Key Points
-
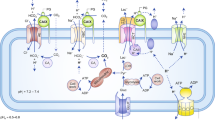
The regulation of pH in tumours involves the interplay of several proteins, including: the carbonic anhydrases (EC 4.2.1.1) CA2, CA9 and CA12; the vacuolar ATPase (V-ATPase); anion exchangers AE1, AE2 and AE3; Na+/HCO3− co-transporters (NBCs); electroneutral Na+-driven Cl−/HCO3− exchanger (NDCBE); the monocarboxylate transporters MCT1, MCT2, MCT3 and MCT4; and Na+/H+ exchanger 1, among others.
-
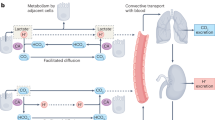
The concerted action of these proteins maintains a slightly alkaline intracellular pH (pHi) and an acidic extracellular pH (pHe) within the tumours, which favours the growth and spread of the primary tumour, leading to the formation of metastases.
-
The inhibition of one or more of these pH regulators with specific inhibitors causes both pHi and pHe values to return to normal, with the consequent impairment of tumour growth. This property represents an antitumour mechanism that is not exploited by the classical anticancer drugs.
-
The inhibition of CA9 and/or CA12 with sulphonamide- or coumarin-based small-molecule inhibitors reverses the effects of tumour acidification, leading to inhibition of cancer cell growth in both primary tumours and metastases. Some of these compounds are in preclinical development. This effect can also be exploited for the imaging and treatment of tumours that overexpress CA9 or CA12. The same effect has been observed with antibodies targeting CA9 (and, more recently, also CA12). Some of these antibodies (for example, cG250) are in Phase III clinical development as antitumour and diagnostic agents.
-
Some sulphonamides also inhibit anion exchangers, whereas proton pump inhibitors of the omeprazole type show antitumour effects by inhibiting V-ATPase, thus interfering with other tumour pH regulators.
-
Potent, specific and non-toxic compounds as well as antibodies that interfere with these proteins may represent valuable new antitumour drugs.
-
Changes in pHi towards basic values lead to the production of splice isoforms of extracellular matrix components at the tumour site, which are ideal targets for antibody-based pharmacodelivery strategies.
Abstract
The high metabolic rate of tumours often leads to acidosis and hypoxia in poorly perfused regions. Tumour cells have thus evolved the ability to function in a more acidic environment than normal cells. Key pH regulators in tumour cells include: isoforms 2, 9 and 12 of carbonic anhydrase, isoforms of anion exchangers, Na+/HCO3− co-transporters, Na+/H+ exchangers, monocarboxylate transporters and the vacuolar ATPase. Both small molecules and antibodies targeting these pH regulators are currently at various stages of clinical development. These antitumour mechanisms are not exploited by the classical cancer drugs and therefore represent a new anticancer drug discovery strategy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Folkman, J. Angiogenesis: an organizing principle for drug discovery? Nature Rev. Drug Discov. 6, 273–286 (2007). This is a comprehensive review on angiogenesis.
Gatenby, R. A. & Gillies, R. J. Why do cancers have high aerobic glycolysis? Nature Rev. Cancer 4, 891–899 (2004). This is an excellent review on the metabolism of cancer cells and the proposal of the somatic evolution theory of cancer.
Ebbesen, P. et al. Taking advantage of tumor cell adaptations to hypoxia for developing new tumor markers and treatment strategies. J. Enzyme Inhib. Med. Chem. 24 (Suppl. 1), 1–39 (2009). This is an updated and comprehensive review on hypoxia and its role in tumorigenesis and how these phenomena can be used to design anticancer drugs.
Fang, J. S., Gillies, R. J. & Gatenby, R. A. Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. Sem. Cancer Biol. 18, 330–337 (2008).
Sonveaux, P. et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 118, 3930–3942 (2008).
Huber, V. et al. Proton dynamics in cancer. J. Transl. Med. 8, 57 (2010).
Swietach, P. et al. Cancer-associated, hypoxia-inducible carbonic anhydrase IX facilitates CO2 diffusion. BJU Int. 101 (Suppl. 4), 22–24 (2008).
Swietach, P. et al. Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growth. J. Biol. Chem. 283, 20473–20483 (2008). This paper demonstrated the role of CA9 in the regulation of pH i in tumours.
Wykoff, C. C. et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 60, 7075–7083 (2000). This was the first report showing that CA9 is overexpressed as a consequence of the HIF1α activation pathway.
Borsi, L. et al. The alternative splicing pattern of the tenascin-C pre-mRNA is controlled by the extracellular pH. J. Biol. Chem. 270, 6243–6245 (1995).
Borsi, L., Allemanni, G., Gaggero, B. & Zardi, L. Extracellular pH controls pre-mRNA alternative splicing of tenascin-C in normal, but not in malignantly transformed, cells. Int. J. Cancer 66, 632–635 (1996).
Kremer, G. & Pouysségur, J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell 13, 472–482 (2008).
Pouysségur, J., Dayan, F. & Mazure, N. M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441, 437–443 (2006). This is an excellent review on the role of various proteins that are involved in the control of pH in tumour cells.
Supuran, C. T. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nature Rev. Drug Discov. 7, 168–181 (2008). This is a comprehensive review on carbonic anhydrases as drug targets.
Supuran, C. T. Carbonic anhydrase inhibitors. Bioorg. Med. Chem. Lett. 20, 3467–3474 (2010).
Pastorek, J. et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and putative helix–loop–helix DNA binding segment. Oncogene 9, 2877–2888 (1994). This was a seminal paper reporting the discovery of the protein that would later be called CA9.
Pérez-Sayáns, M. et al. V-ATPase inhibitors and implication in cancer treatment. Cancer Treat. Rev. 35, 707–713 (2009).
Sterling, D., Brown, N. J. D., Supuran, C. T. & Casey, J. R. The functional and physical relationship between the DRA bicarbonate transporter and carbonic anhydrase II. Am. J. Physiol. Cell. Physiol. 283, C1522–C1529 (2002).
Morgan, P. E., Supuran, C. T. & Casey, J. R. Carbonic anhydrase inhibitors that directly inhibit anion transport by the human Cl−/HCO3− exchanger, AE1. Mol. Membr. Biol. 21, 423–433 (2004).
Halestrap, A. P. & Price, N. T. The proton-linked mono-carboxylate transporter (MCT) family: structure, function and regulation. Biochem. J. 343, 281–299 (1999).
Enerson, B. E. & Drewes, L. R. Molecular features, regulation, and function of monocarboxylate transporters: implications for drug delivery. J. Pharm. Sci. 92, 1531–1544 (2003).
Parks, S. K., Chiche, J. & Pouysségur, J. pH control mechanisms of tumor survival and growth. J. Cell. Physiol. 226, 299–308 (2010).
Scozzafava, A., Mastrolorenzo, A. & Supuran, C. T. Modulation of carbonic anhydrase activity and its applications in therapy. Expert Opin. Ther. Pat. 14, 667–702 (2004).
Scozzafava, A., Mastrolorenzo, A. & Supuran, C. T. Carbonic anhydrase inhibitors and activators and their use in therapy. Expert Opin. Ther. Pat. 16, 1627–1664 (2006).
Supuran, C. T. Carbonic anhydrase inhibition/activation: trip of a scientist around the world in the search of novel chemotypes and drug targets. Curr. Pharm. Des. 16, 3233–3245 (2010).
Tureci, O. et al. Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc. Natl Acad. Sci. USA 95, 7608–7613 (1998). This paper reported the discovery of the second tumour-associated carbonic anhydrase, CA12.
Švastová, E. et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 577, 439–445 (2004). This was a proof-of-concept study on the role of CA9 in the acidification of tumours. It suggested that CA9 might be useful as a therapeutic target and for imaging hypoxic tumours.
Hilvo, M. et al. Biochemical characterization of CA IX: one of the most active carbonic anhydrase isozymes. J. Biol. Chem. 283, 27799–27809 (2008).
Scheurer, S. B. et al. Modulation of gene expression by hypoxia in umbilical vein endothelial cells: a transcriptomic and proteomic study. Proteomics 4, 1737–1760 (2004).
Innocenti, A. et al. The proteoglycan region of the tumor-associated carbonic anhydrase isoform IX acts as an intrinsic buffer optimizing CO2 hydration at acidic pH values characteristic of solid tumors. Bioorg. Med. Chem. Lett. 19, 5825–5828 (2009).
Alterio, V. et al. Crystal structure of the extracellular catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc. Natl Acad. Sci. USA 106, 16233–16238 (2009). This was a report of the three-dimensional structure of CA9, which was determined using X-ray crystallography.
Vullo, D. et al. Carbonic anhydrase inhibitors. Inhibition of the transmembrane isozyme XII with sulfonamides — a new target for the design of antitumor and antiglaucoma drugs? Bioorg. Med. Chem. Lett. 15, 963–969 (2005).
Pastorekova, S. et al. Carbonic anhydrase inhibitors: the first selective, membrane-impermeant inhibitors targeting the tumor-associated isozyme IX. Bioorg. Med. Chem. Lett. 14, 869–873 (2004).
Pastorekova, S., Parkkila, S., Pastorek, J. & Supuran, C. T. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J. Enzyme Inhib. Med. Chem. 19, 199–229 (2004).
Cecchi, A. et al. Carbonic anhydrase inhibitors. Design of fluorescent sulfonamides as probes of tumor-associated carbonic anhydrase IX that inhibit isozyme IX-mediated acidification of hypoxic tumors. J. Med. Chem. 48, 4834–4841 (2005).
Thiry, A., Dogné, J.-M., Masereel, B. & Supuran, C. T. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol. Sci. 27, 566–573 (2006).
Guler, O. O., De Simone, G. & Supuran, C. T. Drug design studies of the novel antitumor targets carbonic anhydrase IX and XII. Curr. Med. Chem. 17, 1516–1526 (2010).
Alterio, V. et al. Carbonic anhydrase inhibitors: X-ray and molecular modeling study for the interaction of a fluorescent antitumor sulfonamide with isozyme II and IX. J. Am. Chem. Soc. 128, 8329–8335 (2006).
De Simone, G. et al. Carbonic anhydrase inhibitors: hypoxia-activatable sulfonamides incorporating disulfide bonds that target the tumor-associated isoform IX. J. Med. Chem. 49, 5544–5551 (2006).
Ahlskog, J. K. J. et al. In vivo targeting of tumor-associated carbonic anhydrases using acetazolamide derivatives. Bioorg. Med. Chem. Lett. 19, 4851–4856 (2009). This was a proof-of-concept study regarding the the anticancer effects of in vivo inhibition of CA9 in tumour-bearing animals.
Maresca, A. et al. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 131, 3057–3062 (2009). This paper reported the discovery of a new class of carbonic anhydrase inhibitors.
Maresca, A. et al. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J. Med. Chem. 53, 335–344 (2010).
Stiti, M. et al. Carbonic anhydrase inhibitor coated gold nanoparticles selectively inhibit the tumor-associated isoform IX over the cytosolic ubiquitous isozymes I and II. J. Am. Chem. Soc. 130, 16130–16131 (2008).
Dubois, L. et al. Imaging the hypoxia surrogate marker CA IX requires expression and catalytic activity for binding fluorescent sulfonamide inhibitors. Radiother. Oncol. 83, 367–373 (2007).
Dubois, L. et al. Imaging of CA IX with fluorescent labelled sulfonamides distinguishes hypoxic and (re)-oxygenated cells in a xenograft tumour model. Radiother. Oncol. 92, 423–428 (2009). This was a proof-of-concept study for the in vivo imaging of hypoxic tumours with sulphonamide-based CA9 inhibitors.
Chiche, J. et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 69, 358–368 (2009). This paper reported the discovery that genetic silencing of CA9 and CA12 has potent antitumour effects.
Lou, Y. et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 71, 3364–3376 (2011). This was the first report to show that sulphonamide and/or coumarin inhibitors potently inhibit the growth of primary tumours and metastases by inhibiting CA9.
Buller, F. et al. Selection of carbonic anhydrase IX inhibitors from one million DNA-encoded compounds. ACS Chem. Biol. 6, 336–344 (2011).
Pacchiano, F. et al. Ureido-substituted benzenesulfon-amides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J. Med. Chem. 54, 1896–1902 (2011).
Dubois, L. et al. Specific inhibition of CA IX activity enhances the therapeutic effect of tumor irradiation. Radiother. Oncol. 99, 424–431 (2011).
Maresca, A., Scozzafava, A. & Supuran, C. T. 7,8-Disubstituted- but not 6,7-disubstituted coumarins selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II in the low nanomolar/subnanomolar range. Bioorg. Med. Chem. Lett. 20, 7255–7258 (2010).
Pearson, M. A. & Fabbro, D. Targeting protein kinases in cancer therapy: a success? Expert. Rev. Anticancer Ther. 4, 1113–1124 (2004).
Parkkila, S. et al. The protein tyrosine kinase inhibitors imatinib and nilotinib strongly inhibit several mammalian α-carbonic anhydrase isoforms. Bioorg. Med. Chem. Lett. 19, 4102–4106 (2009). This paper reported the discovery that imatinib and nilotinib exert potent inhibitory effects on carbonic anhydrase, and that this may be an additional antitumour mechanism of these drugs.
Parkkila, S. et al. Carbonic anhydrase II. A novel biomarker for gastrointestinal stromal tumors. Mod. Pathol. 23, 743–750 (2010).
Battke, C. et al. Generation and characterization of the first inhibitory antibody targeting tumour-associated carbonic anhydrase XII. Cancer Immunol. Immunother. 60, 649–658 (2011).
Stillebroer, A. B. et al. Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur. Urol. 58, 75–83 (2010).
Winter, G. & Milstein, C. Man-made antibodies. Nature 349, 293–299 (1991).
Winter, G., Griffiths, A. D., Hawkins, R. E. & Hoogenboom, H. R. Making antibodies by phage display technology. Annu. Rev. Immunol. 12, 433–455 (1994).
Carter, P. J. Potent antibody therapeutics by design. Nature Rev. Immunol. 6, 343–357 (2010).
Chrastina, A. et al. Biodistribution and pharmacokinetics of 125I-labeled monoclonal antibody M75 specific for carbonic anhydrase IX, an intrinsic marker of hypoxia, in nude mice xenografted with human colorectal carcinoma. Int. J. Cancer 105, 873–881 (2003).
Siebels, M. et al. A clinical phase I/II trial with the monoclonal antibody cG250 (RENCAREX®) and interferon-α-2a in metastatic renal cell carcinoma patients. World J. Urol. 29, 121–126 (2011). These preliminary data reported the successful use of a CA9-specific monoclonal antibody as an anticancer drug in patients with renal carcinoma.
Steffens, M. G. et al. In vivo and in vitro characterizations of three 99mTc-labeled monoclonal antibody G250 preparations. J. Nucl. Med. 40, 829–836 (1999).
van Schaijk, F. G. et al. Pretargeting with bispecific anti-renal cell carcinoma x anti-DTPA(In) antibody in 3 RCC models. J. Nucl. Med. 46, 495–501 (2005).
Low, P. & Kularatne, S. A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 13, 256–262 (2009).
Fais, S. Proton pump inhibitor-induced tumour cell death by inhibition of a detoxification mechanism. J. Intern. Med. 267, 515–525 (2010). This paper described an unorthodox way of managing tumour acidification with a class of drugs that were originally designed for the treatment of peptic ulcers.
Spugnini, E., Citro, G. & Fais, S. Proton pump inhibitors as anti vacuolar-ATPases drugs: a novel anticancer strategy. J. Exp. Clin. Cancer Res. 29, 44 (2010).
Ohta, T. et al. Bafilomycin A1 induces apoptosis in the human pancreatic cancer cell line Capan-1. J. Pathol. 185, 324–330 (1998).
De Milito, A. et al. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int. J. Cancer 127, 207–219 (2010).
Horn, J. The proton-pump inhibitors: similarities and differences. Clin. Ther. 22, 266–280 (2000).
Denny, W. A. & Wilson, W. R. Considerations for the design of nitrophenyl mustards as agents with selective toxicity for hypoxic tumor cells. J. Med. Chem. 29, 879–887 (1986).
Johnson, D. E. & Casey, J. R. in Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications (eds Supuran, C. T. & Winum, J. Y.) 415–437 (Wiley, Hoboken, New Jersey, 2009). This book provides an updated review on HCO 3− transporters and the metabolons in which they are involved.
Sowah, D. & Casey, J. R. An intramolecular transport metabolon: fusion of carbonic anhydrase II to the COOH terminus of the Cl−/HCO3− exchanger, AE1. Am. J. Physiol. Cell Physiol. 301, C336–C346 (2011).
Morgan, P. E. et al. Interactions of transmembrane carbonic anhydrase, CA IX, with bicarbonate transporters. Am. J. Physiol. Cell. Physiol. 293, C738–C748 (2007).
Jessen, F., Sjøholm, C. & Hoffmann, E. K. Identification of the anion exchange protein of ehrlich cells: a kinetic analysis of the inhibitory effects of 4, 4′-diisothiocyano-2,2′-stilbene-disulfonic acid (DIDS) and labeling of membrane proteins with 3H-DIDS. J. Membr. Biol. 92, 195–205 (1986).
Liu, C. J. et al. Anion exchanger inhibitor DIDS induces human poorly-differentiated malignant hepatocellular carcinoma HA22T cell apoptosis. Mol. Cell. Biochem. 308, 117–125 (2008).
Supuran, C. T., Scozzafava, A. & Casini, A. Carbonic anhydrase inhibitors. Med. Res. Rev. 23, 146–189 (2003).
Supuran, C. T., Casini, A., Mastrolorenzo, A. & Scozzafava, A. COX-2 selective inhibitors, carbonic anhydrase inhibition and anticancer properties of sulfonamides belonging to this class of pharmacological agents. Mini Rev. Med. Chem. 4, 625–632 (2004).
Yamagata, M. & Tannock, I. F. The chronic administration of drugs that inhibit the regulation of intracellular pH: in vitro and anti-tumour effects. Br. J. Cancer 73, 1328–1334 (1996).
Di Sario, A. et al. Selective inhibition of ion transport mechanisms regulating intracellular pH reduces proliferation and induces apoptosis in cholangiocarcinoma cells. Dig. Liver Dis. 39, 60–69 (2007).
Wong, P., Lee, C. & Tannock, I. F. Reduction of intracellular pH as a strategy to enhance the pH-dependent cytotoxic effects of melphalan for human breast cancer cells. Clin. Cancer Res. 11, 3553–3557 (2005).
Lagarde, A. E., Franchi, A. J., Paris, S. & Pouysségur, J. M. Effect of mutations affecting Na+:H+ antiport activity on tumorigenic potential of hamster lung fibroblasts. J. Cell. Biochem. 36, 249–260 (1988).
Harley, W. et al. Dual inhibition of sodium-mediated proton and calcium efflux triggers non-apoptotic cell death in malignant gliomas. Brain Res. 1363, 159–169 (2010).
Masereel, B., Pochet, L. & Laeckmann, D. An overview of inhibitors of Na+/H+ exchanger. Eur. J. Med. Chem. 38, 547–554 (2003).
Theroux, P. et al. Inhibition of the sodium-hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations. Circulation 102, 3032–3038 (2000).
Feron, O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother. Oncol. 92, 329–333 (2009).
Ullah, M. S., Davies, A. J. & Halestrap, A. P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J. Biol. Chem. 281, 9030–9037 (2006).
Chiche, J. et al. In vivo pH in metabolic-defective Ras-transformed fibroblast tumors: key role of the monocarboxylate transporter, MCT4, for inducing an alkaline intracellular pH. Int. J. Cancer 30 May 2011 (doi:10.1002/ijc.26125).
Khandoudi, N. et al. Inhibition of the cardiac electrogenic sodium bicarbonate cotransporter reduces ischemic injury. Cardiovasc. Res. 52, 387–396 (2001).
De Giusti, V. et al. Antibodies against the cardiac sodium/bicarbonate cotransporter (NBCe1) as a pharmacological tool. Br. J. Pharmacol. 19 May 2011 (doi: 10.1111/j.1476-5381.2011.01496).
Chiappe de Cingolani, G. E. et al. Involvement of AE3 isoform of Na+-independent Cl−/HCO3− exchanger in myocardial pHi recovery from intracellular alkalinization. Life Sci. 78, 3018–3026 (2006).
Brack, S. S., Silacci, M., Birchler, M. & Neri, D. Tumor-targeting properties of novel antibodies specific to the large isoform of tenascin-C. Clin. Cancer Res. 12, 3200–3208 (2006).
Trachsel, E. et al. A human mAb specific to oncofetal fibronectin selectively targets chronic skin inflammation in vivo. J. Invest. Dermatol. 127, 881–886 (2007).
Schwager, K. et al. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immuno-cytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res. Ther. 11, R142 (2009).
Schwager, K. et al. The antibody-mediated targeted delivery of interleukin-10 inhibits endometriosis in a syngeneic mouse model. Human Reprod. 26, 2344–2352 (2011).
Pedretti, M. et al. Comparative immunohistochemistry of L19 and F16 in non-small cell lung cancer and mesothelioma: two human antibodies investigated in clinical trials in patients with cancer. Lung Cancer 64, 28–33 (2009).
Fiechter, M. et al. Comparative in vivo analysis of the atherosclerotic plaque targeting properties of eight human monoclonal antibodies. Atherosclerosis 214, 325–330 (2011).
Chiquet-Ehrismann, R. & Tucker, R. P. Tenascins and the importance of adhesion modulation. Cold Spring Harb. Perspect. Biol. 3, a004960 (2011).
Neri, D. & Bicknell, R. Tumour vascular targeting. Nature Rev. Cancer 5, 436–446 (2005). This is a comprehensive review on targeting the tumour vasculature.
Carnemolla, B. et al. Identification of a glioblastoma-associated tenascin-C isoform by a high affinity recombinant antibody. Am. J. Pathol. 154, 1345–1352 (1999).
Silacci, M. et al. Human monoclonal antibodies to domain C of tenascin-C selectively target solid tumors in vivo. Protein Eng. Des. Sel. 19, 471–478 (2006).
Berndt, A. et al. A comparative analysis of oncofetal fibronectin and tenascin-C incorporation in tumour vessels using human recombinant SIP format antibodies. Histochem. Cell. Biol. 133, 467–475 (2010).
Castellani, P. et al. The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. Int. J. Cancer 59, 612–618 (1994).
Kaczmarek, J. et al. Distribution of oncofetal fibronectin isoforms in normal, hyperplastic and neoplastic human breast tissues. Int. J. Cancer 59, 11–16 (1994).
Carnemolla, B. et al. Phage antibodies with pan-species recognition of the oncofoetal angiogenesis marker fibronectin ED-B domain. Int. J. Cancer 68, 397–405 (1996).
Castellani, P. et al. Differentiation between high- and low-grade astrocytoma using a human recombinant antibody to the extra domain-B of fibronectin. Am. J. Pathol. 161, 1695–1700 (2002).
Rybak, J. N. et al. The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Res. 67, 10948–10957 (2007).
Sauer, S. et al. Expression of the oncofetal ED-B-containing fibronectin isoform in hematologic tumors enables ED-B-targeted 131I-L19SIP radioimmunotherapy in Hodgkin lymphoma patients. Blood 113, 2265–2274 (2009).
Schliemann, C. et al. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL2. Blood 113, 2275–2283 (2009).
Schliemann, C. et al. Three clinical-stage tumor targeting antibodies reveal differential expression of oncofetal fibronectin and tenascin-C isoforms in human lymphoma. Leuk. Res. 33, 1718–1722 (2009).
Castronovo, V. et al. A chemical proteomics approach for the identification of accessible antigens expressed in human kidney cancer. Mol. Cell. Proteomics 5, 2083–2091 (2006).
Borgia, B. et al. A proteomic approach for the identification of vascular markers of liver metastasis. Cancer Res. 70, 309–318 (2010).
Schliemann, C. et al. In vivo biotinylation of the vasculature in B-cell lymphoma identifies BST-2 as a target for antibody-based therapy. Blood 115, 736–744 (2010).
Soltermann, A. et al. Prognostic significance of epithelial–mesenchymal and mesenchymal–epithelial transition protein expression in non-small cell lung cancer. Clin. Cancer Res. 14, 7430–7437 (2008).
Acknowledgements
Research in our laboratories is financed by a European Union grant of the Seventh Framework Programme (Metoxia; C.T.S.), the ETH Zürich (D.N.), the Swiss National Science Foundation (D.N.), the SwissBridge Foundation (D.N.) and the Stammbach Foundation (D.N.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Claudiu T. Supuran is an inventor on patents of carbonic anhydrase inhibitors, and Dario Neri is an inventor on patents of fibronectin and tenascin antibodies. Dario Neri is also a co-founder and shareholder of Philogen.
Glossary
- Warburg effect
-
The ability of cancer cells to predominantly produce energy by a high rate of glycolysis followed by lactic acid fermentation in the cytosol, rather than by glycolysis followed by oxidation of pyruvate in the mitochondria, as most normal cells do.
- Orthotopic tumours
-
Tumours that occur at the normal place in the body in an animal model of cancer (for example, mouse orthotopic mammary tumours develop in the mammary gland of the animal).
- Metabolon
-
A temporary structural–functional complex formed between sequential enzymes or proteins of a metabolic pathway. It is held together by non-covalent interactions and structural elements of the cell, such as integral membrane proteins and proteins of the cytoskeleton.
Rights and permissions
About this article
Cite this article
Neri, D., Supuran, C. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 10, 767–777 (2011). https://doi.org/10.1038/nrd3554
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3554
This article is cited by
-
Genetic requirements for repair of lesions caused by single genomic ribonucleotides in S phase
Nature Communications (2023)
-
Metabolic targeting of malignant tumors: a need for systemic approach
Journal of Cancer Research and Clinical Oncology (2023)
-
Tumor-responsive dynamic nanoassemblies for boosted photoimmunotherapy
Nano Research (2023)
-
The synthesis and study of carbonic anhydrase activity of sulfonamide-containing dibenzo[1,4]thiazepines
Chemistry of Heterocyclic Compounds (2023)
-
Investigating the Use of Coumarin Derivatives as Lasers
Journal of Fluorescence (2023)