Key Points
-
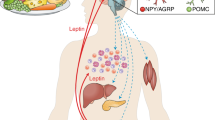
Leptin is a hormone that is produced by adipose tissue and regulates appetite, body weight, neuroendocrine functions and glycaemia.
-
In this Review, we first discuss data from leptin-based clinical trials. The results from these trials indicate that leptin therapy fails to improve metabolic defects in people who have elevated levels of circulating leptin and hence are leptin-resistant. Conversely, regardless of the disease context, leptin therapy is very effective in improving metabolic imbalances in individuals who have severe hypoleptinaemia.
-
The defects underlying leptin resistance could include impaired transport of leptin across the blood–brain barrier, impaired neuronal leptin signalling in target neurons and altered signalling in downstream targets. Evidence suggests that activation of inflammatory pathways and endoplasmic reticulum stress contribute to the development of leptin resistance.
-
In the presence of insulin, the glucose-lowering effects of leptin are probably mediated by leptin receptor (LEPR)-expressing neurons within the arcuate nucleus of the hypothalamus: specifically, by pro-opiomelanocortin (POMC)-expressing neurons. The neurocircuitry controlled by POMC-expressing neurons could be exploited to lower glycaemia in patients with type 2 diabetes.
-
In the absence of insulin, the glucose-lowering effects of leptin are probably also mediated by LEPR-expressing neurons within the brain, but the biochemical identity of these neurons is still unknown. Once identified, this brain-controlled pathway could be exploited to improve hyperglycaemia in patients with type 1 diabetes.
-
A better understanding of the leptin–CNS (central nervous system)–glycaemia pathway is clearly needed, as this may provide opportunities for the identification of new drug targets and therapeutics that can circumvent the obstacle of leptin resistance and ultimately help to improve the quality and length of life of the millions of people suffering from obesity and/or diabetes.
Abstract
Since the discovery of leptin in 1994, we now have a better understanding of the cellular and molecular mechanisms underlying its biological effects. In addition to its established anti-obesity effects, leptin exerts antidiabetic actions that are independent of its regulation of body weight and food intake. In particular, leptin can correct diabetes in animal models of type 1 and type 2 diabetes. In addition, long-term leptin replacement therapy improves glycaemic control, insulin sensitivity and plasma triglycerides in patients with severe insulin resistance due to lipodystrophy. These results have spurred enthusiasm for the use of leptin therapy to treat diabetes. Here, we review the current understanding of the glucoregulatory functions of leptin, emphasizing its central mechanisms of action and lessons learned from clinical studies, and discuss possible therapeutic applications of leptin in the treatment of type 1 and type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brown, M. S. & Goldstein, J. L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 (2008).
Danaei, G. et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378, 31–40 (2011).
Czyzyk, A. & Szczepanik, Z. Diabetes mellitus and cancer. Eur. J. Intern. Med. 11, 245–252 (2000).
Mazzone, T., Chait, A. & Plutzky, J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet 371, 1800–1809 (2008).
Daneman, D. Type 1 diabetes. Lancet 367, 847–858 (2006).
Cryer, P. E. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med. 350, 2272–2279 (2004).
Borchers, A. T., Uibo, R. & Gershwin, M. E. The geoepidemiology of type 1 diabetes. Autoimmun. Rev. 9, A355–A365 (2010).
Larsen, J. et al. Silent coronary atheromatosis in type 1 diabetic patients and its relation to long-term glycemic control. Diabetes 51, 2637–2641 (2002).
Orchard, T. J. et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 26, 1374–1379 (2003).
Hoerger, T. J., Segel, J. E., Gregg, E. W. & Saaddine, J. B. Is glycemic control improving in U.S. adults? Diabetes Care 31, 81–86 (2008).
Friedman, J. M. Leptin at 14 y of age: an ongoing story. Am. J. Clin. Nutr. 89, 973S–979S (2009).
Flier, J. S. & Maratos-Flier, E. Lasker lauds leptin. Cell 143, 9–12 (2010).
Lee, G. H. et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379, 632–635 (1996). This paper reports the cloning of the murine Lepr gene and shows that a mutation in this gene causes the metabolic imbalance observed in db/db mice.
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Donato, J. Jr. et al. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J. Clin. Invest. 121, 355–368 (2011).
Berglund, E., Vianna, C. R., Coppari, R. & Elmquist, J. Direct leptin action on POMC neurons regulates hepatic insulin sensitivity in mice. J. Clin. Invest. 122, 1000–1009 (2012).
Huo, L. et al. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 9, 537–547 (2009). This study is the first to indicate that LEPRs on POMC-expressing neurons have the capacity to mediate the effects of leptin on glucose homeostasis.
Shimomura, I., Hammer, R. E., Ikemoto, S., Brown, M. S. & Goldstein, J. L. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401, 73–76 (1999). This study demonstrates that the insulin-resistant state can be reversed by leptin administration in the context of lipodystrophy in mice.
Petersen, K. F. et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Invest. 109, 1345–1350 (2002).
Oral, E. A. et al. Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 346, 570–578 (2002). This study demonstrates that the insulin-resistant state can be reversed by leptin administration in the context of lipodystrophy in humans.
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999).
Farooqi, I. S. & O'Rahilly, S. Leptin: a pivotal regulator of human energy homeostasis. Am J. Clin. Nutr. 89, 980S–984S (2009).
Paz-Filho, G., Wong, M. L. & Licinio, J. Ten years of leptin replacement therapy. Obes. Rev. 12, e315–e323 (2011).
Maffei, M. et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nature Med. 1, 1155–1161 (1995).
Heymsfield, S. B. et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282, 1568–1575 (1999).
Hukshorn, C. J. et al. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J. Clin. Endocrinol. Metab. 85, 4003–4009 (2000).
Roth, J. D. et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc. Natl Acad. Sci. USA 105, 7257–7262 (2008).
Ravussin, E. et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity 17, 1736–1743 (2009).
Ebihara, K. et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J. Clin. Endocrinol. Metab. 92, 532–541 (2007).
Chong, A. Y., Lupsa, B. C., Cochran, E. K. & Gorden, P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia 53, 27–35 (2010).
Haque, W. A., Shimomura, I., Matsuzawa, Y. & Garg, A. Serum adiponectin and leptin levels in patients with lipodystrophies. J. Clin. Endocrinol. Metab. 87, 2395 (2002).
Simha, V., et al. Comparison of efficacy and safety of leptin replacement therapy in moderately and severely hypoleptinemic patients with familial partial lipodystrophy of the Dunnigan variety. J. Clin. Endocrinol. Metab. 97, 785–792 (2012).
Grinspoon, S. & Carr, A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N. Engl. J. Med. 352, 48–62 (2005).
Sekhar, R. V. et al. Leptin replacement therapy does not improve the abnormal lipid kinetics of hypoleptinemic patients with HIV-associated lipodystrophy syndrome. Metabolism 27 Apr 2012 (doi:10.1016/j.metabol.2012.03.013).
Falutz, J. et al. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled Phase 3 trials with safety extension data. J. Clin. Endocrinol. Metab. 95, 4291–4304 (2010).
Javor, E. D. et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology 41, 753–760 (2005).
Chou, S. H. et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc. Natl Acad. Sci. USA 108, 6585–6590 (2011).
Sienkiewicz, E. et al. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism 60, 1211–1221 (2011).
Welt, C. K. et al. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 351, 987–997 (2004).
Fujikawa, T., Chuang, J. C., Sakata, I., Ramadori, G. & Coppari, R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc. Natl Acad. Sci. USA 107, 17391–17396 (2010).
Yu, X., Park, B. H., Wang, M. Y., Wang, Z. V. & Unger, R. H. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc. Natl Acad. Sci. USA 105, 14070–14075 (2008). This study is the first to suggest that the effects of leptin on glucose homeostasis can be independent of the effects of insulin.
Park, J. Y. et al. Type 1 diabetes associated with acquired generalized lipodystrophy and insulin resistance: the effect of long-term leptin therapy. J. Clin. Endocrinol. Metab. 93, 26–31 (2008).
Cummings, B. P. et al. Subcutaneous administration of leptin normalizes fasting plasma glucose in obese type 2 diabetic UCD-T2DM rats. Proc. Natl Acad. Sci. USA 108, 14670–14675 (2011).
Morton, G. J. et al. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2, 411–420 (2005).
Coppari, R. et al. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 1, 63–72 (2005). This study is the first to indicate that LEPR-expressing neurons in the ARH can mediate the effects of leptin on glucose homeostasis.
Mittendorfer, B. et al. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes 60, 1474–1477 (2011).
Moon, H. S. et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes 60, 1647–1656 (2011).
Ahima, R. S. et al. Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252 (1996).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994). This paper reports the discovery of leptin and demonstrates that a mutation in the gene encoding leptin causes the metabolic imbalance seen in ob/ob mice.
Montague, C. T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387, 903–908 (1997).
Halaas, J. L. et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995).
Bjørbæk, C. Central leptin receptor action and resistance in obesity. J. Investig. Med. 57, 789–794 (2009).
Frederich, R. C. et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nature Med. 1, 1311–1314 (1995).
Caro, J. F. et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348, 159–161 (1996).
Banks, W. A., DiPalma, C. R. & Farrell, C. L. Impaired transport of leptin across the blood–brain barrier in obesity. Peptides 20, 1341–1345 (1999).
Banks, W. A. & Farrell, C. L. Impaired transport of leptin across the blood–brain barrier in obesity is acquired and reversible. Am. J. Physiol. 285, e10–e15 (2003).
Wilsey, J., Zolotukhin, S., Prima, V. & Scarpace, P. J. Central leptin gene therapy fails to overcome leptin resistance associated with diet-induced obesity. Am. J. Physiol. 285, R1011–R1020 (2003).
Bjørbæk, C., Uotani, S., da Silva, B. & Flier, J. S. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J. Biol. Chem. 272, 32686–32695 (1997).
Bates, S. H. et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421, 856–859 (2003).
Munzberg, H., Flier, J. S. & Bjørbæk, C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145, 4880–4889 (2004).
Enriori, P. J. et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 5, 181–194 (2007).
Gamber, K. M. et al. Over-expression of leptin receptors in hypothalamic POMC neurons increases susceptibility to diet-induced obesity. PLoS ONE 7, e30485 (2012).
Balthasar, N. et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–991 (2004).
van de Wall, E. et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149, 1773–1785 (2008).
Banks, W. A. The blood–brain barrier as a cause of obesity. Curr. Pharm. Des. 14, 1606–1614 (2008).
Herde, M. K., Geist, K., Campbell, R. E. & Herbison, A. E. Gonadotropin-releasing hormone neurons extend complex highly branched dendritic trees outside the blood–brain barrier. Endocrinology 152, 3832–3841 (2011).
Faouzi, M. et al. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology 148, 5414–5423 (2007).
Bjørbæk, C., Elmquist, J. K., Frantz, J. D., Shoelson, S. E. & Flier, J. S. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol. Cell 1, 619–625 (1998).
Howard, J. K. et al. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nature Med. 10, 734–738 (2004).
Mori, H. et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nature Med. 10, 739–743 (2004).
Kievit, P. et al. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 4, 123–132 (2006).
Loh, K. et al. Elevated hypothalamic TCPTP in obesity contributes to cellular leptin resistance. Cell Metab. 14, 684–699 (2011).
Bence, K. K. et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nature Med. 12, 917–924 (2006).
Hotamisligil, G. S., Arner, P., Caro, J. F., Atkinson, R. L. & Spiegelman, B. M. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J. Clin. Invest. 95, 2409–2415 (1995).
Gregor, M. F. & Hotamisligil, G. S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 (2011).
Zabolotny, J. M. et al. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J. Biol. Chem. 283, 14230–14241 (2008).
Zhang, X. et al. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008).
Knight, Z. A., Hannan, K. S., Greenberg, M. L. & Friedman, J. M. Hyperleptinemia is required for the development of leptin resistance. PLoS ONE 5, e11376 (2010).
Benomar, Y. et al. Leptin but not ciliary neurotrophic factor (CNTF) induces phosphotyrosine phosphatase-1B expression in human neuronal cells (SH-SY5Y): putative explanation of CNTF efficacy in leptin-resistant state. Endocrinology 150, 1182–1191 (2009).
Bjørbæk, C., El-Haschimi, K., Frantz, J. D. & Flier, J. S. The role of SOCS-3 in leptin signaling and leptin resistance. J. Biol. Chem. 274, 30059–30065 (1999).
Ingalls, A. M., Dickie, M. M. & Snell, G. D. Obese, a new mutation in the house mouse. J. Hered. 41, 317–318 (1950).
Hummel, K. P., Dickie, M. M. & Coleman, D. L. Diabetes, a new mutation in the mouse. Science 153, 1127–1128 (1966).
Tartaglia, L. A. et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 83, 1263–1271 (1995). This paper is the first to report the cloning of LEPRs.
Pelleymounter, M. A. et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269, 540–543 (1995). This study is the first to suggest that the effects of leptin on glucose homeostasis are direct and not secondary to its effects on food intake or body weight.
Schwartz, M. W. et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes 45, 531–535 (1996).
Lin, C. Y., Higginbotham, D. A., Judd, R. L. & White, B. D. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. Am. J. Physiol. 282, e1084–e1091 (2002).
Chinookoswong, N., Wang, J. L. & Shi, Z. Q. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes 48, 1487–1492 (1999).
Cone, R. D. Anatomy and regulation of the central melanocortin system. Nature Neurosci. 8, 571–578 (2005).
Bagnol, D. et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J. Neurosci. 19, RC26 (1999).
Kishi, T. et al. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J. Comp. Neurol. 457, 213–235 (2003).
Rossi, J. et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 13, 195–204 (2011).
Xu, Y., Elmquist, J. K. & Fukuda, M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann. NY Acad. Sci. 1243, 1–14 (2011).
Williams, K. W. et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J. Neurosci. 30, 2472–2479 (2010).
Choudhury, A. I. et al. The role of insulin receptor substrate 2 in hypothalamic and β cell function. J. Clin. Invest. 115, 940–950 (2005).
Claret, M. et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J. Clin. Invest. 117, 2325–2336 (2007).
Williams, K. W., Coppari, R. & Elmquist, J. K. “AMPing up” our understanding of the hypothalamic control of energy balance. J. Clin. Invest. 117, 2089–2092 (2007).
Al-Qassab, H. et al. Dominant role of the p110β isoform of PI3K over p110α in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 10, 343–354 (2009).
Low, M. J. Role of proopiomelanocortin neurons and peptides in the regulation of energy homeostasis. J. Endocrinol. Invest. 27, 95–100 (2004).
Kristensen, P. et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393, 72–76 (1998).
Foo, K. S., Brismar, H. & Broberger, C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience 156, 563–579 (2008).
Meister, B. et al. Hypothalamic proopiomelanocortin (POMC) neurons have a cholinergic phenotype. Eur. J. Neurosci. 24, 2731–2740 (2006).
Hentges, S. T., Otero-Corchon, V., Pennock, R. L., King, C. M. & Low, M. J. Proopiomelanocortin expression in both GABA and glutamate neurons. J. Neurosci. 29, 13684–13690 (2009).
Piper, M. L., Unger, E. K., Myers, M. G. Jr & Xu, A. W. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol. Endocrinol. 22, 751–759 (2008).
Robertson, S. et al. Insufficiency of Janus kinase 2-autonomous leptin receptor signals for most physiologic leptin actions. Diabetes 59, 782–790 (2010).
Coleman, D. L. & Hummel, K. P. The influence of genetic background on the expression of the obese (Ob) gene in the mouse. Diabetologia 9, 287–293 (1973).
Banno, R. et al. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J. Clin. Invest. 120, 720–734 (2010).
Ibrahim, N. et al. Hypothalamic proopiomelanocortin neurons are glucose responsive and express KATP channels. Endocrinology 144, 1331–1340 (2003).
Parton, L. E. et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449, 228–232 (2007). This study describes the crucial molecular component and physiological relevance of glucose-sensing mechanisms in hypothalamic neurons.
Hill, J. W. et al. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J. Clin. Invest. 118, 1796–1805 (2008).
Hill, J. W. et al. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology 150, 4874–4882 (2009).
Malaisse, W., Malaisse-Lagae, F., Wright, P. H. & Ashmore, J. Effects of adrenergic and cholinergic agents upon insulin secretion in vitro. Endocrinology 80, 975–978 (1967).
Ramadori, G., et al. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 14, 301–312 (2011).
Pocai, A., Obici, S., Schwartz, G. J. & Rossetti, L. A brain–liver circuit regulates glucose homeostasis. Cell Metab. 1, 53–61 (2005).
Buettner, C. et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nature Med. 14, 667–675 (2008).
Uyama, N., Geerts, A. & Reynaert, H. Neural connections between the hypothalamus and the liver. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 280, 808–820 (2004).
Kalsbeek, A., Fliers, E., Hofman, M. A., Swaab, D. F. & Buijs, R. M. Vasopressin and the output of the hypothalamic biological clock. J. Neuroendocrinol. 22, 362–372 (2010).
Elias, C. F. et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21, 1375–1385 (1998).
Banno, R. et al. Central administration of melanocortin agonist increased insulin sensitivity in diet-induced obese rats. FEBS Lett. 581, 1131–1136 (2007).
Heijboer, A. C. et al. Intracerebroventricular administration of melanotan II increases insulin sensitivity of glucose disposal in mice. Diabetologia 48, 1621–1626 (2005).
da Silva, A. A., do Carmo, J. M., Freeman, J. N., Tallam, L. S. & Hall, J. E. A functional melanocortin system may be required for chronic CNS-mediated antidiabetic and cardiovascular actions of leptin. Diabetes 58, 1749–1756 (2009).
Leckstrom, A., Lew, P. S., Poritsanos, N. J. & Mizuno, T. M. Treatment with a melanocortin agonist improves abnormal lipid metabolism in streptozotocin-induced diabetic mice. Neuropeptides 45, 123–129 (2011).
Cone, R. D. Studies on the physiological functions of the melanocortin system. Endocr. Rev. 27, 736–749 (2006).
Taylor, G. W. Periodontal treatment and its effects on glycemic control: a review of the evidence. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 87, 311–316 (1999).
van den Hoek, A. M. et al. Leptin deficiency per se dictates body composition and insulin action in ob/ob mice. J. Neuroendocrinol. 20, 120–127 (2008).
German, J. et al. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 150, 4502–4511 (2009).
Harlan, S. M. et al. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ. Res. 108, 808–812 (2011).
Li, J. H. et al. Hepatic muscarinic acetylcholine receptors are not critically involved in maintaining glucose homeostasis in mice. Diabetes 58, 2776–2787 (2009).
Hedbacker, K. et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab. 11, 11–22 (2010).
Levi, J. et al. Acute disruption of leptin signaling in vivo leads to increased insulin levels and insulin resistance. Endocrinology 152, 3385–3395 (2011).
Kojima, S. et al. Central leptin gene therapy, a substitute for insulin therapy to ameliorate hyperglycemia and hyperphagia, and promote survival in insulin-deficient diabetic mice. Peptides 30, 962–966 (2009).
Hidaka, S. et al. Chronic central leptin infusion restores hyperglycemia independent of food intake and insulin level in streptozotocin-induced diabetic rats. FASEB J. 16, 509–518 (2002).
Wang, M.-Y. et al. Leptin therapy in insulin-deficient type I diabetes. Proc. Natl Acad. Sci. USA 107, 4813–4819 (2010).
Koch, L. et al. Central insulin action regulates peripheral glucose and fat metabolism in mice. J. Clin. Invest. 118, 2132–2147 (2008).
Chen, L., Philippe, J. & Unger, R. H. Glucagon responses of isolated α cells to glucose, insulin, somatostatin, and leptin. Endocr. Pract. 17, 819–825 (2011).
Denroche, H. C. et al. Leptin therapy reverses hyperglycemia in mice with streptozotocin-induced diabetes, independent of hepatic leptin signaling. Diabetes 60, 1414–1423 (2011).
Unger, R. H. & Cherrington, A. D. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J. Clin. Invest. 122, 4–12 (2012).
Lee, Y., Wang, M. Y., Du, X. Q., Charron, M. J. & Unger, R. H. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 60, 391–397 (2011).
German, J. P. et al. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes 59, 1626–1634 (2010).
Garg, A. Lipodystrophies: genetic and acquired body fat disorders. J. Clin. Endocrinol. Metab. 96, 3313–3325 (2011).
Rahmouni, K. & Haynes, W. G. Leptin and the cardiovascular system. Recent Prog. Horm. Res. 59, 225–244 (2004).
Seth, R., Knight, W. D. & Overton, J. M. Combined amylin-leptin treatment lowers blood pressure and adiposity in lean and obese rats. Int. J. Obes. 35, 1183–1192 (2011).
Matarese, G. et al. Leptin accelerates autoimmune diabetes in female NOD mice. Diabetes 51, 1356–1361 (2002).
Engelman, J. A., Luo, J. & Cantley, L. C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature Rev. 7, 606–619 (2006).
Wong, K. K., Engelman, J. A. & Cantley, L. C. Targeting the PI3K signaling pathway in cancer. Curr. Opin. Genet. Dev. 20, 87–90 (2010).
Bowker, S. L., Majumdar, S. R., Veugelers, P. & Johnson, J. A. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 29, 254–258 (2006).
Farooqi, I. S. et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 110, 1093–1103 (2002).
Shetty, G. K. et al. Leptin administration to overweight and obese subjects for 6 months increases free leptin concentrations but does not alter circulating hormones of the thyroid and IGF axes during weight loss induced by a mild hypocaloric diet. Eur. J. Endocrinol. 165, 249–254 (2011).
Vadacca, M., Margiotta, D. P., Navarini, L. & Afeltra, A. Leptin in immuno-rheumatological diseases. Cell. Mol. Immunol. 8, 203–212 (2011).
Takeda, S. et al. Leptin regulates bone formation via the sympathetic nervous system. Cell 111, 305–317 (2002).
Ducy, P. et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100, 197–207 (2000).
Moller, D. E. Metabolic disease drug discovery — “hitting the target” is easier said than done. Cell Metab. 15, 19–24 (2012).
Bailey, C. J. & Turner, R. C. Metformin. N. Engl. J. Med. 334, 574–579 (1996).
Shaw, R. J. et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 (2005).
Owen, M. R., Doran, E. & Halestrap, A. P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 (2000).
Mihaylova, M. M. et al. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell 145, 607–621 (2011).
El-Mir, M. Y. et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275, 223–228 (2000).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001).
Rotella, C. M., Monami, M. & Mannucci, E. Metformin beyond diabetes: new life for an old drug. Curr. Diabetes Rev. 2, 307–315 (2006).
Selvin, E. et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch. Intern. Med. 168, 2070–2080 (2008).
Lehmann, J. M. et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ). J. Biol. Chem. 270, 12953–12956 (1995).
Semple, R. K., Chatterjee, V. K. & O'Rahilly, S. PPARγ and human metabolic disease. J. Clin. Invest. 116, 581–589 (2006).
Willson, T. M., Lambert, M. H. & Kliewer, S. A. Peroxisome proliferator-activated receptor γ and metabolic disease. Annu. Rev. Biochem. 70, 341–367 (2001).
Choi, J. H. et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature 466, 451–456 (2010).
Home, P. D. et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373, 2125–2135 (2009).
Joosen, A. M., Bakker, A. H., Gering, M. J. & Westerterp, K. R. The effect of the PPARγ ligand rosiglitazone on energy balance regulation. Diabetes Metab. Res. Rev. 22, 204–210 (2006).
Larsen, P. J. et al. Differential influences of peroxisome proliferator-activated receptors γ and -α on food intake and energy homeostasis. Diabetes 52, 2249–2259 (2003).
Kahn, S. E. et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 355, 2427–2443 (2006).
Festuccia, W. T. et al. Peroxisome proliferator-activated receptor-γ-mediated positive energy balance in the rat is associated with reduced sympathetic drive to adipose tissues and thyroid status. Endocrinology 149, 2121–2130 (2008).
Shimizu, H. et al. Troglitazone reduces plasma leptin concentration but increases hunger in NIDDM patients. Diabetes Care 21, 1470–1474 (1998).
Lu, M. et al. Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nature Med. 17, 618–622 (2011).
Ryan, K. K. et al. A role for central nervous system PPAR-γ in the regulation of energy balance. Nature Med. 17, 623–626 (2011).
Choi, J. H. et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature 477, 477–481 (2011).
Woodcock, J., Sharfstein, J. M. & Hamburg, M. Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. N. Engl. J. Med. 363, 1489–1491 (2010).
Tahrani, A. A., Bailey, C. J., Del Prato, S. & Barnett, A. H. Management of type 2 diabetes: new and future developments in treatment. Lancet 378, 182–197 (2011).
Lebovitz, H. E. Type 2 diabetes mellitus — current therapies and the emergence of surgical options. Nature Rev. Endocrinol. 7, 408–419 (2011).
Nathan, D. M. et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 353, 2643–2653 (2005).
Maahs, D. M. & Rewers, M. Mortality and renal disease in type 1 diabetes mellitus — progress made, more to be done. J. Clin. Endocrinol. Metab. 91, 3757–3759 (2006).
Steffes, M. W., Sibley, S., Jackson, M. & Thomas, W. β-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 26, 832–836 (2003).
Bluestone, J. A., Herold, K. & Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464, 1293–1300 (2010).
Horton, J. D., Goldstein, J. L. & Brown, M. S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131 (2002).
Liu, H. Y. et al. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM). J. Biol. Chem. 284, 27090–27100 (2009).
Shulman, G. I. Cellular mechanisms of insulin resistance. J. Clin. Invest. 106, 171–176 (2000).
Randle, P. J., Garland, P. B., Hales, C. N. & Newsholme, E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789 (1963).
Boden, G. & Shulman, G. I. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β-cell dysfunction. Eur. J. Clin. Invest. 32 (Suppl. 3), 14–23 (2002).
Cryer, P. E. Mechanisms of sympathoadrenal failure and hypoglycemia in diabetes. J. Clin. Invest. 116, 1470–1473 (2006).
Cryer, P. E. The barrier of hypoglycemia in diabetes. Diabetes 57, 3169–3176 (2008).
Cryer, P. E. Hypoglycemia: still the limiting factor in the glycemic management of diabetes. Endocr. Pract. 14, 750–756 (2008).
Cryer, P. E. Preventing hypoglycaemia: what is the appropriate glucose alert value? Diabetologia 52, 35–37 (2009).
Agarwal, A. K. & Garg, A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-γ gene in a patient with familial partial lipodystrophy. J. Clin. Endocrinol. Metab. 87, 408–411 (2002).
Gandotra, S. et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. N. Engl. J. Med. 364, 740–748 (2011).
Hudon, S. E. et al. HIV-protease inhibitors block the enzymatic activity of purified Ste24p. Biochem. Biophys. Res. Commun. 374, 365–368 (2008).
Apostolova, N., Blas-Garcia, A. & Esplugues, J. V. Mitochondrial toxicity in HAART: an overview of in vitro evidence. Curr. Pharm. Des. 17, 2130–2144 (2011).
Devos, R. et al. Ligand-independent dimerization of the extracellular domain of the leptin receptor and determination of the stoichiometry of leptin binding. J. Biol. Chem. 272, 18304–18310 (1997).
Ghilardi, N. & Skoda, R. C. The leptin receptor activates Janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol. Endocrinol. 11, 393–399 (1997).
Kurzer, J. H. et al. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-Bβ. Mol. Cell. Biol. 24, 4557–4570 (2004).
Mistrik, P., Moreau, F. & Allen, J. M. BiaCore analysis of leptin–leptin receptor interaction: evidence for 1:1 stoichiometry. Anal. Biochem. 327, 271–277 (2004).
Couturier, C. & Jockers, R. Activation of the leptin receptor by a ligand-induced conformational change of constitutive receptor dimers. J. Biol. Chem. 278, 26604–26611 (2003).
Ingley, E. & Klinken, S. P. Cross-regulation of JAK and Src kinases. Growth Factors 24, 89–95 (2006).
Li, M., Li, Z., Morris, D. L. & Rui, L. Identification of SH2B2β as an inhibitor for SH2B1- and SH2B2α-promoted Janus kinase-2 activation and insulin signaling. Endocrinology 148, 1615–1621 (2007).
Bjørbæk, C. et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J. Biol. Chem. 276, 4747–4755 (2001).
De Souza, D. et al. SH2 domains from suppressor of cytokine signaling-3 and protein tyrosine phosphatase SHP-2 have similar binding specificities. Biochemistry 41, 9229–9236 (2002).
Banks, A. S., Davis, S. M., Bates, S. H. & Myers, M. G. Jr. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 275, 14563–14572 (2000).
Fukuda, M. et al. Monitoring FoxO1 localization in chemically identified neurons. J. Neurosci. 28, 13640–13648 (2008).
Cao, Y. et al. PDK1-Foxo1 in agouti-related peptide neurons regulates energy homeostasis by modulating food intake and energy expenditure. PLoS ONE 6, e18324 (2011).
Auernhammer, C. J., Bousquet, C. & Melmed, S. Autoregulation of pituitary corticotroph SOCS-3 expression: characterization of the murine SOCS-3 promoter. Proc. Natl Acad. Sci. USA 96, 6964–6969 (1999).
Guo, L., Munzberg, H., Stuart, R. C., Nillni, E. A. & Bjørbæk, C. N-acetylation of hypothalamic α-melanocyte-stimulating hormone and regulation by leptin. Proc. Natl Acad. Sci. USA 101, 11797–11802 (2004).
Munzberg, H., Huo, L., Nillni, E. A., Hollenberg, A. N. & Bjørbæk, C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology 144, 2121–2131 (2003).
Kitamura, T. et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nature Med. 12, 534–540 (2006).
Plum, L. et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nature Med. 15, 1195–1201 (2009).
van den Brink, G. R. et al. Leptin signaling in human peripheral blood mononuclear cells, activation of p38 and p42/44 mitogen-activated protein (MAP) kinase and p70 S6 kinase. Mol. Cell Biol. Res. Commun. 4, 144–150 (2000).
Roux, P. P., Ballif, B. A., Anjum, R., Gygi, S. P. & Blenis, J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl Acad. Sci. USA 101, 13489–13494 (2004).
Gong, Y. et al. The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J. Biol. Chem. 282, 31019–31027 (2007).
Cowley, M. A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Won, J. C. et al. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity 17, 1861–1865 (2009).
De Souza, C. T. et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146, 4192–4199 (2005).
El-Haschimi, K., Pierroz, D. D., Hileman, S. M., Bjørbæk, C. & Flier, J. S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Invest. 105, 1827–1832 (2000).
Acknowledgements
The authors of this article are supported by grants from the American Heart Association (0930366N to R.C.), the American Diabetes Association (7-09-BS-17 and 7-12-BS-010 to C.B.), the Richard and Susan Smith Family Foundation Pinnacle Program Project (7-05-PPG-02 to C.B.), the Boston Obesity Nutrition Research Center (DK46200 to C.B.) and by grants from the US National Institutes of Health (DK080836 to R.C.; DK60673, DK65743 and DK94040 to C.B.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Glossary
- Type 2 diabetes
-
An illness characterized by insulin resistance, elevated blood levels of glucose, insulin and lipids, and estimated to affect more than 300 million people worldwide.
- Type 1 diabetes
-
An illness characterized by the loss of pancreatic β-cells, lack of insulin, hyperglycaemia, cachexia and ketoacidosis, and estimated to affect millions of people worldwide.
- Lipodystrophy
-
A rare condition that can be inherited or acquired, and is typically characterized by varying adipose tissue loss and distribution.
- Hyperleptinaemia
-
A condition in which leptin levels in the blood are elevated, typically in obesity.
- Leptin resistance
-
A condition in which endogenous and exogenous leptin is less effective at mediating its actions: for example, at reducing food intake or lowering glucose and lipids levels in the blood.
- Hypoleptinaemia
-
A condition in which the level of leptin in the blood is below normal owing to reduced fat mass: for example, in patients with anorexia, lipodystrophy or hypothalamic amenorrhea.
- Amylin
-
A hormone that is co-secreted with insulin from pancreatic β-cells; amylin slows gastric emptying and promotes satiety.
- Euglycaemia
-
Normal levels of glucose in the blood.
- Amenorrhea
-
A condition characterized by the absence of menstrual periods in a woman of reproductive age.
- Common obesity
-
A condition that is characterized by increased body weight due to excess adipose mass, as well as hyperleptinaemia, and is associated with an increased risk of developing type 2 diabetes, cardiovascular disease, cancer and non-alcoholic fatty liver disease.
- First-order neurons
-
In a neurocircuitry aimed at orchestrating responses to changes in a circulating cue, first-order neurons are equipped with the molecular tools to monitor the levels of the circulating cue (for example, they express the cognate receptor for the circulating ligand).
- Second-order neurons
-
In a neurocircuitry aimed at orchestrating responses to changes in a circulating cue, second-order neurons receive direct synaptic inputs from first-order neurons.
- Koletsky rats
-
Rats that are homozygous for a nonsense mutation in the leptin receptor gene, causing lack of leptin receptor function and hence altered metabolic homeostasis.
- Hepatic vagotomy
-
Severing of the hepatic branch of the vagus nerve, resulting in lack of vagal efferent and afferent innervations of the liver.
- Insulin-like growth factor binding protein 2
-
(IGFBP2). A protein that is primarily produced by the liver and that may mediate a proportion of the antidiabetic actions of leptin.
- Alloxan
-
A pyrimidine derivative taken up by cells via facilitated transport through glucose transporter 2; this compound is used in research laboratories to destroy insulin-producing cells in animals.
- Polyuria
-
Abnormally elevated levels of urine production; a condition that can be seen in patients with uncontrolled diabetes.
- Hyperketonaemia
-
A condition characterized by a high level of ketone bodies in the blood; this can be seen after prolonged fasting, the use of a ketogenic diet or due to lack of insulin, typically in individuals with type 1 diabetes.
- Pancreatic α-cells
-
Endocrine cells of the pancreas that secrete the hormone glucagon; the proper functionality of these cells is crucial for preventing a life-threatening reduction in blood glucose levels.
Rights and permissions
About this article
Cite this article
Coppari, R., Bjørbæk, C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov 11, 692–708 (2012). https://doi.org/10.1038/nrd3757
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3757
This article is cited by
-
Association of general and central obesity, and their changes with risk of knee osteoarthritis: a nationwide population-based cohort study
Scientific Reports (2023)
-
Arcuate AgRP, but not POMC neurons, modulate paraventricular CRF synthesis and release in response to fasting
Cell & Bioscience (2022)
-
Hepatic non-parenchymal S100A9-TLR4-mTORC1 axis normalizes diabetic ketogenesis
Nature Communications (2022)
-
Prophylactic Application of MA-[d-Leu-4]-OB3, a Small Molecule Synthetic Peptide Leptin Mimetic, Prevents or Slows the Progression of Obesity, Insulin Resistance, and Cognitive Impairment in a Mouse Model of Type 2 Diabetes Mellitus
International Journal of Peptide Research and Therapeutics (2021)
-
Leptin brain entry via a tanycytic LepR–EGFR shuttle controls lipid metabolism and pancreas function
Nature Metabolism (2021)