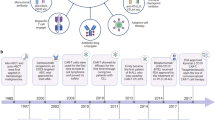
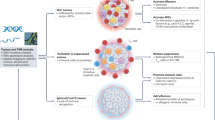
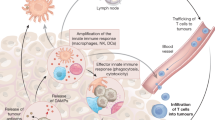
Key Points
-
Clinical development of immunotherapy has been hampered by the complexity of defining the optimal dose and schedule, and the lack of financial support.
-
There is an urgent need for assays that can adequately monitor the induced antitumour immune response and predict the response to immunotherapy.
-
The kinetics of antitumour responses are considerably different between immunotherapy and cytotoxic chemotherapy, as initial progression and even the appearance of new lesions may precede tumour shrinkage following immunotherapy.
-
The clinical applicability of immunotherapy has recently been boosted by the positive results of various approaches, such as CTLA4-specific antibody therapy.
-
Recent findings on a positive interaction between immunotherapy and chemotherapy warrant further investigations
Abstract
Our insight into antitumour immune responses has increased considerably during the past decades, yet the development of immunotherapy as a treatment modality for cancer has been hampered by several factors. These include difficulties in the selection of the optimal dose and schedule, the methods of evaluation, and financial support. Although durable clinical remissions have been observed with various immunotherapeutic strategies, the percentage of patients who benefited from these interventions has remained too small to justify the general use of such strategies. However, the recent positive results of clinical trials with novel immunoactive drugs as well as the unexpected finding of a positive interaction between immunotherapy and chemotherapy may herald a new era for the immunotherapy of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Coley, W. B. The treatment of inoperable sarcoma with the mixed toxins of erysipelas and bacillus prodigiosus: immediate and final results in one hundred and forty cases. JAMA 31, 389–395 (1898).
Gajewski, T. F., Louahed, J. & Brichard, V. G. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 16, 399–403 (2010).
Giaccone, G. et al. A Phase I study of the natural killer T-cell ligand α-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer Res. 8, 3702–3709 (2002).
Garland, S. M. et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 356, 1928–1943 (2007).
Paavonen, J. et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374, 301–314 (2009).
Kenter, G. G. et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 361, 1838–1847 (2009).
Moore, P. S. & Chang, Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nature Rev. Cancer 10, 878–889 (2010).
Kaufman, H. L. et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 17, 718–730 (2010).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
De Francesco, L. Landmark approval for Dendreon's cancer vaccine. Nature Biotech. 28, 531–532 (2010).
Schuster, S. J. et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J. Clin. Oncol. 31 May 2011 (doi:10.1200/JCO.2010.33.3005).
Schwartzentruber, D. J. et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N. Engl. J. Med. 364, 2119–2127 (2011).
Kantoff, P. W. et al. Overall survival analysis of a Phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 28, 1099–1105 (2010).
Vansteenkiste, J. et al. Final results of a multi-center, double-blind, randomized, placebo controlled Phase II study to assess the efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in stage IB/II non-small cell lung cancer (NSCLC). J. Clin. Oncol. (Meeting Abstracts) 25, S7554 (2007).
Dougan, M. & Dranoff, G. Immune therapy for cancer. Annu. Rev. Immunol. 27, 83–117 (2009).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nature Med. 10, 909–915 (2004).
Medical Research Council Renal Cancer Collaborators. Interferon-α and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Lancet 353, 14–17 (1999).
Eggermont, A. M. et al. Post-surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): randomised controlled trial. Lancet 366, 1189–1196 (2005).
Peggs, K. S., Quezada, S. A., Chambers, C. A., Korman, A. J. & Allison, J. P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206, 1717–1725 (2009).
van Elsas, A., Hurwitz, A. A. & Allison, J. P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190, 355–366 (1999).
Ribas, A. et al. Antitumor activity in melanoma and anti-self responses in a Phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J. Clin. Oncol. 23, 8968–8977 (2005).
Weber, J. S. et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J. Clin. Oncol. 26, 5950–5956 (2008).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010). This was the first evidence of the survival benefit of CTLA4-specific antibody therapy with ipilimumab in metastatic melanoma.
Ribas A. et al. Phase III, open-label, randomized, comparative study of tremelimumab (CP-675,206) and chemotherapy (temozolomide [TMZ] or dacarbazine [DTIC]) in patients with advanced melanoma. J. Clin. Oncol. (Meeting Abstracts) 26, LBA9011 (2008).
Robert, C. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 5 Jun 2011 (doi:10.1056/NEJMoa1104621).
Zou, W. & Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nature Rev. Immunol. 8, 467–477 (2008).
Thompson, R. H. et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 66, 3381–3385 (2006).
Curiel, T. J. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Med. 10, 942–949 (2004).
Bates, G. J. et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin. Oncol. 24, 5373–5380 (2006).
Salama, P. et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 27, 186–192 (2009).
Farinha, P. et al. The architectural pattern of FOXP3-positive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood 115, 289–295 (2010).
de Vries, I. J. et al. Frequency of circulating Tregs with demethylated FOXP3 intron 1 in melanoma patients receiving tumor vaccines and potentially Treg-depleting agents. Clin. Cancer Res. 17, 841–848 (2011).
Dannull, J. et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Invest. 115, 3623–3633 (2005).
Jacobs, J. F. et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a Phase I/II study in metastatic melanoma patients. Clin. Cancer Res. 16, 5067–5078 (2010).
Rech, A. J. & Vonderheide, R. H. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann. NY Acad. Sci. 1174, 99–106 (2009).
Litzinger, M. T. et al. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood 110, 3192–3201 (2007).
Sylvester, R. J. et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur. Urol. 57, 766–773 (2010).
Heslop, H. E. et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 115, 925–935 (2010).
Dudley, M. E. et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298, 850–854 (2002). This research showed that the adoptive transfer of tumour-reactive T cells in patients with melanoma induces tumour regressions as well as autoimmune melanocyte destruction.
Dudley, M. E. et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 23, 2346–2357 (2005).
Besser, M. J. et al. Clinical responses in a Phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin. Cancer Res. 16, 2646–2655 (2010).
Morgan, R. A. et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314, 126–129 (2006). This research showed that the adoptive transfer of autologous lymphocytes that are transduced with a retrovirus encoding a T cell receptor in patients with melanoma results in durable engraftment and objective tumour responses.
Johnson, L. A. et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114, 535–546 (2009).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Lamers, C. H. et al. Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol. Immunother. 56, 1875–1883 (2007).
Robbins, P. F. et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924 (2011).
Kochenderfer, J. N. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010).
de Vries, I. J. et al. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J. Clin. Oncol. 23, 5779–5787 (2005). In patients with metastatic melanoma who were treated with autologous antigen-pulsed dendritic cells, a correlation was shown between clinical outcome and antitumour reactivity in delayed-type hypersensitivity skin biopsy samples.
Schuler-Thurner, B. et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J. Exp. Med. 195, 1279–1288 (2002).
Banchereau, J. et al. Immune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 61, 6451–6458 (2001).
Janetzki, S. et al. “MIATA” — minimal information about T cell assays. Immunity 31, 527–528 (2009).
Lurquin, C. et al. Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J. Exp. Med. 201, 249–257 (2005).
World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. (WHO, Geneva, 1979).
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 92, 205–216 (2000).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Weber, J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol. Immunother. 58, 823–830 (2009).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Hoos, A. et al. Improved endpoints for cancer immunotherapy trials. J. Natl Cancer Inst. 102, 1388–1397 (2010).
Zitvogel, L. et al. The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest. 118, 1991–2001 (2008).
Bast, R. C. Jr et al. Regression of established tumors and induction of tumor immunity by intratumor chemotherapy. J. Natl Cancer Inst. 56, 829–832 (1976).
Turk, J. L., Parker, D. & Poulter, L. W. Functional aspects of the selective depletion of lymphoid tissue by cyclophosphamide. Immunology 23, 493–501 (1972).
Lutsiak, M. E. et al. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 105, 2862–2868 (2005).
Nowak, A. K., Robinson, B. W. & Lake, R. A. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 63, 4490–4496 (2003). In this article (as well as in Ref. 86) the possible synergistic effect of cytotoxic chemotherapy and immunotherapy is demonstrated for the first time.
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature Med. 13, 54–61 (2007).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature Med. 13, 1050–1059 (2007).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nature Med. 15, 1170–1178 (2009). The seminal studies in Refs 64–66 provide definite proof that cytotoxic chemotherapy and radiotherapy can induce an immunogenic form of tumour cell death.
Tesniere, A. et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 29, 482–491 (2009).
Nistico, P. et al. Chemotherapy enhances vaccine-induced antitumor immunity in melanoma patients. Int. J. Cancer 124, 130–139 (2008).
Lesterhuis, W. J. et al. A pilot study on the immunogenicity of dendritic cell vaccination during adjuvant oxaliplatin/capecitabine chemotherapy in colon cancer patients. Br. J. Cancer 103, 1415–1421 (2010).
Nierkens, S. et al. In vivo colocalization of antigen and CpG within dendritic cells is associated with the efficacy of cancer immunotherapy. Cancer Res. 68, 5390–5396 (2008).
Blander, J. M. & Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440, 808–812 (2006).
Rakhra, K. et al. CD4+ T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell 18, 485–498 (2010).
Flaherty, K. T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010).
Milas, L. et al. CpG oligodeoxynucleotide enhances tumor response to radiation. Cancer Res. 64, 5074–5077 (2004).
Rosenberg, S. A. et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N. Engl. J. Med. 316, 889–897 (1987).
West, W. H. et al. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N. Engl. J. Med. 316, 898–905 (1987).
Schadendorf, D. et al. Immunotherapy of distant metastatic disease. Ann. Oncol. 20 (Suppl. 6), vi41–vi50 (2009).
Zhang, H. et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nature Med. 11, 1238–1243 (2005).
Palucka, K., Banchereau, J. & Mellman, I. Designing vaccines based on biology of human dendritic cell subsets. Immunity 33, 464–478 (2010).
Lesterhuis, W. J. et al. Dendritic cell vaccines in melanoma: from promise to proof? Crit. Rev. Oncol. Hematol. 66, 118–134 (2008).
de Vries, I. J. et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nature Biotech. 23, 1407–1413 (2005).
Nestle, F. O. et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nature Med. 4, 328–332 (1998). This was the first demonstration of the clinical efficacy of vaccination with autologous antigen-loaded dendritic cells in patients with cancer.
Schadendorf, D. et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized Phase III trial of the DC study group of the DeCOG. Ann. Oncol. 17, 563–570 (2006).
Shurin, G. V., Tourkova, I. L., Kaneno, R. & Shurin, M. R. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J. Immunol. 183, 137–144 (2009).
Alfaro, C. et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br. J. Cancer 100, 1111–1119 (2009).
Nowak, A. K. et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J. Immunol. 170, 4905–4913 (2003).
Gassner, F. J. et al. Fludarabine modulates composition and function of the T cell pool in patients with chronic lymphocytic leukaemia. Cancer Immunol. Immunother. 60, 75–85 (2010).
Bracci, L. et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin. Cancer Res. 13, 644–653 (2007).
Vincent, J. et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70, 3052–3061 (2010).
Zhao, J. et al. Selective depletion of CD4+CD25+Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res. 70, 4850–4858 (2010).
Beyer, M. et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood 106, 2018–2025 (2005).
Desar, I. M. et al. Sorafenib reduces the percentage of tumour infiltrating regulatory T cells in renal cell carcinoma patients. Int. J. Cancer 129, 507–512 (2010).
Correale, P. et al. Treatment of colon and breast carcinoma cells with 5-fluorouracil enhances expression of carcinoembryonic antigen and susceptibility to HLA-A(*)02.01 restricted, CEA-peptide-specific cytotoxic T cells in vitro. Int. J. Cancer 104, 437–445 (2003).
Ramakrishnan, R. et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J. Clin. Invest. 120, 1111–1124 (2010).
Burnet, M. Cancer — a biological approach. I. The processes of control. Br. Med. J. 1, 779–786 (1957).
Zinkernagel, R. M. & Doherty, P. C. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature 251, 547–548 (1974).
Zinkernagel, R. M. & Doherty, P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature 248, 701–702 (1974).
Steinman, R. M. & Cohn, Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 137, 1142–1162 (1973).
Bevan, M. J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 143, 1283–1288 (1976).
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 (1995).
Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J. M. & Hoffmann, J. A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 (1996).
van der Bruggen, P. et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254, 1643–1647 (1991).
Kawakami, Y. et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc. Natl Acad. Sci. USA 91, 3515–3519 (1994).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A. Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Morales, A., Eidinger, D. & Bruce, A. W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 116, 180–183 (1976).
Bindon, C. et al. Clearance rates and systemic effects of intravenously administered interleukin 2 (IL-2) containing preparations in human subjects. Br. J. Cancer 47, 123–133 (1983).
Kirkwood, J. M. et al. Comparison of intramuscular and intravenous recombinant α-2 interferon in melanoma and other cancers. Ann. Intern. Med. 103, 32–36 (1985).
Rosenberg, S. A. et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 313, 1485–1492 (1985).
Lienard, D., Ewalenko, P., Delmotte, J. J., Renard, N. & Lejeune, F. J. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J. Clin. Oncol. 10, 52–60 (1992).
van Seters, M. et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N. Engl. J. Med. 358, 1465–1473 (2008).
Thomas, E. D., Lochte, H. L. Jr, Lu, W. C. & Ferrebee, J. W. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 257, 491–496 (1957).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Den Brok, M. H. et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 64, 4024–4029 (2004).
Nathan, P. D. & Eisen, T. G. The biological treatment of renal-cell carcinoma and melanoma. Lancet Oncol. 3, 89–96 (2002).
Acknowledgements
W.J.L. is supported by a Translation Research Fellowship of the Dutch Cancer Society and by the Netherlands Organization for Scientific Research (Grant 920-03-250).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Cytotoxic T lymphocyte
-
Cytotoxic (CD8+) lymphocytes can kill tumour cells following recognition of tumour-associated antigens that are presented by major histocompatibility complex class I.
- Cytotoxic T lymphocyte-associated antigen 4
-
(CTLA4). A co-inhibitory molecule that is expressed by T cells. Binding of its ligands B7.1 or B7.2 on antigen-presenting cells results in negative regulation of T cell activity.
- Toll-like receptor 4
-
(TLR4). A member of the Toll-like receptor family of innate immune receptors that recognize molecular patterns of microbes or danger signals derived from tissue damage.
- NLRP3
-
NOD-, LRR and pyrin domain-containing 3. This is a pyrin-like protein that is involved in inflammation and immune responses.
- Crosspresentation
-
The mechanism by which certain APCs take up, process and present extracellular antigens on MHC class I molecules to stimulate cytotoxic T cells. This property is atypical, as most cells exclusively present peptides from endogenous proteins on MHC class I molecules.
- Immunogenic cell death
-
The process of immunogenic cell death takes place when dying tumour cells provide an alerting or activating signal to the immune system.
Rights and permissions
About this article
Cite this article
Lesterhuis, W., Haanen, J. & Punt, C. Cancer immunotherapy – revisited. Nat Rev Drug Discov 10, 591–600 (2011). https://doi.org/10.1038/nrd3500
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3500
This article is cited by
-
Prognostic value of EBV DNA and platelet-to-lymphocyte ratio in patients with non-metastatic nasopharyngeal carcinoma: a retrospective study
BMC Cancer (2023)
-
Responsive biomaterials: optimizing control of cancer immunotherapy
Nature Reviews Materials (2023)
-
Metabolic tagging of extracellular vesicles and development of enhanced extracellular vesicle based cancer vaccines
Nature Communications (2023)
-
Application of Nano-Delivery Systems in Lymph Nodes for Tumor Immunotherapy
Nano-Micro Letters (2023)
-
Bio-adhesive Macroporous Hydrogels for In Situ Recruitment and Modulation of Dendritic Cells
Cellular and Molecular Bioengineering (2023)