Key Points
-
Alzheimer's disease (AD) is the largest unmet medical need in neurology. Current drugs improve symptoms, but do not have profound disease-modifying effects.
-
Formal demonstration of efficacy in disease modification requires trials of extended duration with a large number of participants.
-
Criteria for the diagnosis of early AD and the inclusion of patients with early AD in clinical trials will be crucial to improve treatment outcomes.
-
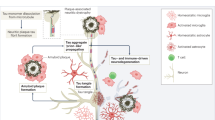
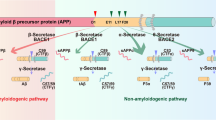
Treatment approaches aimed at the production and clearance of the amyloid-β peptide (Aβ) — a cardinal feature of AD that is thought to be important in disease pathogenesis — are the most advanced, with four drugs currently in Phase III.
-
Among the anti-Aβ therapeutics small-molecule drug development is focused on β-secretase and γ-secretase inhibitors. Clinical trials of both inhibitor classes are underway.
-
Active and passive immunization are being pursued in several ongoing clinical studies to clear Aβ monomers and/or deposits.
-
Approaches to block the progression of tau pathology are at an earlier stage of development than anti-Aβ efforts. It is hoped that tau therapeutics will provide benefit throughout the course of the disease, but generally accepted tractable targets have yet to emerge.
-
AD pathology has an inflammatory component, but there is currently no consensus about whether and how it should be targeted therapeutically.
-
In addition, a number of clinical treatment approaches are based on the idea that a metabolic defect that is not directly reflected in the hallmarks of AD pathology may have a major role in the disease process.
Abstract
Alzheimer's disease is the largest unmet medical need in neurology. Current drugs improve symptoms, but do not have profound disease-modifying effects. However, in recent years, several approaches aimed at inhibiting disease progression have advanced to clinical trials. Among these, strategies targeting the production and clearance of the amyloid-β peptide — a cardinal feature of Alzheimer's disease that is thought to be important in disease pathogenesis — are the most advanced. Approaches aimed at modulating the abnormal aggregation of tau filaments (another key feature of the disease), and those targeting metabolic dysfunction, are also being evaluated in the clinic. This article discusses recent progress with each of these strategies, with a focus on anti-amyloid strategies, highlighting the lessons learned and the challenges that remain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Davis, K. L. & Samuels, S. C. in Pharmacological Management of Neurological and Psychiatric Disorders (eds Enna, S. J. & Coyle, J. T.) 267–316 (McGraw-Hill, New York, 1998).
Alzheimer, A. Über eine eigenartige Erkrankung der Hirnrinde. Centralblatt fur Nervenheilkunde Psychiatrie 30, 177–179 (1907) (in German). Alzheimer's first description of the disease — a classic.
McGeer, P. L. & McGeer, E. NSAIDs and Alzheimer's disease: epidemiological, animal model and clinical studies. Neurobiol. Aging 28, 639–647 (2007).
Cruts, M. & Van Broeckhoven, C. Molecular genetics of Alzheimer's disease. Ann. Med. 30, 560–565 (1998).
Rovelet-Lecrux, A. et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nature Genet. 38, 24–26 (2006).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923 (1993).
Mayeux, R. in Handbook of Clinical Neurology (eds Duyckaerts, C. & Litvan, I.) 195–205 (2008).
Hsiao, K. et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996).
SantaCruz, K. et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481 (2005).
Selkoe, D. J. & Schenk, D. Alzheimer's disease: molecular understanding predicts amyloid-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 43, 545–584 (2003).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002). An influential review of the amyloid hypothesis.
Walsh, D. M. & Selkoe, D. J. Aβ oligomers — a decade of discovery. J. Neurochem. 101, 1172–1184 (2007).
Haass, C. et al. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359, 322–325 (1992).
Dovey, H. F. et al. Functional γ-secretase inhibitors reduce β-amyloid peptide levels in brain. J. Neurochem. 76, 173–181 (2001).
DeStrooper, B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active γ-secretase complex. Neuron 38, 9–12 (2003).
Parks, A. L. & Curtis, D. Presenilin diversifies its portfolio. Trends Genet. 23, 140–150 (2007).
De Strooper, B. et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522 (1999). First description of the Notch-γ secretase connection.
Wong, G. T. et al. Chronic treatment with the γ-secretase inhibitor LY-411,575 inhibits β-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J. Biol. Chem. 279, 12876–12882 (2004).
Milano, J. et al. Modulation of Notch processing by γ-secretase inhibitors causes intestinal goblet cellmetaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci. 82, 341–358 (2004).
Fleisher, A. S. et al. Phase 2 safety trial targeting amyloid β production with a γ-secretase inhibitor in Alzheimer disease. Arch. Neurol. 65, 1031–1038 (2008).
Bateman, R. J. et al. A γ-secretase inhibitor decreases amyloid-β production in the central nervous system. Ann. Neurol. 66, 48–54 (2009).
Martone, R. et al. GSI-953 (begacestat): a novel, selective thiophene sulfonamide inhibitor of APP γ-secretase for the treatment of Alzheimer's disease. J. Pharmacol. Exp. Ther. 331, 598–608 (2009).
Imbimbo, B. P. Alzheimer's disease: γ-secretase inhibitors. Drug Discov. Today 5, 169–175 (2008).
Jarrett, J. T., Berger, E. P. & Lansbury, P. T. Jr. The carboxy terminus of the β amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry 32, 4693–4697 (1993).
Weggen, S. et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414, 212–216 (2001).
Leuchtenberger, S., Beher, D. & Weggen, S. Selective modulation of Aβ42 production in Alzheimer's disease: non-steroidal anti-inflammatory drugs and beyond. Curr. Pharm. Des. 12, 1–19 (2006).
Kukar, T. L. et al. Substrate-targeting γ-secretase modulators. Nature 453, 925–929 (2008).
McGeer, P. L., Schulzer, M. & McGeer, E. G. Arthritis and antiinflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiological studies. Neurology 47, 425–432 (1996).
Green, R.C., Schneider, L. S, Hendrix, S.B., Zavitz, K.H. & Swabb, E. Safety and efficacy of tarenflurbil in subjects with mild Alzheimer's disease: results from an 18-month multi-center phase 3 trial. Alzheimers Dement. 4 (Suppl. 2), T165.
Galasko, D. R. et al. Safety, tolerability, pharmacokinetics, and Aβ levels after short-term administration of R-flurbiprofen in healthy elderly individuals. Alzheimer Dis. Assoc. Disord. 21, 292–299 (2007).
Citron, M. β-Secretase inhibition for the treatment of Alzheimer's disease — promise and challenge. Trends Pharmacol. Sci. 25, 59–112 (2004).
Velliquette, R. A., O'Connor, T. & Vassar, R. Energy inhibition elevates β-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer's disease pathogenesis. J. Neurosci. 25, 10874–10883 (2005).
Willem, M. et al. Control of peripheral nerve myelination by the β-secretase BACE1. Sciencexpress 1–7 (2006).
Hu, X. et al. BACE1 modulates myelination in the central and peripheral nervous system. Nature Neurosci. 9, 1520–1525 (2006).
Sankaranarayanan, S. et al. In vivo β-secretase 1 inhibition leads to brain Aβ lowering and increased α-secretase processing of amyloid precursor protein without effect on neuregulin-1. J. Pharmacol. Exp. Ther. 324, 957–969 (2008).
Hu, X. et al. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 22, 2970–2980 (2008).
Harrison, S. M. et al. BACE1 (β-secretase) transgenic and knockout mice: identification of neurochemical deficits and behavioral changes. Mol. Cell. Neurosci. 24, 646–655 (2003).
Laird, F. M. et al. BACE1, a major determinant of selective vulnerability of the brain to amyloid-β amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J. Neurosci. 25, 11693–11709 (2005).
Gerlai, R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 19, 177–181 (1996).
CoMentis. Press release 28 Jul 2008: CoMentis and Astellas to present Alzheimer's disease research at International Conference on Alzheimer's Disease (ICAD). CoMentis website [online], (2008).
Leung, D., Abbenante, G. & Fairlie, D. P. Protease inhibitors: current status and future prospects. J. Med. Chem. 43, 305–341 (2000).
Durham, T. B. & Shepherd, T. A. Progress toward the discovery and development of efficacious BACE inhibitors. Curr. Opin. Drug Discov. Develop. 9, 776–791 (2006). A review summarizing the medicinal chemistry challenges of β-secretase inhibitor development.
Nitsch, R. M., Slack, B. E., Wurtman, R. J. & Growdon, J. H. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science 258, 304–307 (1992).
Hock, C. et al. Treatment with the selective muscarinic M1 agonist talsaclidine decreases cerebrospinal fluid levels of Aβ42 in patients with Alzheimer's disease. Amyloid 10, 1–6 (2003).
Gervais, F. et al. Targeting soluble Aβ peptide with tramiprosate for the treatment of brain amyloidosis. Neurobiol. Aging 28, 537–547 (2007).
Aisen, P. S. et al. Clinical data on Alzhemed after 12 months in patients with mild to moderate Alzheimer's disease. Neurobiol. Aging 25, S20.
McLaurin, J. et al. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nature Med. 12, 801–808 (2006).
Frederickson, C. J., Koh, J. Y. & Bush, A. I. The neurobiology of zinc in health and disease. Nature 6, 449–462 (2005).
Cherny, R. A. et al. Treatment with a copper–zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron 30, 665–676 (2001).
Lannfelt, L. et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 7, 779–786 (2008).
Eckman, E. A. & Eckman, C. B. Aβ-degrading enzymes: modulators of Alzheimer's disease pathogenesis and targets for therapeutic intervention. Biochem. Soc. Trans. 23, 1101–1105 (2005).
Jacobsen, S. et al. Catabolic clearance of Aβ following treatment with Pai-1 inhibitors. Neurodegen. Dis. 4 (Suppl. 1), 22 (2007).
Deane, R., Wu, Z. & Zlokovic, B. V. RAGE (yin) versus LRP (yang) balance regulates Alzheimer amyloid β-peptide clearance through transport across the blood–brain barrier. Stroke 35 (11 Suppl.1), 2628–2631 (2004).
Dodel, R. et al. Human antibodies against amyloid β peptide: a potential treatment for Alzheimer's disease. Ann. Neurol. 52, 253–256 (2002).
Schenk, D. et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177 (1999). First high-profile publication to discuss Aβ immunization as a therapeutic approach.
Morgan, D. et al. Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature 408, 982–985 (2000).
Janus, C. et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature 408, 979–982 (2000).
Hrncic, R. et al. Antibody-mediated resolution of light chain-associated amyloid deposits. Am. J. Pathol. 157, 1239–1246 (2000).
Bard, F. et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature Med. 6, 916–919 (2000).
Frenkel, D., Katz, O. & Solomon, B. Immunization against Alzheimer's β-amyloid plaques via EFRH phage administration. Proc. Natl Acad. Sci. USA 97, 11455–11459 (2000).
DeMattos, R. B. et al. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. USA 98, 8850–8855 (2001).
Dodart, J. C. et al. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer's disease model. Nature Neurosci. 5, 452–457 (2002).
Racke, M. M. et al. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid β. J. Neurosci. 25, 629–636 (2005).
Thakker, D. R. et al. Intracerebroventricular amyloid-β antibodies reduce cerebral amyloid angiopathy and associated micro-hemorrhages in aged Tg2576 mice. Proc. Natl Acad. Sci. USA 106, 4501–4506 (2009).
Siemers, E. R. et al. P4-346: Safety, tolerability and biomarker effects of an Aβ monoclonal antibody administered to patients with Alzheimer's disease. Alzheimers Dement. 4 (Suppl. 1), T774 (2008).
Tsakanikas, D., Shah, K., Flores, C., Assuras, S. & Relkin, N. R. P4-351: Effects of uninterrupted intravenous immunoglobulin treatment of Alzheimer's disease for nine months. Alzheimers Dement. 4 (Suppl. 1), T776 (2008).
Salloway, S. et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer's disease. Neurology 73, 2061–2070 (2009).
Brody, D. L. & Holtzman, D. M. Active and passive immunotherapy for neurodegenerative disorders. Ann. Rev. Neurosci. 31, 175–193 (2008).
Holmes, C. et al. Long-term effects of Aβ42 immunisation in Alzheimer's disease: follow up of a randomised, placebo-controlled phase I trial. Lancet 372, 216–223 (2008).
Small, S. A. & Duff, K. Linking Aβ and tau in late-onset Alzheimer's disease: a dual pathway hypothesis. Neuron 60, 534–542 (2009).
Vellas, B. et al. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr. Alzheimer Res. 6, 144–151 (2009).
Lambert, J. C. et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nature Genet. 41, 1094–1099 (2009).
Wyss-Coray, T. Inflammation in Alzheimer's disease: driving force, bystander or beneficial response. Nature Med. 12, 1005–1015 (2006). An excellent review of the complicated role of inflammation in AD.
Heneka, M. T. & Landreth, G. E. PPARs in the brain. Biochem. Biophys. Acta 1771, 1031–1045 (2007).
Harrington, C. et al. Effects of rosiglitazone-extended release as adjunctive therapy to acetylcholinesterase inhibitors over 48 weeks on cognition in Apoe4-stratified subjects with mild-to-moderate Alzheimer's disease. Alzheimers Dementia 5, (Suppl. 1), e17–e18 (2009).
Liang, X. et al. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer's disease. J. Neurosci. 25, 10180–10187 (2005).
Thal, D. et al. Alzheimer-related tau-pathology in the perforant path target zone and in the hippocampal stratum oriens and radiatum correlates with onset and degree of dementia. Exp. Neurol. 163, 98–110 (2000).
Hutton, M. et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998).
Goedert, M., Klug, A. & Crowther, R. Tau protein, the paired helical filament and Alzheimer's disease. J. Alzheimers Dis. 9, 195–207 (2006). An excellent review of tau biology.
Schneider, A. & Mandelkow, E. Tau-based treatment strategies in neurodegenerative diseases. Neurotherapeutics 5, 443–457 (2008).
Lee, V. & Trojanowski, J. Progress from Alzheimer's tangles to pathological tau points towards more effective therapies now. J. Alzheimers Dis. 9, 257–262 (2006).
Bulic, B. et al. Development of tau aggregation inhibitors for Alzheimer's disease. Angew. Chem. Int. Ed. 48, 1740–1752 (2009).
Wischik, C., Bentham, P., Wischik, D. & Seng, K. O3-04-07: Tau aggregation inhibitor (TAI) therapy with rember™ arrests disease progression in mild and moderate Alzheimer's disease over 50 weeks. Alzheimers Dement. 4 (Suppl. 1), T167 (2008).
Mahley, R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240, 622–630 (1988).
Bertram, L. & Tanzi, R. E. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nature Rev. Neurosci. 9, 768–778 (2008).
Bu, G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nature Rev. Neurosci. 10, 333–344 (2009).
Mahley, R. W., Weisgraber, K. H. & Huang, Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc. Natl Acad. Sci. USA 103, 5644–5651 (2006).
Fagan, A. M. et al. Human and murine ApoE markedly alters Aβ metabolism before and after plaque formation in a mouse model of Alzheimer's disease. Neurobiol. Dis. 9, 305–318 (2002). An important animal model study describing in vivo effects of APOE isoforms on Aβ metabolism.
Cao, G., Bales, K. R., DeMattos, R. B. & Paul, S. M. Liver X receptor-mediated gene regulation and cholesterol homeostasis in brain: relevance to Alzheimer's disease therapeutics. Curr. Alzheimer Res. 4, 179–184 (2007).
Vanhanen, M. et al. Association of metabolic syndrome with Alzheimer disease. Neurology 67, 843–847 (2006).
Wolozin, B. Cholesterol and the biology of Alzheimer's disease. Neuron 41, 7–10 (2004).
Fassbender, K. et al. Simvastatin strongly reduces levels of Alzheimer's disease β-amyloid peptides Aβ42 and Aβ40 in vitro and in vivo. Proc. Natl Acad. Sci. USA 98, 5856–5861 (2001).
Puglielli, L. et al. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid β-peptide. Nature Cell Biol. 3, 905–912 (2001).
Kandiah, N. & Feldman, H. H. Therapeutic potential of statins in Alzheimer's disease. J. Neurol. Sci. 283, 230–234 (2009).
Mazziotta, J. C., Frackowiak, R. S. & Phelps, M. E. The use of positron emission tomography in the clinical assesment of dementia. Semin. Nucl. Med. 22, 233–246 (1992).
Reiman, E. M. et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the ɛ4 allele for apolipoprotein E. N. Engl. J. Med. 334, 752–758 (1996).
Reiman, E. M. et al. Declining brain activity in cognitively normal apolipoprotein E ɛ4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc. Natl. Acad. Sci. USA 98, 3334–3339 (2001).
Craft, S. Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis. Assoc. Disord. 20, 298–301 (2006).
Doody, R. S. et al. Effect of dimebon on cognition, activities of daily living, behaviour and global function in patients with mild-to-moderate Alzheimer's disease: a randomised, double-blind, placebo-controlled study. Lancet 372, 207–215 (2008).
Bachurin, S. et al. Antihistamine agent dimebon as a novel neuroprotector and cognition enhancer. Ann. NY Acad. Sci. 939, 425–435 (2001).
Medivation. Press release 3 Mar 2010: Pfizer and Medivation announce results from two Phase 3 studies in Dimebon (latrepirdine*) Alzheimer's disease clinical development program. Medivation website [online], (2010).
Jack, C. R. et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain 132, 1355–1365 (2009). A widely discussed study discussing the temporal sequence of biomarker changes in AD — important for drug development.
Dubois, B. et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746 (2007). An important paper suggesting diagnostic criteria for early AD — crucial for efforts to treat AD earlier.
Winblad, B. & Wimo, A. Pharmacoeconomics in Alzheimer's disease. Neurodegenerative Dis. 4, 5 (2007).
Alzheimer's Association. 2009 Alzheimer's disease facts and figures. Alzheimers Dement. 5, 234–270 (2009).
Aisen, P. S. Development of a disease-modifying treatment for Alzheimer's disease: Alzhemed. Alzheimers Dement. 2, 153–154 (2006).
Mohs, R. C., Kawas, C. & Carrillo, M. C. Optimal design of clinical trials for drugs designed to slow the course of Alzheimer's disease. Alzheimers Dement. 2, 131–139 (2006).
Bateman, R. J. Aβ turnover in human subjects. Alzheimers Dement. 4 (Suppl. 1), T123–T124 (2008).
Acknowledgements
I would like to thank R. Mohs and E. Siemers for helpful discussions. Special thanks to J. B. Lindborg for tracking everything in this rapidly moving field.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Martin Citron is an employee and shareholder of Eli Lilly and Company.
Related links
Rights and permissions
About this article
Cite this article
Citron, M. Alzheimer's disease: strategies for disease modification. Nat Rev Drug Discov 9, 387–398 (2010). https://doi.org/10.1038/nrd2896
Issue Date:
DOI: https://doi.org/10.1038/nrd2896
This article is cited by
-
Disease modification in inflammatory skin disorders: opportunities and challenges
Nature Reviews Drug Discovery (2023)
-
Homogeneity Estimation in Multivariate Generalized Linear Models
Communications in Mathematics and Statistics (2023)
-
Disease-Modifying Therapies for Alzheimer's Disease: More Questions than Answers
Neurotherapeutics (2022)
-
Mitochondria: how eminent in ageing and neurodegenerative disorders?
Human Cell (2022)
-
Pyridazinones containing the (4-methoxyphenyl)piperazine moiety as AChE/BChE inhibitors: design, synthesis, in silico and biological evaluation
Medicinal Chemistry Research (2022)