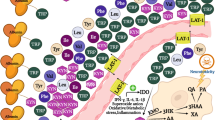
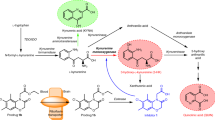
Key Points
-
Kynurenic acid has potentially neuroprotective actions, such as antagonism at NMDA (N-methyl-D-aspartate) receptors, inhibition of glutamate release and free radical scavenging. Pharmacological manipulations to harness the beneficial effects of this blood–brain barrier-impermeable agent include increasing the availability of its precursor L-kynurenine, modulation of the kynurenine pathway enzymes towards the synthesis of kynurenic acid, as well as the systemic administration of kynurenic acid analogues that have improved pharmacokinetic characteristics.
-
Most of the kynurenines are neuroactive; they have important roles in the functioning of glutamate receptors and in free radical production. NMDA receptor-mediated excitotoxicity and excessive free radical production are involved in neurodegenerative diseases such as Huntington's disease. The kynurenine pathway is altered in Huntington's disease to favour the production of toxic metabolites, and the possible therapeutic potential of its pharmacological modulation is currently under experimental investigation.
-
Glutamatergic neurotransmission is essential for the spinal and trigeminal processing of pain. Kynurenic acid has several antiglutamatergic properties. Therefore, the elevation of kynurenic acid levels could have therapeutic value in pain syndromes, including migraine. In this disorder, increases in kynurenic acid levels could suppress trigeminal and higher-order nociceptive neurons, modulate migraine generator nuclei in the brainstem and inhibit cortical spreading depression.
-
The activation of indoleamine 2,3-dioxygenase triggers a complex immunomodulatory response, which is involved in the mediation of physiological and pathological immune tolerance. The immunosuppressive effect of this enzyme is attributable to tryptophan depletion and the actions of downstream kynurenine metabolites. There is evidence to indicate that indoleamine 2,3-dioxygenase is activated in several inflammatory and autoimmune conditions, most probably serving as a self-protecting mechanism.
-
Experimental and indirect evidence suggests that the kynurenine pathway is overactivated in multiple sclerosis. As most of the immunotolerogenic metabolites of the kynurenine pathway exert neurotoxic and/or oligotoxic properties, the influence of this phenomenon on the pathogenesis and progression of multiple sclerosis necessitates further investigation.
-
In experimental models of multiple sclerosis, the activation of indoleamine 2,3-dioxygenase has shown beneficial effects; indeed, this mechanism may underlie the therapeutic potential of interferon-β in multiple sclerosis. Structurally similar synthetic derivatives of kynurenines have shown disease-modifying effects in recent clinical trials. The complex anti-inflammatory and neuroprotective properties of kynurenic acid and its analogues suggest that experimental screening of such compounds is warranted.
Abstract
Various pathologies of the central nervous system (CNS) are accompanied by alterations in tryptophan metabolism. The main metabolic route of tryptophan degradation is the kynurenine pathway; its metabolites are responsible for a broad spectrum of effects, including the endogenous regulation of neuronal excitability and the initiation of immune tolerance. This Review highlights the involvement of the kynurenine system in the pathology of neurodegenerative disorders, pain syndromes and autoimmune diseases through a detailed discussion of its potential implications in Huntington's disease, migraine and multiple sclerosis. The most effective preclinical drug candidates are discussed and attention is paid to currently under-investigated roles of the kynurenine pathway in the CNS, where modulation of kynurenine metabolism might be of therapeutic value.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wolf, H. The effect of hormones and vitamin B6 on urinary excretion of metabolites of the kynurenine pathway. Scand. J. Clin. Lab. Invest. Suppl. 136, 1–186 (1974).
Pardridge, W. M. Blood–brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem. Res. 23, 635–644 (1998).
Gal, E. M. & Sherman, A. D. Synthesis and metabolism of L-kynurenine in rat brain. J. Neurochem. 30, 607–613 (1978).
Fukui, S., Schwarcz, R., Rapoport, S. I., Takada, Y. & Smith, Q. R. Blood–brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J. Neurochem. 56, 2007–2017 (1991).
Guillemin, G. J. Quinolinic acid, the inescapable neurotoxin. FEBS J. 279, 1356–1365 (2012). This is a comprehensive review about the possible modes of action through which quinolinic acid can contribute to degenerative CNS conditions.
Santamaria, A. & Rios, C. MK-801, an N-methyl-D-aspartate receptor antagonist, blocks quinolinic acid-induced lipid peroxidation in rat corpus striatum. Neurosci. Lett. 159, 51–54 (1993).
Frumento, G. et al. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 196, 459–468 (2002).
Szalardy, L. et al. Mitochondrial disturbances, tryptophan metabolites and neurodegeneration: medicinal chemistry aspects. Curr. Med. Chem. 19, 1899–1920 (2012).
Terness, P. et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J. Exp. Med. 196, 447–457 (2002).
Mizdrak, J., Hains, P. G., Truscott, R. J., Jamie, J. F. & Davies, M. J. Tryptophan-derived ultraviolet filter compounds covalently bound to lens proteins are photosensitizers of oxidative damage. Free Radic. Biol. Med. 44, 1108–1119 (2008).
Szalardy, L. et al. Manipulating kynurenic acid levels in the brain — on the edge between neuroprotection and cognitive dysfunction. Curr. Top. Med. Chem. 12, 1797–1806 (2012). This article discusses the relationship between cognitive impairment and kynurenic acid levels in the brain, in the context of the various pharmacological approaches available to modulate the kynurenine pathway.
Prescott, C., Weeks, A. M., Staley, K. J. & Partin, K. M. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci. Lett. 402, 108–112 (2006).
Rozsa, E., Robotka, H., Vecsei, L. & Toldi, J. The Janus-face kynurenic acid. J. Neural. Transm. 115, 1087–1091 (2008).
Hilmas, C. et al. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: physiopathological implications. J. Neurosci. 21, 7463–7473 (2001).
Marchi, M., Risso, F., Viola, C., Cavazzani, P. & Raiteri, M. Direct evidence that release-stimulating α7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J. Neurochem. 80, 1071–1078 (2002).
Wang, J. et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 281, 22021–22028 (2006).
Lugo-Huitron, R. et al. On the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 33, 538–547 (2011).
Winn, P., Stone, T. W., Latimer, M., Hastings, M. H. & Clark, A. J. A comparison of excitotoxic lesions of the basal forebrain by kainate, quinolinate, ibotenate, N-methyl-D-aspartate or quisqualate, and the effects on toxicity of 2-amino-5-phosphonovaleric acid and kynurenic acid in the rat. Br. J. Pharmacol. 102, 904–908 (1991).
Nozaki, K. & Beal, M. F. Neuroprotective effects of L-kynurenine on hypoxia-ischemia and NMDA lesions in neonatal rats. J. Cereb. Blood Flow Metab. 12, 400–407 (1992).
Sas, K. et al. Kynurenine diminishes the ischemia-induced histological and electrophysiological deficits in the rat hippocampus. Neurobiol. Dis. 32, 302–308 (2008).
Gigler, G. et al. Neuroprotective effect of L-kynurenine sulfate administered before focal cerebral ischemia in mice and global cerebral ischemia in gerbils. Eur. J. Pharmacol. 564, 116–122 (2007).
Robotka, H. et al. Neuroprotection achieved in the ischaemic rat cortex with L-kynurenine sulphate. Life Sci. 82, 915–919 (2008).
Kemp, J. A. et al. 7-chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc. Natl Acad. Sci. USA 85, 6547–6550 (1988).
Zwilling, D. et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell 145, 863–874 (2011). This is the first report of a peripherally acting KMO inhibitor providing protection in transgenic models of neurodegeneration.
Stone, T. W. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol. Sci. 21, 149–154 (2000).
Fulop, F. et al. Syntheses, transformations and pharmaceutical applications of kynurenic acid derivatives. Curr. Med. Chem. 16, 4828–4842 (2009).
Borza, I. et al. Kynurenic acid amides as novel NR2B selective NMDA receptor antagonists. Bioorg. Med. Chem. Lett. 17, 406–409 (2007).
Marosi, M. et al. A novel kynurenic acid analogue: a comparison with kynurenic acid. An in vitro electrophysiological study. J. Neural. Transm. 117, 183–188 (2010).
Gellert, L. et al. Neuroprotection with a new kynurenic acid analog in the four-vessel occlusion model of ischemia. Eur. J. Pharmacol. 667, 182–187 (2011).
Zadori, D. et al. Neuroprotective effects of a novel kynurenic acid analogue in a transgenic mouse model of Huntington's disease. J. Neural. Transm. 118, 865–875 (2011). This is the first report of a systemically administered kynurenic acid analogue having neuroprotective properties in a transgenic mouse model of Huntington's disease.
Guidetti, P., Wu, H. Q. & Schwarcz, R. In situ produced 7-chlorokynurenate provides protection against quinolinate- and malonate-induced neurotoxicity in the rat striatum. Exp. Neurol. 163, 123–130 (2000). This is the first report showing that a halogenated kynurenic acid prodrug has a protective effect in toxin-mediated models of Huntington's disease.
Beal, M. F. et al. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature 321, 168–171 (1986). This pioneering report shows that intrastriatal administration of quinolinic acid evokes selective neurodegeneration in a pattern that closely resembles that seen in Huntington's disease.
Guidetti, P., Luthi-Carter, R. E., Augood, S. J. & Schwarcz, R. Neostriatal and cortical quinolinate levels are increased in early grade Huntington's disease. Neurobiol. Dis. 17, 455–461 (2004).
Shear, D. A., Dong, J., Gundy, C. D., Haik-Creguer, K. L. & Dunbar, G. L. Comparison of intrastriatal injections of quinolinic acid and 3-nitropropionic acid for use in animal models of Huntington's disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 22, 1217–1240 (1998).
Schwarcz, R., Okuno, E., White, R. J., Bird, E. D. & Whetsell, W. O. Jr. 3-hydroxyanthranilate oxygenase activity is increased in the brains of Huntington disease victims. Proc. Natl Acad. Sci. USA 85, 4079–4081 (1988).
Sathyasaikumar, K. V. et al. Dysfunctional kynurenine pathway metabolism in the R6/2 mouse model of Huntington's disease. J. Neurochem. 113, 1416–1425 (2010).
Beal, M. F. et al. Kynurenic acid concentrations are reduced in Huntington's disease cerebral cortex. J. Neurol. Sci. 108, 80–87 (1992).
Beal, M. F., Matson, W. R., Swartz, K. J., Gamache, P. H. & Bird, E. D. Kynurenine pathway measurements in Huntington's disease striatum: evidence for reduced formation of kynurenic acid. J. Neurochem. 55, 1327–1339 (1990).
Jauch, D. et al. Dysfunction of brain kynurenic acid metabolism in Huntington's disease: focus on kynurenine aminotransferases. J. Neurol. Sci. 130, 39–47 (1995).
Heyes, M. P. et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 115, 1249–1273 (1992).
Luchowski, P., Luchowska, E., Turski, W. A. & Urbanska, E. M. 1-methyl-4-phenylpyridinium and 3-nitropropionic acid diminish cortical synthesis of kynurenic acid via interference with kynurenine aminotransferases in rats. Neurosci. Lett. 330, 49–52 (2002).
Csillik, A. et al. Effect of 3-nitropropionic acid on kynurenine aminotransferase in the rat brain. Exp. Neurol. 177, 233–241 (2002).
Sapko, M. T. et al. Endogenous kynurenate controls the vulnerability of striatal neurons to quinolinate: implications for Huntington's disease. Exp. Neurol. 197, 31–40 (2006).
Zeron, M. M. et al. Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington's disease. Neuron 33, 849–860 (2002).
Giorgini, F., Guidetti, P., Nguyen, Q., Bennett, S. C. & Muchowski, P. J. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nature Genet. 37, 526–531 (2005).
Campesan, S. et al. The kynurenine pathway modulates neurodegeneration in a Drosophila model of Huntington's disease. Curr. Biol. 21, 961–966 (2011).
Harris, C. A. et al. Modulation of striatal quinolinate neurotoxicity by elevation of endogenous brain kynurenic acid. Br. J. Pharmacol. 124, 391–399 (1998).
Santamaria, A. et al. Systemic dL-kynurenine and probenecid pretreatment attenuates quinolinic acid-induced neurotoxicity in rats. Neuropharmacology 35, 23–28 (1996).
Hardingham, G. E., Fukunaga, Y. & Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nature Neurosci. 5, 405–414 (2002). This paper discusses the opposing downstream effects of synaptic and extrasynaptic NMDA receptor activation, in terms of neuronal survival, and the potential roles they have in neurodegeneration.
Okamoto, S. et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nature Med. 15, 1407–1413 (2009).
Peres, M. F. et al. Cerebrospinal fluid glutamate levels in chronic migraine. Cephalalgia 24, 735–739 (2004).
Cananzi, A. R., D'Andrea, G., Perini, F., Zamberlan, F. & Welch, K. M. Platelet and plasma levels of glutamate and glutamine in migraine with and without aura. Cephalalgia 15, 132–135 (1995).
Pietrobon, D. Familial hemiplegic migraine. Neurotherapeutics 4, 274–284 (2007).
Anttila, V. et al. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nature Genet. 42, 869–873 (2010).
Ligthart, L. et al. Meta-analysis of genome-wide association for migraine in six population-based European cohorts. Eur. J. Hum. Genet. 19, 901–907 (2011).
Chasman, D. I. et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nature Genet. 43, 695–698 (2011).
Pardutz, A. et al. Kynurenines and headache. J. Neural. Transm. 119, 285–296 (2012).
Knyihar-Csillik, E. et al. Kynurenine aminotransferase in the supratentorial dura mater of the rat: effect of stimulation of the trigeminal ganglion. Exp. Neurol. 186, 242–247 (2004).
Fejes, A., Pardutz, A., Toldi, J. & Vecsei, L. Kynurenine metabolites and migraine: experimental studies and therapeutic perspectives. Curr. Neuropharmacol. 9, 376–387 (2011).
Vamos, E. et al. Kynurenate derivative attenuates the nitroglycerin-induced CamKIIα and CGRP expression changes. Headache 50, 834–843 (2010). This study demonstrates the efficiency of a kynurenic acid analogue in mitigating some characteristic histochemical alterations in an experimental migraine model.
Knyihar-Csillik, E. et al. Kynurenine in combination with probenecid mitigates the stimulation-induced increase of c-fos immunoreactivity of the rat caudal trigeminal nucleus in an experimental migraine model. J. Neural. Transm. 114, 417–421 (2007).
Knyihar-Csillik, E. et al. Prevention of electrical stimulation-induced increase of c-fos immunoreaction in the caudal trigeminal nucleus by kynurenine combined with probenecid. Neurosci. Lett. 418, 122–126 (2007).
Knyihar-Csillik, E. et al. The kynurenate analog SZR-72 prevents the nitroglycerol-induced increase of c-fos immunoreactivity in the rat caudal trigeminal nucleus: comparative studies of the effects of SZR-72 and kynurenic acid. Neurosci. Res. 61, 429–432 (2008).
Vamos, E. et al. L-kynurenine combined with probenecid and the novel synthetic kynurenic acid derivative attenuate nitroglycerin-induced nNOS in the rat caudal trigeminal nucleus. Neuropharmacology 57, 425–429 (2009).
Kiss, C., Shepard, P. D., Bari, F. & Schwarcz, R. Cortical spreading depression augments kynurenate levels and reduces malonate toxicity in the rat cortex. Brain Res. 1002, 129–135 (2004).
Faria, L. C. & Mody, I. Protective effect of ifenprodil against spreading depression in the mouse entorhinal cortex. J. Neurophysiol. 92, 2610–2614 (2004).
Wang, Y. et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nature Med. 16, 279–285 (2010).
Sas, K., Csete, K., Vecsei, L. & Papp, J. G. Effect of systemic administration of L-kynurenine on corticocerebral blood flow under normal and ischemic conditions of the brain in conscious rabbits. J. Cardiovasc. Pharmacol. 42, 403–409 (2003).
Akerman, S., Williamson, D. J., Kaube, H. & Goadsby, P. J. Nitric oxide synthase inhibitors can antagonize neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. Br. J. Pharmacol. 137, 62–68 (2002).
Fujigaki, S. et al. L-tryptophan-L-kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in γ interferon-gene-deficient mice: cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase. Infect. Immun. 70, 779–786 (2002).
Prandota, J. Recurrent headache as the main symptom of acquired cerebral toxoplasmosis in nonhuman immunodeficiency virus-infected subjects with no lymphadenopathy: the parasite may be responsible for the neurogenic inflammation postulated as a cause of different types of headaches. Am. J. Ther. 14, 63–105 (2007).
Koseoglu, E., Yazar, S. & Koc, I. Is Toxoplasma gondii a causal agent in migraine? Am. J. Med. Sci. 338, 120–122 (2009).
Mandi, Y. & Vecsei, L. The kynurenine system and immunoregulation. J. Neural. Transm. 119, 197–209 (2011).
Kwidzinski, E. & Bechmann, I. IDO expression in the brain: a double-edged sword. J. Mol. Med. 85, 1351–1359 (2007). This article comprehensively reviews the possible roles of IDO activation in the CNS, focusing on the pathology of multiple sclerosis.
Bozza, S. et al. A crucial role for tryptophan catabolism at the host/Candida albicans interface. J. Immunol. 174, 2910–2918 (2005).
Potula, R. et al. Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in an animal model of HIV-1 encephalitis. Blood 106, 2382–2390 (2005).
Hwu, P. et al. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J. Immunol. 164, 3596–3599 (2000).
Mellor, A. L. et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int. Immunol. 16, 1391–1401 (2004).
Baban, B. et al. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int. Immunol. 17, 909–919 (2005).
Munn, D. H. et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 189, 1363–1372 (1999).
Lee, G. K. et al. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 107, 452–460 (2002).
Fallarino, F. et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 9, 1069–1077 (2002).
Bauer, T. M. et al. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl. Int. 18, 95–100 (2005).
Della Chiesa, M. et al. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 108, 4118–4125 (2006).
Fallarino, F. et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naive T cells. J. Immunol. 176, 6752–6761 (2006).
Pallotta, M. T. et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nature Immunol. 12, 870–878 (2011).
Belladonna, M. L. et al. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J. Immunol. 177, 130–137 (2006).
Yan, Y. et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. 185, 5953–5961 (2010).
Mezrich, J. D. et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 185, 3190–3198 (2010).
Forouzandeh, F., Jalili, R. B., Germain, M., Duronio, V. & Ghahary, A. Differential immunosuppressive effect of indoleamine 2,3-dioxygenase (IDO) on primary human CD4+ and CD8+ T cells. Mol. Cell. Biochem. 309, 1–7 (2008).
Kanai, M., Hiroshi, F. & Toshikazu, N. Implication of tryptophan 2,3-dioxygenase and its novel variants in the hippocampus and cerebellum during the developing and adult brain. Int. J. Tryptophan Res. 3, 141–149 (2010).
Guillemin, G. J. et al. Characterization of the kynurenine pathway in human neurons. J. Neurosci. 27, 12884–12892 (2007).
Ball, H. J. et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene 396, 203–213 (2007).
Metz, R. et al. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 67, 7082–7087 (2007).
Lob, S. et al. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood 111, 2152–2154 (2008).
Lob, S. et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol. Immunother. 58, 153–157 (2009).
Aranami, T. & Yamamura, T. Th17 cells and autoimmune encephalomyelitis (EAE/MS). Allergol. Int. 57, 115–120 (2008).
Sakurai, K., Zou, J. P., Tschetter, J. R., Ward, J. M. & Shearer, G. M. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 129, 186–196 (2002).
Kwidzinski, E. et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 19, 1347–1349 (2005).
Platten, M. et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science 310, 850–855 (2005). This study demonstrates that an orally active synthetic anthranilic acid derivative reverses paralysis in mice with experimental autoimmune encephalitis.
Mai, J., Wang, H. & Yang, X. F. Th 17 cells interplay with Foxp3+ Tregs in regulation of inflammation and autoimmunity. Front. Biosci. 15, 986–1006 (2010).
Xiao, B. G. et al. Therapeutic potential of IFN-γ-modified dendritic cells in acute and chronic experimental allergic encephalomyelitis. Int. Immunol. 16, 13–22 (2004).
Platten, M., Ho, P. P. & Steinman, L. Anti-inflammatory strategies for the treatment of multiple sclerosis — tryptophan catabolites may hold the key. Inflammation 3, 401–408 (2006).
Polman, C. et al. Treatment with laquinimod reduces development of active MRI lesions in relapsing MS. Neurology 64, 987–991 (2005).
Comi, G. et al. Effect of laquinimod on MRI-monitored disease activity in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 371, 2085–2092 (2008).
Comi, G. et al. Pooled analyses from the ALLEGRO and BRAVO trials on the safety and tolerability of laquinimod as a multiple sclerosis treatment. Neurology 78, Abstract P04. 132 (2012).
O'Connor, P. W. et al. A Phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 66, 894–900 (2006).
O'Connor, P. et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N. Engl. J. Med. 365, 1293–1303 (2011).
Confavreux, C. et al. Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years. Mult. Scler. 18, 1278–1289 (2012).
Flanagan, E. M., Erickson, J. B., Viveros, O. H., Chang, S. Y. & Reinhard, J. F. Jr. Neurotoxin quinolinic acid is selectively elevated in spinal cords of rats with experimental allergic encephalomyelitis. J. Neurochem. 64, 1192–1196 (1995).
Chiarugi, A., Cozzi, A., Ballerini, C., Massacesi, L. & Moroni, F. Kynurenine 3-mono-oxygenase activity and neurotoxic kynurenine metabolites increase in the spinal cord of rats with experimental allergic encephalomyelitis. Neuroscience 102, 687–695 (2001).
Alberati-Giani, D., Ricciardi-Castagnoli, P., Kohler, C. & Cesura, A. M. Regulation of the kynurenine metabolic pathway by interferon-γ in murine cloned macrophages and microglial cells. J. Neurochem. 66, 996–1004 (1996).
Xiao, B. G., Liu, X. & Link, H. Antigen-specific T cell functions are suppressed over the estrogen-dendritic cell-indoleamine 2,3-dioxygenase axis. Steroids 69, 653–659 (2004).
Lim, C. K., Brew, B. J., Sundaram, G. & Guillemin, G. J. Understanding the roles of the kynurenine pathway in multiple sclerosis progression. Int. J. Tryptophan Res. 3, 157–167 (2010).
Monaco, F., Fumero, S., Mondino, A. & Mutani, R. Plasma and cerebrospinal fluid tryptophan in multiple sclerosis and degenerative diseases. J. Neurol. Neurosurg. Psychiatry 42, 640–641 (1979).
Ott, M., Demisch, L., Engelhardt, W. & Fischer, P. A. Interleukin-2, soluble interleukin-2-receptor, neopterin, L-tryptophan and β2-microglobulin levels in CSF and serum of patients with relapsing-remitting or chronic-progressive multiple sclerosis. J. Neurol. 241, 108–114 (1993).
Amirkhani, A. et al. Interferon-β affects the tryptophan metabolism in multiple sclerosis patients. Eur. J. Neurol. 12, 625–631 (2005). This paper shows that administration of IFNβ to patients with multiple sclerosis is associated with a subsequent increase in IDO activity in the bloodstream.
Rejdak, K. et al. Astrocytic activation in relation to inflammatory markers during clinical exacerbation of relapsing-remitting multiple sclerosis. J. Neural. Transm. 114, 1011–1015 (2007).
Rejdak, K. et al. Decreased level of kynurenic acid in cerebrospinal fluid of relapsing-onset multiple sclerosis patients. Neurosci. Lett. 331, 63–65 (2002).
Hartai, Z. et al. Kynurenine metabolism in multiple sclerosis. Acta Neurol. Scand. 112, 93–96 (2005).
Baran, H. et al. Lowered kynurenine aminotransferase activities in CNS of MS patients. Program No. 481.12 In: Society for Neuroscience 30th Annual Meeting (New Orleans, 2000).
Guillemin, G. J. et al. IFN-β1b induces kynurenine pathway metabolism in human macrophages: potential implications for multiple sclerosis treatment. J. Interferon Cytokine Res. 21, 1097–1101 (2001).
Sardar, A. M. & Reynolds, G. P. Frontal cortex indoleamine-2,3-dioxygenase activity is increased in HIV-1-associated dementia. Neurosci. Lett. 187, 9–12 (1995).
Cammer, W. Oligodendrocyte killing by quinolinic acid in vitro. Brain Res. 896, 157–160 (2001).
Tiszlavicz, Z. et al. Different inhibitory effects of kynurenic acid and a novel kynurenic acid analogue on tumour necrosis factor-α (TNF-α) production by mononuclear cells, HMGB1 production by monocytes and HNP1-3 secretion by neutrophils. Naunyn Schmiedebergs Arch. Pharmacol. 383, 447–455 (2011).
Varga, G. et al. N-methyl-D-aspartate receptor antagonism decreases motility and inflammatory activation in the early phase of acute experimental colitis in the rat. Neurogastroenterol. Motil. 22, 217–225 (2010).
Kaszaki, J. et al. Kynurenic acid inhibits intestinal hypermotility and xanthine oxidase activity during experimental colon obstruction in dogs. Neurogastroenterol. Motil. 20, 53–62 (2008).
Mazzoni, O. et al. Synthesis and pharmacological evaluation of some 4-oxo-quinoline-2-carboxylic acid derivatives as anti-inflammatory and analgesic agents. Arch. Pharm. 343, 561–569 (2010).
Fallarini, S., Magliulo, L., Paoletti, T., de Lalla, C. & Lombardi, G. Expression of functional GPR35 in human iNKT cells. Biochem. Biophys. Res. Commun. 398, 420–425 (2010).
Cantorna, M. T., Zhao, J. & Yang, L. Vitamin D, invariant natural killer T-cells and experimental autoimmune disease. Proc. Nutr. Soc. 71, 62–66 (2012).
Gigli, G., Caielli, S., Cutuli, D. & Falcone, M. Innate immunity modulates autoimmunity: type 1 interferon-β treatment in multiple sclerosis promotes growth and function of regulatory invariant natural killer T cells through dendritic cell maturation. Immunology 122, 409–417 (2007).
Oki, S. & Miyake, S. Invariant natural killer T (iNKT) cells in asthma: a novel insight into the pathogenesis of asthma and the therapeutic implication of glycolipid ligands for allergic diseases. Allergol. Int. 56, 7–14 (2007).
Opitz, C. A. et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203 (2011).
Han, Q. Li, J. & Li, J. pH dependence, substrate specificity and inhibition of human kynurenine aminotransferase I. Eur. J. Biochem. 271, 4804–4814 (2004).
Schwarcz, R., Bruno, J. P., Muchowski, P. J. & Wu, H. Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nature Rev. Neurosci. 13, 465–477 (2012).
Alkondon, M. et al. Age dependency of inhibition of α7 nicotinic receptors and tonically active N-methyl-D-aspartate receptors by endogenously produced kynurenic acid in the brain. J. Pharmacol. Exp. Ther. 337, 572–582 (2011).
Luchowska, E. et al. Endogenous level of kynurenic acid and activities of kynurenine aminotransferases following transient global ischemia in the gerbil hippocampus. Pol. J. Pharmacol. 55, 443–447 (2003).
Darlington, L. G. et al. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur. J. Neurosci. 26, 2211–2221 (2007).
Gold, A. B. et al. The relationship between indoleamine 2,3-dioxygenase activity and post-stroke cognitive impairment. J. Neuroinflamm. 8, 17 (2011).
Saito, K., Nowak, T. S. Jr., Markey, S. P. & Heyes, M. P. Mechanism of delayed increases in kynurenine pathway metabolism in damaged brain regions following transient cerebral ischemia. J. Neurochem. 60, 180–192 (1993).
Cozzi, A., Carpenedo, R. & Moroni, F. Kynurenine hydroxylase inhibitors reduce ischemic brain damage: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3,4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2yl]-benzenesulfonamide (Ro 61–8048) in models of focal or global brain ischemia. J. Cereb. Blood Flow Metab. 19, 771–777 (1999).
Baran, H., Jellinger, K. & Deecke, L. Kynurenine metabolism in Alzheimer's disease. J. Neural. Transm. 106, 165–181 (1999).
Hartai, Z. et al. Decreased serum and red blood cell kynurenic acid levels in Alzheimer's disease. Neurochem. Int. 50, 308–313 (2007).
Widner, B. et al. Degradation of tryptophan in neurodegenerative disorders. Adv. Exp. Med. Biol. 467, 133–138 (1999).
Widner, B. et al. Tryptophan degradation and immune activation in Alzheimer's disease. J. Neural. Transm. 107, 343–353 (2000).
Guillemin, G. J., Brew, B. J., Noonan, C. E., Takikawa, O. & Cullen, K. M. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer's disease hippocampus. Neuropathol. Appl. Neurobiol. 31, 395–404 (2005).
Guillemin, G. J., Smythe, G. A., Veas, L. A., Takikawa, O. & Brew, B. J. Aβ1–42 induces production of quinolinic acid by human macrophages and microglia. NeuroReport 14, 2311–2315 (2003).
Rahman, A. et al. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS ONE 4, e6344 (2009).
Carrillo-Mora, P., Mendez-Cuesta, L. A., Perez- De La Cruz, V., Fortoul- van Der Goes, T. I. & Santamaria, A. Protective effect of systemic L-kynurenine and probenecid administration on behavioural and morphological alterations induced by toxic soluble amyloid beta (25–35) in rat hippocampus. Behav. Brain Res. 210, 240–250 (2010).
Wu, H. Q., Lee, S. C. & Schwarcz, R. Systemic administration of 4-chlorokynurenine prevents quinolinate neurotoxicity in the rat hippocampus. Eur. J. Pharmacol. 390, 267–274 (2000).
Ogawa, T. et al. Kynurenine pathway abnormalities in Parkinson's disease. Neurology 42, 1702–1706 (1992).
Widner, B., Leblhuber, F. & Fuchs, D. Increased neopterin production and tryptophan degradation in advanced Parkinson's disease. J. Neural. Transm. 109, 181–189 (2002).
Knyihar-Csillik, E. et al. Decreased expression of kynurenine aminotransferase-I (KAT-I) in the substantia nigra of mice after 1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine (MPTP) treatment. Neuroscience 126, 899–914 (2004).
Lee do, Y. et al. Kynurenic acid attenuates MPP+-induced dopaminergic neuronal cell death via a Bax-mediated mitochondrial pathway. Eur. J. Cell Biol. 87, 389–397 (2008).
Miranda, A. F., Boegman, R. J., Beninger, R. J. & Jhamandas, K. Protection against quinolinic acid-mediated excitotoxicity in nigrostriatal dopaminergic neurons by endogenous kynurenic acid. Neuroscience 78, 967–975 (1997).
Silva-Adaya, D. et al. Protective effect of L-kynurenine and probenecid on 6-hydroxydopamine-induced striatal toxicity in rats: implications of modulating kynurenate as a protective strategy. Neurotoxicol. Teratol. 33, 303–312 (2011).
Merino, M., Vizuete, M. L., Cano, J. & Machado, A. The non-NMDA glutamate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione and 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline, but not NMDA antagonists, block the intrastriatal neurotoxic effect of MPP+. J. Neurochem. 73, 750–757 (1999).
Pearson, S. J. & Reynolds, G. P. Increased brain concentrations of a neurotoxin, 3-hydroxykynurenine, in Huntington's disease. Neurosci. Lett. 144, 199–201 (1992).
Guidetti, P., Reddy, P. H., Tagle, D. A. & Schwarcz, R. Early kynurenergic impairment in Huntington's disease and in a transgenic animal model. Neurosci. Lett. 283, 233–235 (2000).
Stoy, N. et al. Tryptophan metabolism and oxidative stress in patients with Huntington's disease. J. Neurochem. 93, 611–623 (2005).
Guidetti, P. et al. Elevated brain 3-hydroxykynurenine and quinolinate levels in Huntington disease mice. Neurobiol. Dis. 23, 190–197 (2006).
Chen, Y. et al. The kynurenine pathway and inflammation in amyotrophic lateral sclerosis. Neurotox. Res. 18, 132–142 (2010).
Ilzecka, J., Kocki, T., Stelmasiak, Z. & Turski, W. A. Endogenous protectant kynurenic acid in amyotrophic lateral sclerosis. Acta Neurol. Scand. 107, 412–418 (2003).
Chen, Y., Brew, B. J. & Guillemin, G. J. Characterization of the kynurenine pathway in NSC-34 cell line: implications for amyotrophic lateral sclerosis. J. Neurochem. 118, 816–825 (2011).
Yamamoto, H., Shindo, I., Egawa, B. & Horiguchi, K. Kynurenic acid is decreased in cerebrospinal fluid of patients with infantile spasms. Pediatr. Neurol. 10, 9–12 (1994).
Yamamoto, H. Studies on CSF tryptophan metabolism in infantile spasms. Pediatr. Neurol. 7, 411–414 (1991).
Langlais, P. J., Wardlow, M. L. & Yamamoto, H. Changes in CSF neurotransmitters in infantile spasms. Pediatr. Neurol. 7, 440–445 (1991).
Yamamoto, H., Murakami, H., Horiguchi, K. & Egawa, B. Studies on cerebrospinal fluid kynurenic acid concentrations in epileptic children. Brain Dev. 17, 327–329 (1995).
Heyes, M. P., Saito, K., Devinsky, O. & Nadi, N. S. Kynurenine pathway metabolites in cerebrospinal fluid and serum in complex partial seizures. Epilepsia 35, 251–257 (1994).
Rozsa, E. et al. The pentylenetetrazole-induced activity in the hippocampus can be inhibited by the conversion of L-kynurenine to kynurenic acid: an in vitro study. Brain Res. Bull. 76, 474–479 (2008).
Nemeth, H. et al. Kynurenine administered together with probenecid markedly inhibits pentylenetetrazol-induced seizures. An electrophysiological and behavioural study. Neuropharmacology 47, 916–925 (2004).
Robotka, H., Nemeth, H., Somlai, C., Vecsei, L. & Toldi, J. Systemically administered glucosamine-kynurenic acid, but not pure kynurenic acid, is effective in decreasing the evoked activity in area CA1 of the rat hippocampus. Eur. J. Pharmacol. 513, 75–80 (2005).
Battaglia, G. et al. Systemically administered D-glucose conjugates of 7-chlorokynurenic acid are centrally available and exert anticonvulsant activity in rodents. Brain Res. 860, 149–156 (2000).
Demeter, I. et al. A novel kynurenic acid analog (SZR104) inhibits pentylenetetrazole-induced epileptiform seizures. An electrophysiological study: special issue related to kynurenine. J. Neural. Transm. 119, 151–154 (2012).
Kocki, T., Wielosz, M., Turski, W. A. & Urbanska, E. M. Enhancement of brain kynurenic acid production by anticonvulsants — novel mechanism of antiepileptic activity? Eur. J. Pharmacol. 541, 147–151 (2006).
Qu, X. X. et al. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp. Neurol. 215, 298–307 (2009).
Christoph, T., Reissmuller, E., Schiene, K., Englberger, W. & Chizh, B. A. Antiallodynic effects of NMDA glycineB antagonists in neuropathic pain: possible peripheral mechanisms. Brain Res. 1048, 218–227 (2005).
Heyliger, S. O., Goodman, C. B., Ngong, J. M. & Soliman, K. F. The analgesic effects of tryptophan and its metabolites in the rat. Pharmacol. Res. 38, 243–250 (1998).
Richter, R. C. & Behbehani, M. M. Evidence for glutamic acid as a possible neurotransmitter between the mesencephalic nucleus cuneiformis and the medullary nucleus raphe magnus in the lightly anesthetized rat. Brain Res. 544, 279–286 (1991).
Beitz, A. J. Relationship of glutamate and aspartate to the periaqueductal gray-raphe magnus projection: analysis using immunocytochemistry and microdialysis. J. Histochem. Cytochem. 38, 1755–1765 (1990).
Olpe, H. R., Steinmann, M. W., Brugger, F. & Pozza, M. F. Excitatory amino acid receptors in rat locus coeruleus. An extracellular in vitro study. Naunyn Schmiedebergs Arch. Pharmacol. 339, 312–314 (1989).
Kalen, P., Strecker, R. E., Rosengren, E. & Bjorklund, A. Regulation of striatal serotonin release by the lateral habenula-dorsal raphe pathway in the rat as demonstrated by in vivo microdialysis: role of excitatory amino acids and GABA. Brain Res. 492, 187–202 (1989).
Reinhard, J. F. Jr. Pharmacological manipulation of brain kynurenine metabolism. Ann. NY Acad. Sci. 1035, 335–349 (2004).
Speciale, C. et al. (R,S)-3,4-dichlorobenzoylalanine (FCE 28833A) causes a large and persistent increase in brain kynurenic acid levels in rats. Eur. J. Pharmacol. 315, 263–267 (1996).
Rover, S., Cesura, A. M., Huguenin, P., Kettler, R. & Szente, A. Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J. Med. Chem. 40, 4378–4385 (1997).
Pellicciari, R. et al. Modulation of the kynurenine pathway in search for new neuroprotective agents. Synthesis and preliminary evaluation of (m-nitrobenzoyl)alanine, a potent inhibitor of kynurenine-3-hydroxylase. J. Med. Chem. 37, 647–655 (1994).
Natalini, B. et al. Synthesis and activity of enantiopure (S) (m-nitrobenzoyl) alanine, potent kynurenine-3-hydroxylase inhibitor. Bioorg. Med. Chem. Lett. 5, 1451–1454 (1995).
Carpenedo, R. et al. Inhibitors of kynurenine hydroxylase and kynureninase increase cerebral formation of kynurenate and have sedative and anticonvulsant activities. Neuroscience 61, 237–243 (1994).
Pellicciari, R. et al. Modulation of the kynurine pathway of tryptophan metabolism in search for neuroprotective agents. Focus on kynurenine-3-hydroxylase. Adv. Exp. Med. Biol. 527, 621–628 (2003).
Walsh, J. L., Todd, W. P., Carpenter, B. K. & Schwarcz, R. 4-halo-3-hydroxyanthranilic acids: potent competitive inhibitors of 3-hydroxy-anthranilic acid oxygenase in vitro. Biochem. Pharmacol. 42, 985–990 (1991).
Linderberg, M. et al. Synthesis and QSAR of substituted 3-hydroxyanthranilic acid derivatives as inhibitors of 3-hydroxyanthranilic acid dioxygenase (3-HAO). Eur. J. Med. Chem. 34, 729–744 (1999).
Walsh, H. A., Leslie, P. L., O'Shea, K. C. & Botting, N. P. 2-amino-4-[3′-hydroxyphenyl]-4-hydroxybutanoic acid; a potent inhibitor of rat and recombinant human kynureninase. Bioorg. Med. Chem. Lett. 12, 361–363 (2002).
Walsh, H. A., O'Shea, K. C. & Botting, N. P. Comparative inhibition by substrate analogues 3-methoxy- and 3-hydroxydesaminokynurenine and an improved 3 step purification of recombinant human kynureninase. BMC Biochem. 4, 13 (2003).
Fitzgerald, D. H., Muirhead, K. M. & Botting, N. P. A comparative study on the inhibition of human and bacterial kynureninase by novel bicyclic kynurenine analogues. Bioorg. Med. Chem. 9, 983–989 (2001).
Lima, S., Kumar, S., Gawandi, V., Momany, C. & Phillips, R. S. Crystal structure of the Homo sapiens kynureninase-3-hydroxyhippuric acid inhibitor complex: insights into the molecular basis of kynureninase substrate specificity. J. Med. Chem. 52, 389–396 (2009).
Tanizawa, K. & Soda, K. The mechanism of kynurenine hydrolysis catalyzed by kynureninase. J. Biochem. 86, 1199–1209 (1979).
Alberati-Giani, D. et al. Isolation and expression of a cDNA clone encoding human kynureninase. Eur. J. Biochem. 239, 460–468 (1996).
Chiarugi, A. & Moroni, F. Effects of mitochondria and o-methoxybenzoylalanine on 3-hydroxyanthranilic acid dioxygenase activity and quinolinic acid synthesis. J. Neurochem. 72, 1125–1132 (1999).
Dua, R. K., Taylor, E. W. & Phillips, R. S. S-aryl-l-cysteine S,S-dioxides: design, synthesis, and evaluation of a new class of inhibitors of kynureninase. J. Am. Chem. Soc. 115, 1264–1270 (1993).
Heiss, C., Anderson, J. & Phillips, R. S. Differential effects of bromination on substrates and inhibitors of kynureninase from Pseudomonas fluorescens. Org. Biomol. Chem. 1, 288–295 (2003).
Drysdale, M. J. & Reinhard, J. F. S-aryl cysteine S,S-dioxides as inhibitors of mammalian kynureninase. Bioorg. Med. Chem. Lett. 8, 133–138 (1998).
Smith, J. R. et al. Novel indoleamine 2,3-dioxygenase-1 inhibitors from a multistep in silico screen. Bioorg. Med. Chem. 20, 1354–1363 (2012).
Yue, E. W. et al. Discovery of potent competitive inhibitors of indoleamine 2,3-dioxygenase with in vivo pharmacodynamic activity and efficacy in a mouse melanoma model. J. Med. Chem. 52, 7364–7367 (2009).
Meininger, D. et al. Purification and kinetic characterization of human indoleamine 2,3-dioxygenases 1 and 2 (IDO1 and IDO2) and discovery of selective IDO1 inhibitors. Biochim. Biophys. Acta 1814, 1947–1954 (2011).
Rohrig, U. F. et al. Rational design of 4-aryl-1,2,3-triazoles for indoleamine 2,3-dioxygenase 1 inhibition. J. Med. Chem. 55, 5270–5290 (2012).
Yu, C. J., Zheng, M. F., Kuang, C. X., Huang, W. D. & Yang, Q. Oren-gedoku-to and its constituents with therapeutic potential in Alzheimer's disease inhibit indoleamine 2,3-dioxygenase activity in vitro. J. Alzheimers Dis. 22, 257–266 (2010).
Matsuno, K. et al. S-benzylisothiourea derivatives as small-molecule inhibitors of indoleamine- 2,3-dioxygenase. Bioorg. Med. Chem. Lett. 20, 5126–5129 (2010).
Eguchi, N., Watanabe, Y., Kawanishi, K., Hashimoto, Y. & Hayaishi, O. Inhibition of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase by β-carboline and indole derivatives. Arch. Biochem. Biophys. 232, 602–609 (1984).
Grant, R. & Kapoor, V. Inhibition of indoleamine 2,3-dioxygenase activity in IFN-γ stimulated astroglioma cells decreases intracellular NAD levels. Biochem. Pharmacol. 66, 1033–1036 (2003).
Peterson, A. et al. Evaluation of substituted β-carbolines as noncompetitive indoleamine 2,3-dioxygenase inhibitors. Med. Chem. Res. 3, 473–482 (1993).
Gaspari, P. et al. Structure–activity study of brassinin derivatives as indoleamine 2,3-dioxygenase inhibitors. J. Med. Chem. 49, 684–692 (2006).
Banerjee, T. et al. A key in vivo antitumor mechanism of action of natural product-based brassinins is inhibition of indoleamine 2,3-dioxygenase. Oncogene 27, 2851–2857 (2008).
Saito, K. et al. 4-chloro-3-hydroxyanthranilate, 6-chlorotryptophan and norharmane attenuate quinolinic acid formation by interferon-γ-stimulated monocytes (THP-1 cells). Biochem. J. 291, 11–14 (1993).
Cao, D. et al. Diindolylmethane inhibits indoleamine 2,3-dioxygenase activity in breast cancer cells. Proc. Amer. Assoc. Cancer Res. 46, Abstract 2461 (2005).
Pereira, A., Vottero, E., Roberge, M., Mauk, A. G. & Andersen, R. J. Indoleamine 2,3-dioxygenase inhibitors from the northeastern pacific marine hydroid Garveia annulata. J. Nature Prod. 69, 1496–1499 (2006).
Dolusic, E. et al. Indol-2-yl ethanones as novel indoleamine 2,3-dioxygenase (IDO) inhibitors. Bioorg. Med. Chem. 19, 1550–1561 (2011).
Rohrig, U. F. et al. Rational design of indoleamine 2,3-dioxygenase inhibitors. J. Med. Chem. 53, 1172–1189 (2010).
Hou, D. Y. et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 67, 792–801 (2007).
Muller, A. J., DuHadaway, J. B., Donover, P. S., Sutanto-Ward, E. & Prendergast, G. C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nature Med. 11, 312–319 (2005).
Vottero, E. et al. Inhibitors of human indoleamine 2,3-dioxygenase identified with a target-based screen in yeast. Biotechnol. J. 1, 282–288 (2006).
Kumar, S. et al. Structure based development of phenylimidazole-derived inhibitors of indoleamine 2,3-dioxygenase. J. Med. Chem. 51, 4968–4977 (2008).
Carr, G. et al. Plectosphaeroic acids A, B, and C, indoleamine 2,3-dioxygenase inhibitors produced in culture by a marine isolate of the fungus Plectosphaerella cucumerina. Org. Lett. 11, 2996–2999 (2009).
Terentis, A. C. et al. The selenazal drug ebselen potently inhibits indoleamine 2,3-dioxygenase by targeting enzyme cysteine residues. Biochemistry 49, 591–600 (2010).
Carr, G., Chung, M. K., Mauk, A. G. & Andersen, R. J. Synthesis of indoleamine 2,3-dioxygenase inhibitory analogues of the sponge alkaloid exiguamine A. J. Med. Chem. 51, 2634–2637 (2008).
Volgraf, M. et al. Biomimetic synthesis of the IDO inhibitors exiguamine A and B. Nature Chem. Biol. 4, 535–537 (2008).
Koblish, H. K. et al. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol. Cancer Ther. 9, 489–498 (2010).
Liu, X. et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 115, 3520–3530 (2010).
Kumar, S. et al. Indoleamine 2,3-dioxygenase is the anticancer target for a novel series of potent naphthoquinone-based inhibitors. J. Med. Chem. 51, 1706–1718 (2008).
John, S., Thangapandian, S., Sakkiah, S. & Lee, K. W. Identification of potent virtual leads to design novel indoleamine 2,3-dioxygenase inhibitors: pharmacophore modeling and molecular docking studies. Eur. J. Med. Chem. 45, 4004–4012 (2010).
Comi, G. et al. Placebo-controlled trial of oral laquinimod for multiple sclerosis. N. Engl. J. Med. 366, 1000–1009 (2012).
Freedman, M. S. et al. Teriflunomide added to interferon-β in relapsing multiple sclerosis: a randomized phase II trial. Neurology 78, 1877–1885 (2012).
Miller, A. E. et al. Pre-specified subgroup analyses of a placebo-controlled phase III trial (TEMSO) of oral teriflunomide in relapsing multiple sclerosis. Mult. Scler. 18, 1625–1632 (2012).
Acknowledgements
The authors' research activities are supported by the projects titled “OTKA (K 75628) and TÁMOP-4.2.2.A-11/1KONV-2012-0052 — Creating the Centre of Excellence at the University of Szeged”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1
Kynurenine alterations and therapeutic implications in psychiatric disorders (PDF 228 kb)
Supplementary information S2
Excitotoxicity and Huntington's disease (PDF 123 kb)
Supplementary information S3
The role of glutamate in trigeminal nociception and migraine pathogenesis (PDF 127 kb)
Supplementary information S4
Molecular modelling of migraine (PDF 114 kb)
Supplementary information S5
Selected inhibitors of kynurenine aminotransferases I and II (PDF 181 kb)
Related links
Related links
FURTHER INFORMATION
László Vécsei and Levente Szalárdy's homepage
Glossary
- Huntington's disease
-
A dominantly inherited neurodegenerative disorder in which there is preferential loss of striatal GABA (γ-aminobutyric acid)-ergic neurons, manifesting in involuntary movements and psychic alterations, and is gradually accompanied by dystonia, pyramidal signs and dementia.
- Migraine
-
A common primary headache characterized by spontaneous attacks of unilateral pulsating pain of moderate to severe intensity, which is aggravated by routine physical activity and generally associated with nausea as well as photo- and phonophobia.
- Immunological tolerance
-
A process in which the immune system actively ignores an antigen and does not elicit a specific immunological attack. The 'target' of tolerance can be either autogeneic or allogeneic.
- Autoimmune diseases
-
A collective of various clinical disorders in which the immune system fails to provide self-tolerance.
- Multiple sclerosis
-
A chronic autoimmune neuroinflammatory disorder of the central nervous system, characterized by demyelination as well as axonal and neuronal degeneration. The disease course is progressive, generally presenting in relapses and remissions.
- Mutant huntingtin
-
Pathognomonic huntingtin protein that develops as a result of the expansion of the CAG repeat in the huntingtin (HTT) gene, resulting in the formation of inclusion bodies and mitochondrial dysfunction. The length of the polyglutamine sequence correlates with disease severity.
- 3-nitropropionic acid
-
The most widely used mitochondrial complex II inhibitor among the neurotoxin-mediated models of Huntington's disease. It evokes preferential striatal neurodegeneration and characteristic motor symptoms in vivo.
- Trigeminovascular system
-
An anatomical and physiological system comprising trigeminal sensory neurons that receive afferentation from meningeal blood vessels. The activation and sensitization of the trigeminovascular system is thought to have a central role in the pathomechanism of migraine.
- Caudal trigeminal nucleus
-
A brainstem structure containing second-order trigeminal sensory neurons that receive nociceptive afferents from the trigeminal ganglia and regulatory afferents from other brainstem structures, including the locus coeruleus and nucleus raphe magnus. The activation and sensitization of this region has a key role in migraine.
- Cortical spreading depression
-
The electrophysiological correlate of the aura phenomenon, which is a perceptual disturbance experienced by 20–25% of patients before a migraine attack.
- First-order nociceptive neurons
-
Pseudounipolar sensory neurons in the trigeminal and dorsal root ganglia that receive noxious stimuli in the periphery and transmit these stimuli to second-order nociceptive neurons. Their functional plasticity contributes to the development of peripheral sensitization in pain syndromes.
- FOS
-
An immediate early gene, the expression of which is widely used as a marker of neuronal activity.
- NMDA hypothesis
-
A concept proposing that alterations in glutamatergic neurotransmission — especially those mediated by NMDA (N-methyl-D-aspartate) receptors — have a central role in the pathogenesis of migraine.
- Dendritic cells
-
Professional antigen-presenting cells that are capable of engulfing, processing and presenting antigens to stimulate naive lymphocytes and promote adaptive immune responses. Certain subpopulations can exert potent T cell-regulatory properties.
- Natural killer cells
-
A major component of the innate immune system. Natural killer cells are involved in the deletion of bacterial, malignantly transformed and virus-infected cells. They are not identical to natural killer T cells.
- Regulatory T cells
-
A CD25+ and forkhead box P3-positive (FOXP3+) subtype of mature CD4+ T cells that are responsible for the suppression and inhibition of the adaptive immune response, and as such are essential in maintaining immune homeostasis and self-tolerance.
- B7
-
A ligand that is expressed on the surface of antigen-presenting cells and is bi-directionally involved in the regulation of immune responses. The interaction of B7 with cytotoxic T lymphocyte antigen 4 (CTLA4) has been implicated in indoleamine 2,3-dioxygenase induction in dendritic cells as well as in the inhibition of T helper 17 (TH17) cell differentiation.
- Experimental autoimmune encephalitis
-
(EAE). The prototype model for T cell-mediated autoimmune disorders. The priming of the neuroimmune system with different central nervous system (CNS)-specific antigens results in distinct models that recapitulate some of the key features of multiple sclerosis.
- TH17 cells
-
T helper 17 cells; a recently identified CD4+ T helper subtype that expresses interleukin-17. These cells are generally associated with autoimmune disorders and also have a potential role in antitumour immune responses.
Rights and permissions
About this article
Cite this article
Vécsei, L., Szalárdy, L., Fülöp, F. et al. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov 12, 64–82 (2013). https://doi.org/10.1038/nrd3793
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3793
This article is cited by
-
Novel effect of topical Roquinimex and its combination with Clobetasol on an imiquimod-induced model of psoriasis in mice
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Cluster headache and kynurenines
The Journal of Headache and Pain (2023)
-
Modulating AHR function offers exciting therapeutic potential in gut immunity and inflammation
Cell & Bioscience (2023)
-
The kynurenic acid analog SZR104 induces cytomorphological changes associated with the anti-inflammatory phenotype in cultured microglia
Scientific Reports (2023)
-
Advances in nanotechnology versus stem cell therapy for the theranostics of multiple sclerosis disease
Applied Nanoscience (2023)