Abstract
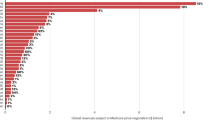
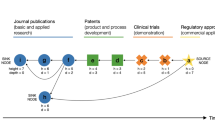
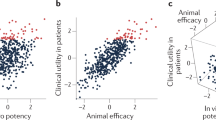
Understanding the factors that promote drug innovation is important both for improvements in health care and for the future of organizations engaged in drug discovery research and development. By identifying the inventors of 252 new drugs approved by the US Food and Drug Administration from 1998 to 2007 and their places of work, and also classifying these drugs according to innovativeness, this study investigates the contribution of different types of organizations and regions to drug innovation during this period. The data indicate that drugs initially discovered in biotechnology companies or universities accounted for approximately half of the scientifically innovative drugs approved, as well as half of those that responded to unmet medical needs, although their contribution to the total number of new drugs was proportionately lower. The biotechnology companies were located mainly in the United States. This article presents a comprehensive analysis of these data and discusses potential contributing factors to the trends observed, with the aim of aiding efforts to promote drug innovation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
12 November 2010
In figure 7, the second column in panels a and b should have been labelled 'NDA/BLA applicant'. In the legend for figure 7, and in the article text related to this figure on p879, the subset of the drugs analysed in panel a are those with peak year sales above US$500 million, rather than mean peak year sales.
References
Pisano, G. P. Science Business: The Promise, the Reality and the Future of Biotech (Harvard Business School Press, Boston, 2006).
Lawrence, S. Biotech drugs blaze a trail. Nature Biotechnol. 24, 736 (2006).
Natanson, L. & van Brunt, J. 20 compounds that defined biotech. Signals Magazine [online] (27 Jun 2008).
Munos, B. Lessons from 60 years of pharmaceutical innovation. Nature Rev. Drug Discov. 8, 959–968 (2009).
Kneller, R. Correspondence: The origin of new drugs. Nature Biotechnol. 23, 529–530 (2005).
Kneller, R. Correspondence: The national origins of new drugs. Nature Biotechnol. 23, 655–656 (2005).
Haffner, M. E. Adopting orphan drugs — two dozen years of treating rare diseases. N. Engl. J. Med. 354, 445–447 (2006).
Kneller, R. W. in Reconfiguring Knowledge Production: Changing Authority Relations in the Sciences and Their Consequences for Intellectual Innovation (eds Whitley, R., Gläser, J. & Engvall, L.) 110–145 (Oxford University Press, Oxford, 2010).
Kneller, R. in Changing Governance of the Sciences: the Advent of Research Evaluation Systems (eds Whitley, R. & Glaser, J.) 51–73 (Springer, Dordecht, 2007).
Normile, D. Older scientists win majority of funding. Science 303, 1746 (2004).
Kneller, R. Bridging Islands: Venture Companies and the Future of Japanese and American Industry 42–167, 232–376 (Oxford University Press, Oxford, 2007).
Mervis, J. And then there was one. Science 321, 1622–1628 (2008).
Saxenian, A. Regional Advantage: Culture and Competition in Silicon Valley and Route 128 1–19, 105–259 (Harvard University Press, Cambridge, 1996).
Hyde, A. Working in Silicon Valley: Economic and Legal Analysis of a High Velocity Labor Market (M. E. Sharpe, Armonk NY, 2003).
Christensen, C. M. The rigid disk drive industry: a history of commercial and technological turbulence. Bus. Hist. Rev. 67, 531–588 (1993).
Zucker, L. G. & Darby, M. R. Costly information: firm transformation, exit or persistent failure. Am. Behav. Sci. 39, 959–974 (1997).
Garnier, J.-P. Rebuilding the R & D engine in Big Pharma. Harvard Bus. Rev. 86, 68–76 (2008).
Saxenian, A. The New Argonauts: Regional Advantage in a Global Economy 1–121, 125–138 (Harvard University Press, Cambridge, 2006).
Frantz, S. 2004 approvals: the demise of the blockbuster? Nature Rev. Drug Discov. 4, 93–94 (2005).
Hughes, B. Pharma pursues novel models for academic collaboration. Nature Rev. Drug Discov. 7, 631–632 (2008).
Jarvis, L. M. New deal: seeking closer ties, drug companies and universities shake up the model for research alliances. Chem. Eng. News 86, 13–20 (2008).
Kneller, R. Autarkic drug discovery in Japanese pharmaceutical companies: insights into national differences in industrial innovation. Res. Policy 32, 1805–1827 (2003).
Rader, R. A. Biopharmaceutical Products in the U.S. and European Markets. 6th edn Vols 1 & 2 (Bioplan Associates, Maryland, 2007).
Savarino, A. A historical sketch of the discovery and development of HIV-1 integrase inhibitors. Expert Opin. Investig. Drugs 15, 1507–1522 (2006).
Sternitzke, C., Bartkowski, A. & Schramm, R. Visualizing patent statistics by means of social network analysis tools. World Patent Inf. 30, 115–131 (2008).
Acknowledgements
I thank researchers (usually the listed patent inventors) who helped to clarify apparent discrepancies between the patent history and the development information for several drugs. The helpful comments of the referees are also greatly appreciated.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author received no financial support for this research other than grants-in-aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT). These grants-in-aid supported research related to the role of start-ups in innovation generally, not specifically in pharmaceuticals. The author consults for two Japanese biotechnology companies, for which his only compensation is equity or stock options. Neither of these companies is mentioned, or specifically alluded to, in this article. He is also an advisor to RIKEN (The Institute of Chemistry and Physics), a basic-research laboratory supported by the Japanese government. Otherwise, he has no interests that might be perceived to influence the results and discussion reported in this article. The author has not shown this paper to, or discussed its contents with, any organization representing biotechnology or pharmaceutical companies.
Supplementary information
Supplementary information Box S1
Additional notes on data sources and analysis methodology (PDF 363 kb)
Supplementary information Table S2
Lists of all 252 drugs with attributions and other key features (XLS 218 kb)
Supplementary information Box S3
Notes on the attribution, classification or characteristics of particular drugs or groups of drugs (PDF 717 kb)
Supplementary information Box S4
Special topic notes (PDF 402 kb)
Related links
Rights and permissions
About this article
Cite this article
Kneller, R. The importance of new companies for drug discovery: origins of a decade of new drugs. Nat Rev Drug Discov 9, 867–882 (2010). https://doi.org/10.1038/nrd3251
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3251
This article is cited by
-
SPARKing academic technologies across the valley of death
Nature Biotechnology (2024)
-
Biopharma innovation trends during COVID-19 and beyond: an evidence from global partnerships and fundraising activities, 2011-2022
Globalization and Health (2023)
-
Role of global public sector research in discovering new drugs and vaccines
The Journal of Technology Transfer (2023)
-
Mind the gap: closing the growing chasm between academia and industry
Nature Biotechnology (2022)
-
Determinants of radical drug innovation: a systematic literature review
Management Review Quarterly (2022)