 28, 9755–9768 (2008).
Yee, B. K. et al. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J. Neurosci. 26, 3169–3181 (2006). This is the first report on mutant mice lacking forebrain neuronal GlyT1, clearly showing the presence of schizophrenia-resilient phenotypes.
Singer, P. et al. Altered mnemonic functions and resistance to N-methyl-D-aspartate receptor antagonism by forebrain conditional knockout of glycine transporter 1. Neuroscience 161, 635–654 (2009).
Rees, M. I. et al. Mutations in the gene encoding GlyT2 (SLC6A5) define a presynaptic component of human startle disease. Nature Genet. 38, 801–806 (2006). This report demonstrates that mutations in the human GLYT2 gene cause startle disease with associated life-threatening neonatal apnoea episodes.
Carta, E. et al. Mutations in the GlyT2 gene (SLC6A5) are a second major cause of startle disease. J. Biol. Chem. 287, 28975–28985 (2012).
Cubelos, B., Giménez, C. & Zafra, F. Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cereb. Cortex 15, 448–459 (2005).
Cubelos, B., Gonzalez-Gonzalez, I. M., Gimenez, C. & Zafra, F. The scaffolding protein PSD-95 interacts with the glycine transporter GLYT1 and impairs its internalization. J. Neurochem. 95, 1047–1058 (2005).
Zafra, F. et al. Glycine transporters are differentially expressed among CNS cells. J. Neurosci. 15, 3952–3969 (1995).
Coyle, J. T., Balu, D., Benneyworth, M., Basu, A. & Roseman, A. Beyond the dopamine receptor: novel therapeutic targets for treating schizophrenia. Dialogues Clin. Neurosci. 12, 359–382 (2010).
Javitt, D. C. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int. Rev. Neurobiol. 78, 69–108 (2007). This paper traces the background of the glutamate hypothesis of schizophrenia up to the recent attempts in glycine augmentation therapy, including the inhibition of glycine reuptake.
Coyle, J. T. NMDA receptor and schizophrenia: a brief history. Schizophr Bull. 38, 920–926 (2012).
Marino, M. J., Knutsen, L. J. & Williams, M. Emerging opportunities for antipsychotic drug discovery in the postgenomic era. J. Med. Chem. 51, 1077–1107 (2008).
Perry, K. W. et al. Neurochemical and behavioral profiling of the selective GlyT1 inhibitors ALX5407 and LY2365109 indicate a preferential action in caudal versus cortical brain areas. Neuropharmacology 55, 743–754 (2008).
Danysz, W. & Parsons, C. G. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol. Rev. 50, 597–664 (1998).
Furukawa, H. & Gouaux, E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J. 22, 2873–2885 (2003).
Kristensen, A. S. et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 63, 585–640 (2011).
Cioffi, C. L., Liu, S. & Wolf, M. A. Chapter 2 — Recent developments in glycine transporter-1 inhibitors. Annu. Rep. Med. Chem. 45, 19–35 (2010).
Möhler, H. et al. Glycine transporter 1 as a potential therapeutic target for schizophrenia-related symptoms: evidence from genetically modified mouse models and pharmacological inhibition. Biochem. Pharmacol. 81, 1065–1077 (2011). This comprehensive review compares different mouse models of GlyT1 disruption. It also explores the possibility of targeting subpopulations of GlyT1 in specific regions of the brain and/or cell types.
Tsai, G. et al. Gene knockout of glycine transporter 1: characterization of the behavioral phenotype. Proc. Natl Acad. Sci. USA 101, 8485–8490 (2004).
Martina, M. et al. Reduced glycine transporter type 1 expression leads to major changes in glutamatergic neurotransmission of CA1 hippocampal neurones in mice. J. Physiol. 563, 777–793 (2005).
Balu, D. T. & Coyle, J. T. Glutamate receptor composition of the post-synaptic density is altered in genetic mouse models of NMDA receptor hypo- and hyperfunction. Brain Res. 1392, 1–7 (2011).
Black, M. D. et al. Procognitive and antipsychotic efficacy of glycine transport 1 inhibitors (GlyT1) in acute and neurodevelopmental models of schizophrenia: latent inhibition studies in the rat. Psychopharmacol. (Berl.) 202, 385–396 (2009).
Boulay, D., Bergis, O., Avenet, P. & Griebel, G. The glycine transporter-1 inhibitor SSR103800 displays a selective and specific antipsychotic-like profile in normal and transgenic mice. Neuropsychopharmacology 35, 416–427 (2010).
Weiner, I. Neural substrates of latent inhibition: the switching model. Psychol. Bull. 108, 442–461 (1990).
Moser, P. C., Hitchcock, J. M., Lister, S. & Moran, P. M. The pharmacology of latent inhibition as an animal model of schizophrenia. Brain Res. Brain Res. Rev. 33, 275–307 (2000).
Yee, B. K. et al. Latent inhibition enhancement by glycine transporter 1 disruption is mediated by anti-dopaminergic mechanism in the nucleus accumbens. Program No. 571.10/GG15. 2010 Neuroscience Meeting Planner [online], (San Diego, California; Society for Neuroscience, 2010).
Gray, J. A., Feldon, J., Rawlins, J. N. P., Smith, A. D. & Hemsley, D. R. The neuropsychology of schizophrenia. Behav. Brain Sci. 14, 1–19 (1991).
Harsing, L. G. Jr et al. The glycine transporter-1 inhibitors NFPS and Org 24461: a pharmacological study. Pharmacol. Biochem. Behav. 74, 811–825 (2003).
Alberati, D. et al. Glycine reuptake inhibitor RG1678: a pharmacologic characterization of an investigational agent for the treatment of schizophrenia. Neuropharmacology 62, 1152–1161 (2012).
Depoortere, R. et al. Neurochemical, electrophysiological and pharmacological profiles of the selective inhibitor of the glycine transporter-1 SSR504734, a potential new type of antipsychotic. Neuropsychopharmacology 30, 1963–1985 (2005).
Singer, P., Feldon, J. & Yee, B. K. Interactions between the glycine transporter 1 (GlyT1) inhibitor SSR504734 and psychoactive drugs in mouse motor behaviour. Eur. Neuropsychopharmacol. 19, 571–580 (2009).
Leonetti, M. et al. 2-chloro-N−[(S)-phenyl [(2S)-piperidin-2-yl] methyl]-3-trifluoromethyl benzamide, monohydrochloride, an inhibitor of the glycine transporter type 1, increases evoked-dopamine release in the rat nucleus accumbens in vivo via an enhanced glutamatergic neurotransmission. Neuroscience 137, 555–564 (2006).
Lidö, H. H., Ericson, M., Marston, H. & Soderpalm, B. A role for accumbal glycine receptors in modulation of dopamine release by the glycine transporter-1 inhibitor Org25935. Front. Psychiatry 2, 8 (2011).
Barch, D. M. & Carter, C. S. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr. Res. 77, 43–58 (2005).
Molander, A. & Soderpalm, B. Glycine receptors regulate dopamine release in the rat nucleus accumbens. Alcohol Clin. Exp. Res. 29, 17–26 (2005).
Singh, S. P. & Singh, V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs 25, 859–885 (2011).
Singer, P., Feldon, J. & Yee, B. K. The glycine transporter 1 inhibitor SSR504734 enhances working memory performance in a continuous delayed alternation task in C57BL/6 mice. Psychopharmacol. (Berl.) 202, 371–384 (2009).
Roberts, B. M. et al. Glycine transporter inhibition reverses ketamine-induced working memory deficits. Neuroreport 21, 390–394 (2010).
Boulay, D. et al. Characterization of SSR103800, a selective inhibitor of the glycine transporter-1 in models predictive of therapeutic activity in schizophrenia. Pharmacol. Biochem. Behav. 91, 47–58 (2008).
Manahan-Vaughan, D., Wildforster, V. & Thomsen, C. Rescue of hippocampal LTP and learning deficits in a rat model of psychosis by inhibition of glycine transporter-1 (GlyT1). Eur. J. Neurosci. 28, 1342–1350 (2008).
Igartua, I., Solis, J. M. & Bustamante, J. Glycine-induced long-term synaptic potentiation is mediated by the glycine transporter GLYT1. Neuropharmacology 52, 1586–1595 (2007).
Martina, M. et al. Glycine transporter type 1 blockade changes NMDA receptor-mediated responses and LTP in hippocampal CA1 pyramidal cells by altering extracellular glycine levels. J. Physiol. 557, 489–500 (2004).
Dubroqua, S. et al. Intact working memory in the absence of forebrain neuronal glycine transporter 1. Behav. Brain Res. 230, 208–214 (2012).
Singer, P., Boison, D., Mohler, H., Feldon, J. & Yee, B. K. Enhanced recognition memory following glycine transporter 1 deletion in forebrain neurons. Behav. Neurosci. 121, 815–825 (2007).
Dubroqua, S., Boison, D., Feldon, J., Mohler, H. & Yee, B. K. Examining the sex- and circadian dependency of a learning phenotype in mice with glycine transporter 1 deletion in two Pavlovian conditioning paradigms. Neurobiol. Learn. Mem. 96, 218–229 (2011).
Shimazaki, T., Kaku, A. & Chaki, S. D-serine and a glycine transporter-1 inhibitor enhance social memory in rats. Psychopharmacol. (Berl.) 209, 263–270 (2010).
Singer, P., Boison, D., Mohler, H., Feldon, J. & Yee, B. K. Deletion of glycine transporter 1 (GlyT1) in forebrain neurons facilitates reversal learning: enhanced cognitive adaptability? Behav. Neurosci. 123, 1012–1027 (2009).
Nong, Y. et al. Glycine binding primes NMDA receptor internalization. Nature 422, 302–307 (2003). This is an important paper demonstrating that the stimulation of the glycine-B site on NMDA receptors may prime these receptors for subsequent internalization via clathrin-dependent endocytosis, which highlights the possibility that glycine and D -serine, which bind to the glycine-B site, may also downregulate NMDA receptor activity.
Nikiforuk, A. et al. Blockade of glycine transporter 1 by SSR-504734 promotes cognitive flexibility in glycine/NMDA receptor-dependent manner. Neuropharmacology 61, 262–267 (2011).
Singer, P., Boison, D., Mohler, H., Feldon, J. & Yee, B. K. Modulation of sensorimotor gating in prepulse inhibition by conditional brain glycine transporter 1 deletion in mice. Eur. Neuropsychopharmacol. 21, 401–413 (2011).
Kopec, K. et al. Glycine transporter (GlyT1) inhibitors with reduced residence time increase prepulse inhibition without inducing hyperlocomotion in DBA/2 mice. Biochem. Pharmacol. 80, 1407–1417 (2010).
Lipina, T., Labrie, V., Weiner, I. & Roder, J. Modulators of the glycine site on NMDA receptors, D-serine and ALX 5407, display similar beneficial effects to clozapine in mouse models of schizophrenia. Psychopharmacol. (Berl.) 179, 54–67 (2005).
Yang, S. Y., Hong, C. J., Huang, Y. H. & Tsai, S. J. The effects of glycine transporter I inhibitor, N-methylglycine (sarcosine), on ketamine-induced alterations in sensorimotor gating and regional brain c-Fos expression in rats. Neurosci. Lett. 469, 127–130 (2010).
Le Pen, G. et al. Prepulse inhibition deficits of the startle reflex in neonatal ventral hippocampal-lesioned rats: reversal by glycine and a glycine transporter inhibitor. Biol. Psychiatry 54, 1162–1170 (2003).
Bullich, S. et al. Biodistribution and radiation dosimetry of the glycine transporter-1 ligand 11C-GSK931145 determined from primate and human whole-body PET. Mol. Imag. Biol. 13, 776–784 (2011).
Hamill, T. G. et al. The synthesis and preclinical evaluation in rhesus monkey of [18F]MK-6577 and [11C]CMPyPB glycine transporter 1 positron emission tomography radiotracers. Synapse 65, 261–270 (2011).
Borroni, E. et al. Pre-clinical characterization of [11C]R05013853 as a novel radiotracer for imaging of the glycine transporter type 1 by positron emission tomography. Neuroimage 75, 291–300 (2013).
Mezler, M. et al. Inhibitors of GlyT1 affect glycine transport via discrete binding sites. Mol. Pharmacol. 74, 1705–1715 (2008).
Papouin, T. et al. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150, 633–646 (2012).
Laughren, T. & Levin, R. Food and Drug Administration commentary on methodological issues in negative symptom trials. Schizophr Bull. 37, 255–256 (2011).
Tsai, G. E. & Lin, P. Y. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr. Pharm. Des. 16, 522–537 (2010).
Lin, C. H., Lane, H. Y. & Tsai, G. E. Glutamate signaling in the pathophysiology and therapy of schizophrenia. Pharmacol. Biochem. Behav. 100, 665–677 (2012).
Javitt, D. C. Glycine transport inhibitors and the treatment of schizophrenia. Biol. Psychiatry 63, 6–8 (2008).
Buchanan, R. W. et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am. J. Psychiatry 164, 1593–1602 (2007).
Heresco-Levy, U. et al. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch. Gen. Psychiatry 56, 29–36 (1999).
Lane, H. Y., Chang, Y. C., Liu, Y. C., Chiu, C. C. & Tsai, G. E. Sarcosine or D-serine add-on treatment for acute exacerbation of schizophrenia: a randomized, double-blind, placebo-controlled study. Arch. Gen. Psychiatry 62, 1196–1204 (2005).
Lane, H. Y. et al. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to clozapine for the treatment of schizophrenia. Biol. Psychiatry 60, 645–649 (2006).
Lane, H. Y. et al. A randomized, double-blind, placebo-controlled comparison study of sarcosine (N-methylglycine) and D-serine add-on treatment for schizophrenia. Int. J. Neuropsychopharmacol. 13, 451–460 (2010).
Tsai, G., Lane, H. Y., Yang, P., Chong, M. Y. & Lange, N. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry 55, 452–456 (2004).
Lane, H. Y. et al. Sarcosine (N-methylglycine) treatment for acute schizophrenia: a randomized, double-blind study. Biol. Psychiatry 63, 9–12 (2008).
Javitt, D. C., Duncan, L., Balla, A. & Sershen, H. Inhibition of system A-mediated glycine transport in cortical synaptosomes by therapeutic concentrations of clozapine: implications for mechanisms of action. Mol. Psychiatry 10, 275–287 (2005).
Leucht, S. et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382, 951–962 (2013).
van Berckel, B. N. et al. Efficacy and tolerance of D-cycloserine in drug-free schizophrenic patients. Biol. Psychiatry 40, 1298–1300 (1996).
Woods, S. W. et al. Glycine treatment of the risk syndrome for psychosis: report of two pilot studies. Eur. Neuropsychopharmacol. 23, 931–940 (2013).
Pinard, E. et al. Selective GlyT1 inhibitors: discovery of [4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfonyl-2-((S)-2,2,2-trifluoro-1- methylethoxy)phenyl]methanone (RG1678), a promising novel medicine to treat schizophrenia. J. Med. Chem. 53, 4603–4614 (2010).
Umbricht, D. et al. Glycine transporter type 1 (GLYT1) inhibitor RG1678: Positive results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Neuropsychopharmacology 35, S320–S321 (2010).
Martin-Facklam, M. et al. Glycine transporter type 1 occupancy by bitopertin: a positron emission tomography study in healthy volunteers. Neuropsychopharmacology 38, 504–512 (2013).
Molander, A., Lidö, H. H., Lof, E., Ericson, M. & Soderpalm, B. The glycine reuptake inhibitor Org25935 decreases ethanol intake and preference in male Wistar rats. Alcohol Alcohol. 42, 11–18 (2007).
Söderpalm, B. & Ericson, M. Neurocircuitry involved in the development of alcohol addiction: the dopamine system and its access points. Curr. Top. Behav. Neurosci. 13, 127–161 (2013). This study proposes a model of alcohol addiction that links the action of glycine and alcohol with the reward pathway, and provides the rationale for glycine transporter reuptake inhibition as a means to reduce alcohol consumption and relapse.
Uslaner, J. M. et al. Inhibition of glycine transporter 1 attenuates nicotine- but not food-induced cue-potentiated reinstatement for a response previously paired with sucrose. Behav. Brain Res. 207, 37–43 (2010).
Achat-Mendes, C., Nic Dhonnchadha, B. A., Platt, D. M., Kantak, K. M. & Spealman, R. D. Glycine transporter-1 inhibition preceding extinction training inhibits reacquisition of cocaine seeking. Neuropsychopharmacology 37, 2837–2845 (2012).
Nic Dhonnchadha, B. A. et al. Inhibiting glycine transporter-1 facilitates cocaine-cue extinction and attenuates reacquisition of cocaine-seeking behavior. Drug Alcohol Depend. 122, 119–126 (2012).
Vengeliene, V., Leonardi-Essmann, F., Sommer, W. H., Marston, H. M. & Spanagel, R. Glycine transporter-1 blockade leads to persistently reduced relapse-like alcohol drinking in rats. Biol. Psychiatry 68, 704–711 (2010).
Lidö, H. H., Stomberg, R., Fagerberg, A., Ericson, M. & Soderpalm, B. The glycine reuptake inhibitor Org 25935 interacts with basal and ethanol-induced dopamine release in rat nucleus accumbens. Alcohol Clin. Exp. Res. 33, 1151–1157 (2009).
Szegedi, A. et al. Evaluation of glycine transporter inhibitor Org 25935 for the prevention of relapse in alcohol-dependent patients: a multisite, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology 38, S314–S446 (2012).
Molander, A., Lof, E., Stomberg, R., Ericson, M. & Soderpalm, B. Involvement of accumbal glycine receptors in the regulation of voluntary ethanol intake in the rat. Alcohol Clin. Exp. Res. 29, 38–45 (2005).
Eggers, E. D. & Berger, A. J. Mechanisms for the modulation of native glycine receptor channels by ethanol. J. Neurophysiol. 91, 2685–2695 (2004).
Spanagel, R. & Vengeliene, V. New pharmacological treatment strategies for relapse prevention. Curr. Top. Behav. Neurosci. 13, 583–609 (2013).
Wee, S. & Koob, G. F. The role of the dynorphin-κ opioid system in the reinforcing effects of drugs of abuse. Psychopharmacol. (Berl.) 210, 121–135 (2010).
Mann, K., Bladstrom, A., Torup, L., Gual, A. & van den Brink, W. Extending the treatment options in alcohol dependence: a randomized controlled study of as-needed nalmefene. Biol. Psychiatry 73, 706–713 (2013).
Spanagel, R. et al. An integrated genome research network for studying the genetics of alcohol addiction. Addict. Biol. 15, 369–379 (2010).
Lidö, H. H., Marston, H., Ericson, M. & Soderpalm, B. The glycine reuptake inhibitor Org24598 and acamprosate reduce ethanol intake in the rat; tolerance development to acamprosate but not to Org24598. Addict. Biol. 17, 897–907 (2012).
Vengeliene, V., Bachteler, D., Danysz, W. & Spanagel, R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology 48, 822–829 (2005).
Gass, J. T. & Olive, M. F. Glutamatergic substrates of drug addiction and alcoholism. Biochem. Pharmacol. 75, 218–265 (2008).
Sesack, S. R. & Grace, A. A. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35, 27–47 (2010).
Engblom, D. et al. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron 59, 497–508 (2008).
Sindrup, S. H., Otto, M., Finnerup, N. B. & Jensen, T. S. Antidepressants in the treatment of neuropathic pain. Bas. Clin. Pharmacol. Toxicol. 96, 399–409 (2005).
Núñez, E., Lopez-Corcuera, B., Vazquez, J., Gimenez, C. & Aragon, C. Differential effects of the tricyclic antidepressant amoxapine on glycine uptake mediated by the recombinant GLYT1 and GLYT2 glycine transporters. Br. J. Pharmacol. 129, 200–206 (2000).
Tanabe, M., Takasu, K., Yamaguchi, S., Kodama, D. & Ono, H. Glycine transporter inhibitors as a potential therapeutic strategy for chronic pain with memory impairment. Anesthesiology 108, 929–937 (2008).
Morita, K. et al. Spinal antiallodynia action of glycine transporter inhibitors in neuropathic pain models in mice. J. Pharmacol. Exp. Ther. 326, 633–645 (2008).
Dohi, T., Morita, K., Kitayama, T., Motoyama, N. & Morioka, N. Glycine transporter inhibitors as a novel drug discovery strategy for neuropathic pain. Pharmacol. Ther. 123, 54–79 (2009). This review examines the evidence supporting the role of glycine reuptake inhibition in pain control and describes the relevant neural circuits of pain processing that we have presented in this article.
Yoshikawa, S., Oguchi, T., Funahashi, Y., de Groat, W. C. & Yoshimura, N. Glycine transporter type 2 (GlyT2) inhibitor ameliorates bladder overactivity and nociceptive behavior in rats. Eur. Urol. 62, 704–712 (2012).
Cheng, W. et al. Intracerebroventricular or intrathecal injection of glycine produces analgesia in thermal nociception and chemical nociception via glycine receptors. Eur. J. Pharmacol. 614, 44–49 (2009).
Wallace, M. S. et al. A randomized, double-blind, placebo-controlled trial of a glycine antagonist in neuropathic pain. Neurology 59, 1694–1700 (2002).
Beyer, C., Komisaruk, B. R., Lopez-Colome, A. M. & Caba, M. Administration of AP5, a glutamate antagonist, unmasks glycine analgesic actions in the rat. Pharmacol. Biochem. Behav. 42, 229–232 (1992).
Centeno, M. V., Mutso, A., Millecamps, M. & Apkarian, A. V. Prefrontal cortex and spinal cord mediated anti-neuropathy and analgesia induced by sarcosine, a glycine-T1 transporter inhibitor. Pain 145, 176–183 (2009).
Kodama, D., Ono, H. & Tanabe, M. Increased hippocampal glycine uptake and cognitive dysfunction after peripheral nerve injury. Pain 152, 809–817 (2011).
Werdehausen, R. et al. Lidocaine metabolites inhibit glycine transporter 1: a novel mechanism for the analgesic action of systemic lidocaine? Anesthesiology 116, 147–158 (2012).
Hermanns, H. et al. Differential effects of spinally applied glycine transporter inhibitors on nociception in a rat model of neuropathic pain. Neurosci. Lett. 445, 214–219 (2008).
Haranishi, Y., Hara, K., Terada, T., Nakamura, S. & Sata, T. The antinociceptive effect of intrathecal administration of glycine transporter-2 inhibitor ALX1393 in a rat acute pain model. Anesth. Analg. 110, 615–621 (2010).
Nishikawa, Y., Sasaki, A. & Kuraishi, Y. Blockade of glycine transporter GlyT2, but not GlyT1, ameliorates dynamic and static mechanical allodynia in mice with herpetic or postherpetic pain. J. Pharmacol. Sci. 112, 352–360 (2010).
Munts, A. G. et al. Intrathecal glycine for pain and dystonia in complex regional pain syndrome. Pain 146, 199–204 (2009).
Waziri, R. Glycine therapy of schizophrenia: some caveats. Biol. Psychiatry 39, 155–156 (1996).
Harsing, L. G. Jr et al. Glycine transporter type-1 and its inhibitors. Curr. Med. Chem. 13, 1017–1044 (2006).
Yang, C. R. & Svensson, K. A. Allosteric modulation of NMDA receptor via elevation of brain glycine and D-serine: the therapeutic potentials for schizophrenia. Pharmacol. Ther. 120, 317–332 (2008).
Ouellet, D., Sutherland, S., Wang, T., Griffini, P. & Murthy, V. First-time-in-human study with GSK1018921, a selective GlyT1 inhibitor: relationship between exposure and dizziness. Clin. Pharmacol. Ther. 90, 597–604 (2011).
Atkinson, B. N. et al. ALX 5407: a potent, selective inhibitor of the hGlyT1 glycine transporter. Mol. Pharmacol. 60, 1414–1420 (2001).
Howard, A. et al. Glycine transporter GLYT1 is essential for glycine-mediated protection of human intestinal epithelial cells against oxidative damage. J. Physiol. 588, 995–1009 (2010).
Javitt, D. C. Is the glycine site half saturated or half unsaturated? Effects of glutamatergic drugs in schizophrenia patients. Curr. Opin. Psychiatry 19, 151–157 (2006).
French, J. A. & Faught, E. Rational polytherapy. Epilepsia 50 (Suppl. 8), 63–68 (2009).
Tamminga, C. A. When is polypharmacy an advantage? Am. J. Psychiatry 168, 663 (2011).
Ritsner, M. S. (ed) Polypharmacy in Psychiatry Practice, Volume II: Use of Polypharmacy in the “Real World” (Springer, 2013).
D'Souza, D. C. et al. Glycine transporter inhibitor attenuates the psychotomimetic effects of ketamine in healthy males: preliminary evidence. Neuropsychopharmacology 37, 1036–1046 (2012).
Pinard, E. et al. Discovery of benzoylpiperazines as a novel class of potent and selective GlyT1 inhibitors. Bioorg. Med. Chem. Lett. 18, 5134–5139 (2008).
Gray, J. A. & Nicoll, R. A. Thinking outside the synapse: glycine at extrasynaptic NMDA receptors. Cell 150, 455–456 (2012).
Mothet, J. P. et al. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc. Natl Acad. Sci. USA 97, 4926–4931 (2000).
Rosenberg, D. et al. Neuronal D-serine and glycine release via the ASC-1 transporter regulates NMDA receptor-dependent synaptic activity. J. Neurosci. 33, 3533–3544 (2013).
Marchetti, M. et al. ATP binding to human serine racemase is cooperative and modulated by glycine. FEBS J. http://dx.doi.org/10.1111/febs.12510 (2013).
Musante, V., Summa, M., Cunha, R. A., Raiteri, M. & Pittaluga, A. Pre-synaptic glycine GlyT1 transporter–NMDA receptor interaction: relevance to NMDA autoreceptor activation in the presence of Mg2+ ions. J. Neurochem. 117, 516–527 (2011).
Duguid, I. C. & Smart, T. G. in Biology of the NMDA Receptor (ed. Dongen, A. M. V. ) 313–328 (CRC Press, 2009).
Gelfin, E. et al. D-serine adjuvant treatment alleviates behavioural and motor symptoms in Parkinson's disease. Int. J. Neuropsychopharmacol. 15, 543–549 (2012).
Schneider, J. S., Tinker, J. P., Van Velson, M. & Giardiniere, M. Effects of the partial glycine agonist D-cycloserine on cognitive functioning in chronic low dose MPTP-treated monkeys. Brain Res. 860, 190–194 (2000).
Jimenez, E. et al. P2Y purinergic regulation of the glycine neurotransmitter transporters. J. Biol. Chem. 286, 10712–10724 (2011).
Barkess, G. et al. The chromatin-binding protein HMGN3 stimulates histone acetylation and transcription across the Glyt1 gene. Biochem. J. 442, 495–505 (2012).
Verissimo, C. et al. Nonketotic hyperglycinemia: a cause of encephalopathy in children. J. Child Neurol. 28, 251–254 (2013).
Applegarth, D. A. & Toone, J. R. Glycine encephalopathy (nonketotic hyperglycinemia): comments and speculations. Am. J. Med. Genet. A 140A, 186–188 (2006).
Conter, C. et al. Genetic heterogeneity of the GLDC gene in 28 unrelated patients with glycine encephalopathy. J. Inherit Metab. Dis. 29, 135–142 (2006).
Mayor, F. Jr et al. Atypical nonketotic hyperglycinemia with a defective glycine transport system in nervous tissue. Neurochem. Pathol. 2, 233–249 (1984).
Jursky, F. & Nelson, N. Developmental expression of the glycine transporters GLYT1 and GLYT2 in mouse brain. J. Neurochem. 67, 336–344 (1996).
Shiang, R. et al. Mutations in the α1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nature Genet. 5, 351–358 (1993).
Chung, S. K. et al. Pathophysiological mechanisms of dominant and recessive GLRA1 mutations in hyperekplexia. J. Neurosci. 30, 9612–9620 (2010).
Chung, S. K. et al. GLRB is the third major gene of effect in hyperekplexia. Hum. Mol. Genet. 22, 927–940 (2013).
James, V. M. et al. Novel missense mutations in the glycine receptor beta subunit gene (GLRB) in startle disease. Neurobiol. Dis. 52, 137–149 (2013).
Gimenez, C. et al. A novel dominant hyperekplexia mutation Y705C alters trafficking and biochemical properties of the presynaptic glycine transporter GlyT2. J. Biol. Chem. 287, 28986–29002 (2012).
Charlier, C. et al. Highly effective SNP-based association mapping and management of recessive defects in livestock. Nature Genet. 40, 449–454 (2008).
Gill, J. L. et al. Startle disease in Irish wolfhounds associated with a microdeletion in the glycine transporter GlyT2 gene. Neurobiol. Dis. 43, 184–189 (2011).
Bohme, I. & Luddens, H. The inhibitory neural circuitry as target of antiepileptic drugs. Curr. Med. Chem. 8, 1257–1274 (2001).
Seiler, N. & Sarhan, S. Synergistic anticonvulsant effects of a GABA agonist and glycine. Gen. Pharmacol. 15, 367–369 (1984).
Halsey, M. J., Little, H. J. & Wardley-Smith, B. Systemically administered glycine protects against strychnine convulsions, but not the behavioural effects of high pressure, in mice. J. Physiol. 408, 431–441 (1989).
Peterson, S. L. Glycine potentiates the anticonvulsant action of diazepam and phenobarbital in kindled amygdaloid seizures of rats. Neuropharmacology 25, 1359–1363 (1986).
Larson, A. A. & Beitz, A. J. Glycine potentiates strychnine-induced convulsions: role of NMDA receptors. J. Neurosci. 8, 3822–3826 (1988).
Semyanov, A., Walker, M. C., Kullmann, D. M. & Silver, R. A. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 27, 262–269 (2004).
Eichler, S. A. et al. Splice-specific roles of glycine receptor α3 in the hippocampus. Eur. J. Neurosci. 30, 1077–1091 (2009).
Chen, R. Q. et al. Role of glycine receptors in glycine-induced LTD in hippocampal CA1 pyramidal neurons. Neuropsychopharmacology 36, 1948–1958 (2011).
Zhang, L. H., Gong, N., Fei, D., Xu, L. & Xu, T. L. Glycine uptake regulates hippocampal network activity via glycine receptor-mediated tonic inhibition. Neuropsychopharmacology 33, 701–711 (2008). This paper shows that inhibition of GlyT1 in the hippocampus facilitates the induction of NMDA receptor-dependent LTP and simultaneously potentiates neuronal inhibition via glycine receptors, thereby indicating that GlyT1 can effectively fine-tune both excitatory and inhibitory activities within the hippocampus through the regulation of glycine trafficking.
Socała, K., Nieoczym, D., Rundfeldt, C. & Wlaz, P. Effects of sarcosine, a glycine transporter type 1 inhibitor, in two mouse seizure models. Pharmacol. Rep. 62, 392–397 (2010).
Kalinichev, M. et al. Glycine transporter 1 (GlyT1) inhibitors exhibit anticonvulsant properties in the rat maximal electroshock threshold (MEST) test. Brain Res. 1331, 105–113 (2010).
Peterson, S. L. Anticonvulsant drug potentiation by glycine in maximal electroshock seizures is mimicked by D-serine and antagonized by 7-chlorokynurenic acid. Eur. J. Pharmacol. 199, 341–348 (1991).
Peterson, S. L. & Schwade, N. D. The anticonvulsant activity of D-cycloserine is specific for tonic convulsions. Epilepsy Res. 15, 141–148 (1993).
Wlaź, P., Baran, H. & Loscher, W. Effect of the glycine/NMDA receptor partial agonist, D-cycloserine, on seizure threshold and some pharmacodynamic effects of MK-801 in mice. Eur. J. Pharmacol. 257, 217–225 (1994).
Xue, J. G. et al. NMDA receptor activation enhances inhibitory GABAergic transmission onto hippocampal pyramidal neurons via presynaptic and postsynaptic mechanisms. J. Neurophysiol. 105, 2897–2906 (2011).
Perry, T. L. & Hansen, S. Amino acid abnormalities in epileptogenic foci. Neurology 31, 872–876 (1981).
Foster, A. C. & Kemp, J. A. Neurobiology. Glycine maintains excitement. Nature 338, 377–378 (1989).
Croucher, M. J. & Bradford, H. F. 7-chlorokynurenic acid, a strychnine-insensitive glycine receptor antagonist, inhibits limbic seizure kindling. Neurosci. Lett. 118, 29–32 (1990).
Bristow, L. J. et al. Anticonvulsant and behavioral profile of L-701,324, a potent, orally active antagonist at the glycine modulatory site on the N-methyl-D-aspartate receptor complex. J. Pharmacol. Exp. Ther. 279, 492–501 (1996).
Stelzer, A., Slater, N. T. & ten Bruggencate, G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature 326, 698–701 (1987).
Endele, S. et al. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nature Genet. 42, 1021–1026 (2010).
Carvill, G. L. et al. GRIN2A mutations cause epilepsy-aphasia spectrum disorders. Nature Genet. 45, 1073–1076 (2013).
Lemke, J. R. et al. Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nature Genet. 45, 1067–1072 (2013).
Lesca, G. et al. GRIN2A mutations in acquired epileptic aphasia and related childhood focal epilepsies and encephalopathies with speech and language dysfunction. Nature Genet. 45, 1061–1066 (2013).
Muller, E., Le-Corronc, H. & Legendre, P. Extrasynaptic and postsynaptic receptors in glycinergic and GABAergic neurotransmission: a division of labor? Front. Mol. Neurosci. 1, 3 (2008).
Li, Y. & Xu, T. L. State-dependent cross-inhibition between anionic GABAA and glycine ionotropic receptors in rat hippocampal CA1 neurons. Neuroreport 13, 223–226 (2002).
Breustedt, J., Schmitz, D., Heinemann, U. & Schmieden, V. Characterization of the inhibitory glycine receptor on entorhinal cortex neurons. Eur. J. Neurosci. 19, 1987–1991 (2004).
Brodie, M. J. & Sills, G. J. Combining antiepileptic drugs — rational polytherapy? Seizure 20, 369–375 (2011).
Greenberg, W. M. et al. Adjunctive glycine in the treatment of obsessive-compulsive disorder in adults. J. Psychiatr. Res. 43, 664–670 (2009).
Wu, P. L., Tang, H. S., Lane, H. Y., Tsai, C. A. & Tsai, G. E. Sarcosine therapy for obsessive compulsive disorder: a prospective, open-label study. J. Clin. Psychopharmacol 31, 369–374 (2011).
Carlsson, M. L. On the role of cortical glutamate in obsessive-compulsive disorder and attention-deficit hyperactivity disorder, two phenomenologically antithetical conditions. Acta Psychiatr. Scand. 102, 401–413 (2000).
Wu, K., Hanna, G. L., Rosenberg, D. R. & Arnold, P. D. The role of glutamate signaling in the pathogenesis and treatment of obsessive-compulsive disorder. Pharmacol. Biochem. Behav. 100, 726–735 (2012).
Kushner, M. G. et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol. Psychiatry 62, 835–838 (2007).
Wilhelm, S. et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am. J. Psychiatry 165, 335–341 (2008).
Ressler, K. J. et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch. Gen. Psychiatry 61, 1136–1144 (2004).
Davis, M., Ressler, K., Rothbaum, B. O. & Richardson, R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol. Psychiatry 60, 369–375 (2006).
Otto, M. W. et al. Efficacy of D-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol. Psychiatry 67, 365–370 (2010).
Guastella, A. J. et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol. Psychiatry 63, 544–549 (2008).
Hofmann, S. G. et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch. Gen. Psychiatry 63, 298–304 (2006).
Nations, K. R. et al. Evaluation of the glycine transporter inhibitor Org 25935 as augmentation to cognitive-behavioral therapy for panic disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 73, 647–653 (2012).
Huang, C. C. et al. Inhibition of glycine transporter-1 as a novel mechanism for the treatment of depression. Biol. Psychiatry http://dx.doi.org/10.1016/j.biopsych.2013.02.020 (2013).
Heresco-Levy, U. et al. A randomized add-on trial of high-dose D-cycloserine for treatment-resistant depression. Int. J. Neuropsychopharmacol. 16, 501–506 (2013).
Burgdorf, J. et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 38, 729–742 (2013).
Catena-Dell'osso, M., Fagiolini, A., Rotella, F., Baroni, S. & Marazziti, D. Glutamate system as target for development of novel antidepressants. CNS Spectr. 18, 188–198 (2013).
Perez-Siles, G. et al. Molecular basis of the differential interaction with lithium of glycine transporters GLYT1 and GLYT2. J. Neurochem. 118, 195–204 (2011).
Poleszak, E. et al. A complex interaction between glycine/NMDA receptors and serotonergic/noradrenergic antidepressants in the forced swim test in mice. J. Neural Transm. 118, 1535–1546 (2011).
Vanhoof, G. et al. Glycine transporter 1 heterozygotes display reduced anxiety in novel environments. Program No. 504.13. 2004 Neuroscience Meeting Planner (San Diego, California; Society for Neuroscience, 2004).
Dubroqua, S. et al. Impacts of forebrain neuronal glycine transporter 1 disruption in the senescent brain: evidence for age-dependent phenotypes in Pavlovian learning. Behav. Neurosci. 124, 839–850 (2010).
Jacobs, B. L., van Praag, H. & Gage, F. H. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol. Psychiatry 5, 262–269 (2000).
Harvey, R. J. et al. GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304, 884–887 (2004). This key study reveals a novel biological role for GlyRα3 in central inflammatory pain sensitization.
Rácz, I., Schütz, B., Abo-Salem, O. M. & Zimmer, A. Visceral, inflammatory and neuropathic pain in glycine receptor α3-deficient mice. Neuroreport 16, 2025–2028 (2005).
Hösl, K. et al. Spinal prostaglandin E receptors of the EP2 subtype and the glycine receptor α3 subunit, which mediate central inflammatory hyperalgesia, do not contribute to pain after peripheral nerve injury or formalin injection. Pain 126, 46–53 (2006).
Harvey, V. L., Caley, A., Muller, U. C., Harvey, R. J. & Dickenson, A. H. A selective role for α3 subunit glycine receptors in inflammatory pain. Front. Mol. Neurosci. 2, 14 (2009).
Morita, K., Kitayama, T., Morioka, N. & Dohi, T. Glycinergic mediation of tactile allodynia induced by platelet-activating factor (PAF) through glutamate-NO-cyclic GMP signalling in spinal cord in mice. Pain 138, 525–536 (2008).
Xiong, W. et al. Cannabinoid potentiation of glycine receptors contributes to cannabis-induced analgesia. Nature Chem. Biol. 7, 296–303 (2011). This pivotal study maps the binding site for cannabinoids on glycine receptors and demonstrates that cannabinoid-induced analgesia is absent in mice lacking GlyRα3 but not in cannabinoid receptor 1 (CB1R)- and CB2R-knockout mice.
Zeilhofer, H. U. et al. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J. Comp. Neurol. 482, 123–141 (2005).
Fuziwara, S., Inoue, K. & Denda, M. NMDA-type glutamate receptor is associated with cutaneous barrier homeostasis. J. Invest. Dermatol. 120, 1023–1029 (2003).
Acknowledgements
R.J.H. is supported by the Medical Research Council (Grants G0500833, G0601585 and J004049) and Action Medical Research (Grant 1966). B.K.Y. is supported by the US National Institutes of Health (NIH) (Grant R01MH083973), and the Legacy Foundation Grant, and would like to acknowledge former support from the Swiss National Science Foundation (Grant: 3100-066855), the Swiss National Center of Competence in Research Neural Plasticity and Repair, and the Swiss Federal Institute of Technology, Zurich.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Interneurons
-
Nerve cells that form short, local (mostly inhibitory) connections with nearby neurons within a single area of the brain. They are to be distinguished from principal or projection neurons that extend axonal connections to distal regions of the nervous system.
- Forebrain
-
An area of the brain that comprises two main sections: the diencephalon and the telencephalon. It covers the entire cerebral cortex, limbic cortices, thalamus and striatum, and governs sensory and motor information processing, language and memory function.
- Affects
-
The experience and expression of feelings or emotions that can be positive or negative; the psychological processes involved are often contrasted with (but cannot be entirely disentangled from) cognition and thought processes.
- Executive functions
-
A broad number of cognitive processes including planning, working memory, attention, problem solving, reasoning, inhibition, mental flexibility and task switching, as well as the initiation and monitoring of these cognitive processes.
- Positive allosteric modulatory sites
-
Binding sites on a receptor or channel that are distinct from the active site. These sites allow regulatory ligands (that have an affinity to these sites) to enhance the biochemical response that is associated with the activation of the active site.
- Glutamate hypothesis
-
A hypothesis of the pathophysiology of schizophrenia that attributes the production of schizophrenia symptoms to the underactivity of the glutamatergic neurotransmission system — in particular, to the transmission of neural signals via NMDA (N-methyl-D-aspartate) receptors.
- Glyt1+/− mice
-
Constitutive heterozygous knockout mice that lack one allele of the glycine transporter 1 gene (Glyt1; also known as Slc6a9), and have GlyT1 expression levels that are ∼50% lower than those of wild-type mice.
- Glyt1fl/fl:CamKIIα–Cre+/− conditional knockout mice
-
Conditional knockout mutant mice in which the expression of the glycine transporter 1 gene (Glyt1; also known as Slc6a9) is disrupted in forebrain principal neurons.
- Telencephalon
-
The largest and most highly developed part of the human brain that is involved in many higher brain functions including intelligence, personality and interpretation of sensory information; often referred to as the cerebrum or cerebral cortex in the literature.
- Nucleus accumbens
-
The main component of the ventral (or limbic) striatum that receives ascending dopaminergic innervation and limbic glutamatergic inputs, and is involved in reward, motivation and attention.
- Clozapine
-
8-chloro-11-(4-methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine); the prototypical second-generation antipsychotic drug, which is associated with fewer motor side effects. It is commonly prescribed to patients with schizophrenia who do not respond to first-generation antipsychotic drugs.
- Glyt1fl/fl:EMX1–Cre+/− mice
-
Mice in which expression of the glycine transporter 1 gene (Glyt1; also known as Slc6a9) in the dorsal telencephalon is disrupted so that all cells in the cerebral cortex including the limbic cortices (the hippocampus and amygdala) lack GlyT1, but GlyT1 expression is retained in the striatum.
- Pavlovian fear conditioning
-
A behavioural paradigm in which organisms learn to anticipate an aversive event by learning to associate the aversive stimulus (for example, an electrical shock) with a neutral stimulus (for example, a tone), which results in the expression of fear responses (for example, freezing or immobility) to the originally neutral stimulus.
- Porsolt forced swim test
-
A rodent test that is used for determining the effects of antidepressant drugs. Such drugs can increase the length of time before a rodent gives up swimming or struggling and begins to float when left in a cylinder of water without any possibility of escaping.
- Prepulse inhibition
-
A cross-species translational paradigm that is sensitive to an early attentional deficit in schizophrenia, which measures the inhibition of a startle response to an intense acoustic stimulus (that is, a pulse) induced by a weak non-startling stimulus (that is, a prepulse).
- Adjunctive design
-
A clinical trial that is designed to test the efficacy of a given treatment that is administered as an add-on to the medications that the patients are already receiving; clinical outcomes are compared with placebo add-on.
- Negative symptom factor score
-
A summary score, derived from the Positive and Negative Syndrome Scale (PANSS), to index the change in the negative symptoms of schizophrenia between the treatment and placebo arms in a clinical trial.
- Complete Freund's adjuvant
-
A water-in-oil emulsion that contains heat-killed mycobacteria or mycobacterial cell wall components, and is used to induce an inflammatory response and pain in animal models.
- Gabapentin
-
(2-[1-(aminomethyl)cyclohexyl]acetic acid). An analogue of GABA (γ-aminobutyric acid) that is currently used for the treatment of neuropathic pain but was originally developed as an anti-epileptic drug.
- Allodynia
-
A form of pain caused by a thermal or physical stimulus that does not normally elicit pain sensation and can occur following injury.
- Epileptic foci
-
Localized areas of the brain where a crucial number of pathologically discharging neurons reside; these neurons have the potential to give rise to widespread brain seizures.
- NMDA autoreceptors
-
NMDA (N-methyl-D-aspartate) receptors that are located on presynaptic nerve cell membranes. They may provide tonic facilitation of neural transmission, contribute to neural plasticity such as long-term depression or be part of a negative feedback loop to reduce glutamate release.
Rights and permissions
About this article
Cite this article
Harvey, R., Yee, B. Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence and pain. Nat Rev Drug Discov 12, 866–885 (2013). https://doi.org/10.1038/nrd3893
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3893
This article is cited by
-
The GlyT1-inhibitor Org 24598 facilitates the alcohol deprivation abolishing and dopamine elevating effects of bupropion + varenicline in rats
Journal of Neural Transmission (2024)
-
Investigating the shared genetic architecture and causal relationship between pain and neuropsychiatric disorders
Human Genetics (2023)
-
Luvadaxistat: A Novel Potent and Selective d-Amino Acid Oxidase Inhibitor Improves Cognitive and Social Deficits in Rodent Models for Schizophrenia
Neurochemical Research (2023)
-
Structural insights into the inhibition of glycine reuptake
Nature (2021)
-
Effects of systemic glycine on accumbal glycine and dopamine levels and ethanol intake in male Wistar rats
Journal of Neural Transmission (2021)