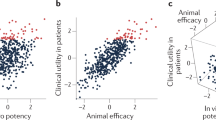
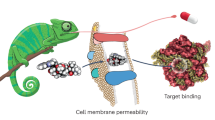
Abstract
Given its position at the heart of small-molecule drug discovery, medicinal chemistry has an important role in tackling the well-known productivity challenges in pharmaceutical research and development. In recent years, extensive analyses of successful and failed discovery compounds and drug candidates have improved our understanding of the role of physicochemical properties in drug attrition. Based on the clarified challenges in finding the 'sweet spot' in medicinal chemistry programmes, we suggest that this goal can be achieved through a combination of first identifying chemical starting points with appropriate 'nature' and then rigorously 'nurturing' them during lead optimization. Here, we discuss scientific, strategic, organizational and cultural considerations for medicinal chemistry practices, with the aim of promoting more effective use of what is already known, as well as a wider appreciation of the risks of pursuing suboptimal compounds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
05 October 2012
Michael M. Hann's postcode was originally incorrect; it has now been updated online.
References
Madea, B., Mußhoff, F. & Berghaus, G. (eds) Verkehrsmedizin: Fahreignung, Fahrsicherheit, Unfallrekonstruktion 435 (Deutscher Ärzte-Verlag, Köln, 2007).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23, 3–25 (1997).
Wenlock, M. C., Austin, R. P., Barton, P., Davis, A. M. & Leeson, P. D. A comparison of physiochemical property profiles of development and marketed oral drugs. J. Med. Chem. 46, 1250–1256 (2003).
Leeson, P. D. & Davis, A. M. Time-related differences in the physical property profiles of oral drugs. J. Med. Chem. 47, 6338–6348 (2004).
Mannhold, R., Poda, G. I., Ostermann, C. & Tetko, I. V. Calculation of molecular lipophilicity: state-of-the-art and comparison of log P methods on more than 96,000 compounds. J. Pharm. Sci. 98, 861–893 (2009).
Oprea, T. I., Davis, A. M., Teague, S. J. & Leeson, P. D. Is there a difference between leads and drugs? A historical perspective. J. Chem. Inf. Comput. Sci. 41, 1308–1315 (2001).
Hann, M. M., Leach, A. R. & Harper, G. Molecular complexity and its impact on the probability of finding leads for drug discovery. J. Chem. Inf. Comput. Sci. 41, 856–864 (2001).
Hann, M. M. & Oprea, T. I. Pursuing the leadlikeness concept in pharmaceutical research. Curr. Opin. Chem. Biol. 8, 255–263 (2004).
Leeson, P. D. & Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nature Rev. Drug Discov. 6, 881–890 (2007).
Waring, M. Lipophilicity in drug discovery. Expert Opin. Drug Discov. 5, 235–248 (2010).
Waring, M. Defining optimum lipophilicity and molecular weight ranges for drug candidates — molecular weight dependent lower logD limits based on permeability. Bioorg. Med. Chem. Lett. 19, 2844–2851 (2009).
Gleeson, M. P. Generation of a set of simple, interpretable ADMET rules of thumb. J. Med. Chem. 51, 817–834 (2008).
Gleeson, M. P., Hersey, A., Montanari, D. & Overington, J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nature Rev. Drug Discov. 10, 197–208 (2011).
Keserü, G. M. 5th Drug Design Lead Discovery Conference 2009: lead finding strategies and optimization case studies. Drugs Future 35, 143–153 (2010).
Hann, M. M. Molecular obesity, potency and other addictions in drug discovery. MedChemComm. 2, 349–355 (2011).
Hopkins, A. L., Groom, C. R. & Alex, A. Ligand efficiency: a useful metric for lead selection. Drug Discov. Today 9, 430–431 (2004).
Keserü, G. M. & Makara, G. M. The influence of lead discovery strategies on the properties of drug candidates. Nature Rev. Drug Discov. 8, 203–212 (2009).
Ferenczy, G. G. & Keserü, G. M. Thermodynamics guided lead discovery and optimization. Drug Discov. Today 15, 919–932 (2010).
Morphy, R. The influence of target family and functional activity on the physicochemical properties of pre-clinical compounds. J. Med. Chem. 49, 2969–2978 (2006).
Vieth, M. & Sutherland, J. J. Dependence of molecular properties on proteomic family for marketed oral drugs. J. Med. Chem. 49, 3451–3453 (2006).
Paolini, G. V., Shapland, R. H. B., van Hoorn, W. P., Mason, J. S. & Hopkins, A. L. Global mapping of pharmacological space. Nature Biotech. 24, 805–815 (2006).
Southan, C., Boppana, K., Jagarlapudi, S. A. R. P. & Muresan, S. Analysis of in vitro bioactivity data extracted from drug discovery literature and patents: ranking 1654 human protein targets by assayed compounds and molecular scaffolds. J. Cheminform. 3, 14 (2011).
Egner, U. & Hillig, R. C. A structural biology view of target drugability. Expert Opin. Drug Discov. 3, 391–401 (2008).
Edfeldt, F. N. B., Folmer, R. H. A. & Breeze, A. L. Fragment screening to predict druggability (ligandability) and lead discovery success. Drug Discov. Today 16, 284–287 (2011).
Hajduk, P. J., Huth, J. R. & Tse, C. Predicting protein druggability. Drug Discov. Today 10, 1675–1682 (2005).
Swinney, D. C. & Anthony, J. How were new medicines discovered? Nature Rev. Drug Discov. 10, 507–519 (2011).
Merelli, X., Bourgeas, R. & Roche, P. Chemical and structural lessons from recent successes in protein–protein interaction inhibition (2P2I). Curr. Opin. Chem. Biol. 15, 1–7 (2011).
Alex, A., Millan, D. S., Perez, M., Wakenhut, F. & Whitlock, G. A. Intramolecular hydrogen bonding to improve membrane permeability and absorption in beyond rule of five chemical space. MedChemComm. 2, 669–674 (2011).
Leeson, P. D. & St-Gallay, S. A. The influence of the 'organizational factor' on compound quality in drug discovery. Nature Rev. Drug Discov. 10, 749–765 (2011).
Tyrchan, C., Blomberg, N., Engkvist, O., Kogej, T. & Muresan, S. Physicochemical property profiles of marketed drugs, clinical candidates and bioactive compounds. Bioorg. Med. Chem. Lett. 19, 6943–6947 (2009).
Murray, C. W. & Rees, D. C. The rise of fragment-based drug discovery. Nature Chem. 1, 187–192 (2009).
Macarron, R. et al. Impact of high-throughput screening in biomedical research. Nature Rev. Drug Discov. 10, 188–195 (2011).
Leach, A. R. & Hann, M. M. Molecular complexity and fragment-based drug discovery: ten years on. Curr. Opin. Chem. Biol. 15, 489–496 (2011).
Olsson, T. S., Williams, M. A., Pitt, W. R. & Ladbury, J. E. The thermodynamics of protein–ligand interaction and solvation: insights for ligand design. J. Mol. Biol. 384, 1002–1017 (2008).
Durrant, J. D. & McCammon, J. A. BINANA: a novel algorithm for ligand-binding characterization. J. Mol. Graph. Model. 29, 888–893 (2011).
Ladbury, J. E., Klebe, G. & Freire, E. Adding calorimetric data to decision making in lead discovery: a hot tip. Nature Rev. Drug Discov. 9, 23–27 (2010).
Brandt, T. et al. Congeneric but still distinct: how closely related trypsin ligands exhibit different thermodynamic and structural properties. J. Mol. Biol. 405, 1170–1187 (2011).
Snyder, P. W. et al. Mechanism of the hydrophobic effect in the biomolecular recognition of arylsulfonamides by carbonic anhydrase. Proc. Natl Acad. Sci. USA 108, 17889–17894 (2011).
Ferenczy, G. G. & Keserü, G. M. Enthalpic efficiency of ligand binding. J. Chem. Inf. Model. 50, 1536–1541 (2010).
Overington, J. P., Al-Lazikani, B. & Hopkins, A. L. How many drug targets are there? Nature Rev. Drug Discov. 5, 993–996 (2006).
Morphy, R. & Rankovic, Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 48, 6523–6543 (2005).
Olsson, T. S., Ladbury, J. E., Pitt, W. R. & Williams, M. A. Extent of enthalpy-entropy compensation in protein–ligand interactions. Protein Sci. 20, 1607–1618 (2011).
Copeland, R. A., Pompliano, D. L. & Meek, T. D. Drug–target residence time and its implications for lead optimization. Nature Rev. Drug Discov. 5, 730–739 (2006).
Schmidtke, P., Luque, F. J., Murray, J. B. & Barril, X. Shielded hydrogen bonds as structural determinants of binding kinetics: application in drug design. J. Am. Chem. Soc. 133, 18903–18910 (2011).
Reynolds, C. H., Tounge, B. A. & Bembenek, S. D. Ligand binding efficiency: trends, physical basis, and implications. J. Med. Chem. 51, 2432–2438 (2008).
Reynolds, C. H. & Holloway, M. K. Thermodynamics of ligand binding and efficiency. ACS Med. Chem. Lett. 2, 433–437 (2011).
Nissink, J. W. M. Simple size-independent measure of ligand efficiency. J. Chem. Inf. Model. 49, 1617–1622 (2009).
Mortenson, P. N. & Murray, C. W. Assessing the lipophilicity of fragments and early hits. J. Comput. Aided Mol. Des. 25, 663–667 (2011).
Wager, T. T. et al. Defining desirable central nervous system drug space through the alignment of molecular properties, in vitro ADME, and safety attributes. ACS Chem. Neurosci. 1, 420–434 (2010).
Tarcsay, A., Nyiri, K. & Keserü, G. M. The impact of lipophilic efficiency on compound quality. J. Med. Chem. 55, 1252–1260 (2012).
Wager, T. T., Hou, X., Verhoest, P. R. & Villalobos, A. Moving beyond rules: the development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chem. Neurosci. 1, 435–449 (2010).
Dack, K. Reducing Attrition Risk: Evolution of an in silico 'Compound Safety Evaluator'. Designing Safer Medicines In Discovery Symposium (Society of Chemical Industry, London, 17 March 2011).
Braggio, S., Montanari, D., Rossi, T. & Ratti, E. Drug efficiency: a new concept to guide lead optimization programs towards the selection of better clinical candidates. Expert Opin. Drug Discov. 5, 609–618 (2010).
Montanari, D. et al. Application of drug efficiency index in drug discovery: a strategy towards low therapeutic dose. Expert Opin. Drug Discov. 6, 913–920 (2011).
Bickerton, G. R., Paolini, G. V., Besnard, J., Muresan, S. & Hopkins, A. L. Quantifying druglikeness and target druggability. Nature Chem. 4, 90–98 (2012).
Stepan, A. F. et al. Structural alert/reactive metabolite concept as applied in medicinal chemistry to mitigate the risk of idiosyncratic drug toxicity: a perspective based on the critical examination of trends in the top 200 drugs marketed in the United States. Chem. Res. Toxicol. 24, 1345–1410 (2011).
Cooper, T. W., Campbell, I. B. & Macdonald, S. J. Factors determining the selection of organic reactions by medicinal chemists and the use of these reactions in arrays (small focused libraries). Angew. Chem. Int. Ed. Engl. 49, 8082–8091 (2010).
Roughley, S. D. & Jordan, A. M. The medicinal chemist's toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Walters, W. P., Green, J., Weiss, J. R. & Murcko, M. A. What do medicinal chemists actually make? A 50-year retrospective. J. Med. Chem. 54, 6405–6416 (2011).
Pitt, W. R., Parry, D. M., Perry, B. G. & Groom, C. R. Heteroaromatic rings of the future. J. Med. Chem. 52, 2952–2963 (2009).
Darvas, F. et al. High pressure, high temperature reactions in continuous flow. Merging discovery and process chemistry. Chemistry Today 27, 40–43 (2009).
Wegner, J., Ceylan, S. & Kirschning, A. Ten key issues in modern flow chemistry. Chem. Commun. (Camb.) 47, 4583–4592 (2011).
Nadin, A., Hattotuwagama, C. & Churcher, I. Lead-oriented synthesis: a new opportunity for synthetic chemistry. Angew. Chem. Int. Ed. Engl. 51, 1114–1122 (2011).
Tan, D. S. Diversity-oriented synthesis: exploring the intersections between chemistry and biology. Nature Chem. Biol. 1, 74–84 (2005).
Jones, S. B., Simmons, B., Mastracchio, A. & MacMillan, D. W. C. Collective synthesis of natural products by means of organocascade catalysis. Nature 475, 183–188 (2011).
Hill, A. P. & Young, R. J. Getting physical in drug discovery: a contemporary perspective on solubility and hydrophobicity. Drug Discov. Today 15, 648–655 (2010).
Young, R. J., Green, D. V., Luscombe, C. N. & Hill, A. P. Getting physical in drug discovery II: the impact of chromatographic hydrophobicity measurements and aromaticity. Drug Discov. Today 16, 822–830 (2011).
Hughes, J. D. et al. Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg. Med. Chem. Lett. 18, 4872–4875 (2008).
Paul, S. M. et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nature Rev. Drug Discov. 9, 203–214 (2010).
Dimitri, N. An assessment of R&D productivity in the pharmaceutical industry. Trends Pharm. Sci. 32, 683–685 (2011).
Butler, J. M. & Dressman, J. B. The developability classification system: application of biopharmaceutics concepts to formulation development. J. Pharm. Sci. 99, 4940–4954 (2010).
Bennani, Y. Drug discovery in the next decade: innovation needed ASAP. Drug Discov. Today 16, 779–792 (2011).
Abad-Zapatero, C. & Metz, J. T. Ligand efficiency indices as guideposts for drug discovery. Drug Discov. Today 10, 464–469 (2005).
Orita, M., Ohno, K. & Niimi, T. Two 'golden ratio' indices in fragment-based drug discovery. Drug Discov. Today 14, 321–328 (2009).
Acknowledgements
The authors thank the many scientists who have contributed to the ideas presented in this article, in particular: A. Leach, D. Green, I. Churcher, C. Dollery, J. Butler, A. Brewster, R. Young, A. Hill and K. Valkó at GlaxoSmithKline; Á. Tarcsay, Zs. Hadady, O. Éliás, G. Szabó, A. Visegrády, J. Éles, Gy. Domány and Gy. T. Balogh at Gedeon Richter; G. G. Ferenczy at Sanofi; P. Leeson at AstraZeneca; and G. Williams at Astex Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.M.K. is an employee of Gedeon Richter. M.M.H. is an employee of GlaxoSmithKline.
Supplementary information
Supplementary information
Finding the sweet spot in medicinal chemistry (PDF 463 kb)
Related links
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Hann, M., Keserü, G. Finding the sweet spot: the role of nature and nurture in medicinal chemistry. Nat Rev Drug Discov 11, 355–365 (2012). https://doi.org/10.1038/nrd3701
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3701
This article is cited by
-
The evolving role of investigative toxicology in the pharmaceutical industry
Nature Reviews Drug Discovery (2023)
-
Focused small molecule library of 5,6,7,8-tetrahydro[1,2,4]triazolo-[4,3-a]pyrazines: a brick for the house of medicinal chemistry
Chemistry of Heterocyclic Compounds (2023)
-
Pharmacoinformatic approach to identify potential phytochemicals against SARS-CoV-2 spike receptor-binding domain in native and variants of concern
Molecular Diversity (2023)
-
Imidazole–Thiazole Hybrid: Synthesis, Brain Penetration, Cytochrome P450 Enzyme and Gastrointestinal Absorption Study
Iranian Journal of Science (2023)
-
Transition metal complexes of pyridazine-based ligand: synthesis, characterization, biological activities, and molecular docking studies
Journal of the Iranian Chemical Society (2023)