Abstract
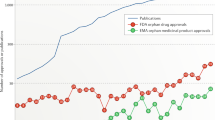
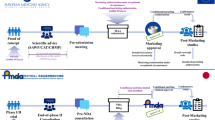
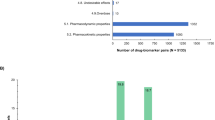
In 2000, regulation on orphan medicinal products was adopted in the European Union with the aim of benefiting patients who suffer from serious, rare conditions for which there is currently no satisfactory treatment. Since then, more than 850 orphan drug designations have been granted by the European Commission based on a positive opinion from the Committee for Orphan Medicinal Products (COMP), and more than 60 orphan drugs have received marketing authorization in Europe. Here, stimulated by the tenth anniversary of the COMP, we reflect on the outcomes and experience gained in the past decade, and contemplate issues for the future, such as catalysing drug development for the large number of rare diseases that still lack effective treatments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
European Commission. Communication from the commission to the European parliament, the council, the European economic and social committee and the committee of the regions on rare diseases: Europe's challenges. European Commission [online], (2008).
Stolk, P., Willemen, M. J. C & Leufkens, H. G. M. Rare essentials: drugs for rare diseases as essential medicines. Bull. World Health Organ. 84, 745–751 (2006).
Aymé, S. EUGLOREH project: the status of health in the European Union: towards a healthier Europe: full report. 5.15. Rare Diseases. EUGLOREH 2007 [online], (2007).
Heemstra, H. E., de Vrueh, R. L., van Weely, S., Büller, H. A. & Leufkens, H. G. Predictors of orphan drug approval in the European Union. Eur. J. Clin. Pharmacol. 64, 545–552 (2008).
Haffner, M., Torrent-Farnell, J. & Maher, P. D. Does orphan drug legislation really answer the needs of patients? Lancet 371, 2041–2044 (2008).
Braun, M. M., Farag-El-Massah, S., Xu, K. & Coté, T. R. Emergence of orphan drugs in the United States: a quantitative assessment of the first 25 years. Nature Rev. Drug Discov. 9, 519–522 (2010).
Westermark, K. The regulation of orphan medicines in the EU: objectives reached and main challenges when facing the future. Pharmaceut. Policy Law 9, 327–342 (2007).
The European Parliament and the Council of the European Union. Regulation (EC) No. 141/2000 of the European Parliament and of the Council of 16 December 1999 on orphan medicinal products. Official J. Eur. Communities L18/1–L18/5 (2000).
Mariz, S., Llinares, J. & Westermark, K. Orphan drugs: EU regulations. BMJ 342, d136 (2011).
Schneider, C. K. et al. Challenges with advanced therapy medicinal products and how to meet them. Nature Rev. Drug Discov. 9, 195–201 (2010).
Regnstrom, J. et al. Factors associated with success of market authorisation applications for pharmaceutical drugs submitted to the European Medicines Agency. Eur. J. Clin. Pharmacol. 66, 39–48 (2010).
EMA. Press release: Regulatory cooperation expanded. European Medicines Agency [online], (2007).
[No authors listed.] Making rare diseases a public-health and research priority. Lancet 371, 1972 (2008).
Parisse-Brassens, J. Improving patient access to orphan drugs in Europe. Eurordis — The Voice of Rare Disease Patients in Europe [online], (2006).
European Parliament. Charter of Fundamental Rights of the European Union. European Parliament [online], (2000).
US FDA. New resource for drug developers: the rare disease repurposing database (RDRD). US FDA [online], (2010).
Buckley, B. M. Clinical trials of orphan medicines. Lancet 371, 2051–2055 (2008).
Eichler, H. G., Pignatti, F., Flamion, B., Leufkens, H. & Breckenridge, A. Balancing early market access to new drugs with the need for benefit/risk data: a mounting dilemma. Nature Rev. Drug Discov. 7, 818–26 (2008).
Liberti, L. et al. Expediting patients' access to medicines by improving the predictability of drug development and the regulatory approval process. Clin. Pharmacol. Ther. 87, 27–31 (2010).
Braiteh, F. & Kurzrock, R. Uncommon tumors and exceptional therapies: paradox or paradigm? Mol. Cancer Ther. 6, 1175–1179 (2007).
Fishman, M. C. & Porter, J. A. Pharmaceuticals: a new grammar for drug discovery. Nature 437, 491–493 (2005).
Acknowledgements
The Chairperson of the COMP would like to specifically acknowledge the invaluable input of COMP colleagues who actively contributed to this work by coordinating the discussions of subheadings and coordinating the participation of the entire committee, namely B. Sepodes (COMP member, nominated by the EMA's recommendation); J. T. i Farnell (COMP member, Spain); F. Rivière, J. Llinares-Garcia and S. Aarum (EMA COMP Scientific Secretariats), L. Greene and B. B. Holm (COMP patients' representatives);and M. Mavris (COMP General Observer, EURORDIS). The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the EMA or one of its committees or working parties.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The author declare no competing financial interests.
Additional information
A full list of authors appears in Box 2.
Related links
Rights and permissions
About this article
Cite this article
The Committee for Orphan Medicinal Products and the European Medicines Agency Scientific Secretariat. European regulation on orphan medicinal products: 10 years of experience and future perspectives. Nat Rev Drug Discov 10, 341–349 (2011). https://doi.org/10.1038/nrd3445
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3445
This article is cited by
-
The importance of the policy framework on orphan drug accessibility
Journal of Public Health (2023)
-
Using four decades of FDA orphan drug designations to describe trends in rare disease drug development: substantial growth seen in development of drugs for rare oncologic, neurologic, and pediatric-onset diseases
Orphanet Journal of Rare Diseases (2021)
-
DDIEM: drug database for inborn errors of metabolism
Orphanet Journal of Rare Diseases (2020)
-
FDA orphan products clinical trial grants: assessment of outcomes and impact on rare disease product development
Orphanet Journal of Rare Diseases (2020)
-
Pharmaceutical compounding of orphan active ingredients in Belgium: how community and hospital pharmacists can address the needs of patients with rare diseases
Orphanet Journal of Rare Diseases (2019)